Abstract
In this work, garden waste Clivia miniata leaves (CL) were used as a feedstock to produce high performance biochar (BCL-K-H) through the pretreatment processes of alkali hydrolysis combined with acid hydrolysis. Specifically, CL is subjected to a two-step pretreatment process to destroy its dense wood fibre structure, and then the pretreatment product is processed into biochar by carbonation activation. The results demonstrated that the physicochemical properties of the modified Clivia miniata leaves biochar (BCL-K-H) prepared by the pretreatment process were altered in comparison with those of Clivia miniata leaves biochar (BCL). The specific surface area increased from 3052.12 to 3555.31 m2/g, and the total pore volume increased from 1.94 to 2.91 cm3/g. The characterisation results demonstrated that the surface of BCL-K-H contained a significant number of reactive functional groups (e.g. hydroxyl, hydrocarbon and carboxyl groups, etc.), which may provide a substantial number of active sites during the adsorption of pollutants and enhance the adsorption performance. The pollutants tetracycline hydrochloride (TH) and synthetic dye Rhodamine B (RhB) were studied to gain insight into biochars’ performance. The maximum adsorption capacity of BCL-K-H for pollutants was found to be 1436.56 mg/g (TH) and 1505.47 mg/g (RhB). The adsorption process of BCL-K-H on the pollutants was found to be in accordance with the proposed second-order kinetic model and the Freundlich isotherm model. This finding suggests that the adsorption process may involve electron transfer between the pollutant and the adsorbent, as well as adsorption on heterogeneous surfaces. The presence of a large number of reactive functional groups (e.g. hydroxyl, hydrocarbon and carboxyl groups, etc.) within the adsorbent material has been postulated to provide a substantial number of active sites during the adsorption of pollutants, thereby enhancing the adsorption performance. Following 10 cycles, the removal of pollutants by BCL and BCL-K-H remained above 60%. All data demonstrate that the pre-treatment of biomass precursors is effective and feasible in further improving the performance of biochar, thus providing a new strategy for the further development of lignocellulosic garden waste.
Similar content being viewed by others
Introduction
Biochar is an innovative functional material characterised by its large specific surface area, intricate pore structure, and abundant active functional groups, making it a widely utilised adsorbent1,2. In recent years, a significant number of biomass resources, including but not limited to corn stalks, cow dung, coffee hulls, and activated sludge, have been utilised in the development of biochar materials. These materials have been shown to exhibit excellent performance in the treatment of water pollutants3,4,5. The conventional methodology for the production of biochar is generally comprised of two primary stages. Firstly, the carbon precursors are obtained. Secondly, the biochar is then prepared6,7. In order to enhance the performance of the resultant biochar, the most effective approach involves modifying the biochar material itself, as well as transforming the biomass carbon precursor or optimising the preparation process parameters. When utilising the same class of biomass carbon precursors, the optimisation of parameters such as carbonization temperature and time, activation temperature and time, and alkali-carbon ratio alone does not result in a significant improvement in biochar performance beyond a certain threshold8,9,10. Consequently, the transformation of carbon precursors emerges as a compelling strategy for enhancing biochar quality.
Garden waste constitutes a distinct category of agricultural waste, originating predominantly from urban landscape management practices. This encompasses the natural decline of trees, grass, and flowers in parks, scenic spots, nurseries, flower markets, and green belts adjacent to roads, as well as plant residues resulting from maintenance activities. The phenomenon under discussion also encompasses the processes of ageing, degradation, dead seedlings, broken branches, inclement weather such as typhoons, weed seedlings from greening construction, and timely renewal of flowering seedlings11. As a form of renewable biomass resource, its yield and reserves are also substantial12,13. In the contemporary era, the utilisation of garden waste as a resource has emerged as a pivotal ___domain for research. According to official sources, the city of Beijing generates approximately 5.2 million tons of landscaping waste on an annual basis. The present study explores the potential for the efficient and valuable utilisation of garden waste. A plethora of studies have been conducted on the subject of garden waste treatment, encompassing a wide range of methodologies such as organic composting treatment, biomass solid fuel preparation, in-situ soil improvement applications, and thermochemical conversion14,15. While these methods can also achieve the objective of eliminating garden waste reserves, their added value is limited, resulting in diminished development power and, consequently, affecting their comprehensive utilisation efficiency. The development of advanced and efficient methods for the further exploitation of the economic potential of garden waste remains a challenge.
Clivia miniata leaves (CL) are a common garden waste. In our previous studies16, we demonstrated the feasibility of this process as a precursor for biochar production from biomass carbon sources. However, further research is required to determine whether this process is sufficiently efficient to support its use as a high value-added product. In recent years, there has been an exploration of the potential for further research to enhance its performance and stimulate its economic potential. CL is a plant-based waste consisting mainly of cellulose, hemicellulose and lignin, which are intertwined to form an intricate lignocellulosic structure. Numerous studies have shown that in most lignocellulose, this complex structure may limit the performance of subsequent biochar preparation17,18. There are many reports about the structure of lignocellulose being broken by pretreatment, mainly focusing on acid hydrolysis, alkali hydrolysis and combined treatment19,20. Lignin is a complex aromatic polymer composed mainly of phenylpropane units, which are connected by C–O and C–C bonds. It is evident that the high pH of hydroxide facilitates its capacity to effectively degrade lignin, thereby promoting its separation from other components21. Hemicellulose, as an important constituent of plant cell walls, is a complex polysaccharide polymer formed by linking a number of glycosidic groups, including D-xylose, D-mannose, D-glucose, D-galactose and L-arabinosyl. It has been reported that under acidic conditions, these glycosidic bonds undergo hydrolytic breakage, leading to the depolymerisation of hemicellulose into low molecular weight sugar units, which results in effective separation from the lignocellulosic matrix22,23. The dense structure of CL feedstock may limit the performance of biochar derived from it, and the modulation of the feedstock structural properties by pretreatment technology is expected to further enhance the performance index of biochar.
In this work, CL was used as the biomass material to destroy the dense structure of lignocellulose by pre-treating to remove some specific components (KOH to remove lignin; HCL to remove hemicellulose) in order to improve the performance of biochar preparation. The impact of pretreatment technology on the physicochemical properties of biomass and biochar was analysed. The pre-treatment process is conducted in two stages of alkali hydrolysis and acid hydrolysis, followed by the preparation of the pre-treatment product as biochar through carbonation-activation. In this study, tetracycline hydrochloride and Rhodamine B were selected as water pollutants to systematically investigate the adsorption performance of biochar. The research encompassed adsorption kinetics, isotherm analysis, thermodynamic studies, and pH effect evaluation to elucidate the underlying adsorption mechanisms. The primary objective was to assess the feasibility of pretreatment processes in enhancing biochar performance, thereby establishing a theoretical foundation for improving the comprehensive utilization efficiency of garden waste and developing high-value-added biochar products.
Experimental details
Materials
CL was obtained from an experimental field at Changchun University in the autumn of 2024. The analytical pure grade hydrochloric acid (HCl, CAS: 7647-01-0), sodium hydroxide (KOH, CAS: 1310-58-3), tetracycline hydrochloride (TH, CAS: 64-75-5), and Rhodamine B (RhB, CAS: 81-88-9) were procured from Shanghai Aladdin Chemical Co., LTD., China.
Biochar preparation methods
The preparation of biochar involves three stages: the pre-treatment process, the carbonization process, and the activation process. The pre-treatment is further divided into two steps: alkaline hydrolysis and acid hydrolysis. During the alkaline hydrolysis, 10.0 g of cleaned, dried, and crushed Clivia miniata leaves (CL) powder is placed in a erlenmeyer flask, and 2% KOH solution is added in a solid-to-liquid ratio of 1:20 (W:V). The erlenmeyer flask is then sealed with kraft paper and placed in an autoclave at 120 °C for 60 min, after which it is washed thoroughly with water until neutral. The resulting solid residue is dried in a blast air oven at 80 °C to constant weight and labeled CL-K. The acid hydrolysis is performed similarly: 10.0 g of the CL-K sample is placed in a erlenmeyer flask, and a 2% HCl solution is added at a 1:20 ratio (W:V), followed by treatment at 120 °C for 60 min. The residue is then dried to neutrality and recorded as CL-K-H for future use. The characterization of the relative contents of cellulose, lignin, and hemicellulose of pretreated samples was shown in Section S1 of Supporting Materials.
The carbonization and activation processes are conducted in a horizontal tube furnace under nitrogen protection. The carbonization was carried out at 600 °C for 60 min with a heating rate of 10 °C/min. Subsequently, the carbonized samples are ground with KOH at a ratio of 1:4 and activated at 700 °C for 60 min. After cooling to room temperature, the residue was washed sequentially with deionized water and a 2% HCl solution until a neutral pH was achieved. The resulting biochar samples were then dried to constant weight in an oven at 80 °C. The carbonized samples were designated as “CCL-K-H” and “CCL”, corresponding to the precursor materials CL-K-H and CL, respectively. Meanwhile, the biochar samples were labeled as “BCL” and “BCL-K-H”, derived from CCL and CCL-K-H, respectively.
Adsorption experiments
Batch adsorption experiments were conducted using a constant temperature shaker operating at a rotational speed of 150 RPM, shielded from light. Specifically, a 10 mg biochar sample was weighed and placed into a erlenmeyer flask containing an antibiotic solution of varying concentrations. At designated time intervals, a specific volume of liquid was drawn with a pipette, and the supernatant was separated using a centrifuge at 12,000 RPM. After adjusting the tested volume with deionized water, the antibiotic concentration in the solution was measured using a UV spectrophotometer (Agilent Cary-300, USA). The biochar adsorption capacity (Qe, mg/g) was calculated as follows:
where the abbreviations C0, Ce, V, and m represent initial concentration, the equilibrium concentrations of solutions, the volume of the solutions, and the mass of the biochar.
Reusability study methods
In each cycle of the experiment, 0.1 g of biochar was added to the solution, which was 200 ml and contained 400 mg/L. Subsequently, the adsorbent was separated from the suspension by centrifugation and washed on multiple occasions with deionised water. The powder was then subjected to a process of re-carbonisation under conditions of nitrogen atmosphere, after which it was reused as an adsorbent in subsequent cycles. Details regarding the characterization tests of the samples can be found in Section S1 of the Supporting Materials.
Result and discussion
Preparation of biochars
The preparation process of biochar is illustrated in Fig. 1a. As illustrated in Fig. 1b, the relative alterations in the composition of lignocellulosic components within the samples were examined before and after the pretreatment process. The relative content concept is a frequently employed one in the determination of lignocellulosic components. It refers to the small molecule polysaccharide substance that is obtained by hydrolysis. The macromolecule content is reversely deduced according to the formula, and then the percentage is calculated with the measured substance24. The relative contents of cellulose, hemicellulose and lignin in the original sample were 37.2%, 28.1% and 12.4%, respectively. After KOH treatment, the relative contents of cellulose and hemicellulose have a significant change (49.4% and 35.2%), while lignin content dramatically decreased. This transformation primarily results from extensive lignin hydrolysis during the alkaline treatment process. After further acid hydrolysis of the treated CL-K, the relative contents of each component changed again, to 73.9%, 14.7% and 3.8%, which could be explained by the hydrolysis of hemicellulose under acid environment. In the pre-treatment process, whether it is alkali hydrolysis of lignin or acid hydrolysis of hemicellulose, the organic polymer of large molecules is converted into small molecules through the fracture of functional keys, and then removed by past ionized water during the cleaning process25. Figure 1c shows the samples after various treatments. It is evident that there are discernible differences between the samples, which serves to reinforce the efficacy of the pre-treatment process.
Characterizations results
The surface morphology and structure of the materials were investigated by means of scanning electron microscopy (Fig. 2). CL exhibited a smooth and flat surface, characterised by a typical wood fibre structure. Following alkali treatment, the surface of CL-K exhibited a rough texture, and the etching marks of CL-K-H became more pronounced after further acid treatment. This suggests that the pretreatment process has caused damage to the wood fibre structure26. It was found that both CCL and CCL-K-H of the carbonized samples underwent a certain degree of dehydration, with CCL-K-H being more sparsely structured than CCL. This phenomenon can be attributed to the pre-treatment process, which appears to have disrupted the compact structure of the wood fibres. Following activation, the structural fragmentation of BCL and BCL-K-H was observed to deepen, indicating that high temperature and activator successfully activated the biochar27. Figure 2f and i show that BCL-K-H has a richer pore structure, which suggests that the pretreatment process improves the efficiency of the activator KOH and provides BCL-K-H with a larger specific surface area.
Thermogravimetric (TG) analysis revealed that in the initial stage of mass loss (from starting temperature to 200 °C), moisture and volatile components were released from the surfaces of CL, CL-K, and CL-K-H, resulting in slight mass loss (Fig. 3a). As the temperature increased further to 500 °C, the components of biomass (lignin, cellulose, and hemicellulose) underwent pyrolysis, leading to a more substantial mass loss28. During the third stage, the rate of mass loss stabilised, primarily due to the ash content of the material29. At a temperature of 600 °C, the residual masses of CL, CL-K, and CL-K-H were determined to be 26.13%, 21.25%, and 17.01%, respectively. This finding suggests that while the pretreatment process disrupts the lignocellulosic fibre structure, it also results in a significant reduction in the thermal stability of the material. Following a comprehensive analysis of the TG results, it was determined that 600 °C would serve as the optimal carbonization temperature for the subsequent experimental procedures.
The Fourier-transform infrared (FT-IR) results indicate that all materials exhibit a characteristic peak in the range of 3430–3450 cm−1, attributed to the -OH stretching vibration (Fig. 3b)30. Peaks observed at 2850–2930 cm−1, 1595–1619 cm−1, and 1049–1084 cm−1 correspond to the aromatic C–H stretching vibration, C=O/C=C bonding stretching vibration, and C–OH stretching vibration, respectively31. The weak peaks observed in the 1100–1550 cm−1 range for CL, CL-K, and CL-K-H are indicative of the typical features of wood fibre structures. Specifically, the weak peaks of CL-K-H in the same range are indicative of the characteristic peaks of lignocellulosic fibre structures, which disappeared following the carbonisation and activation processes. This suggests that the high-temperature pyrolysis decomposed and transformed some functional groups32. The X-ray diffraction (XRD) results (Fig. 3c) reveal the crystalline structure of the materials. The diffraction peaks of CL, CL-K, and CL-K-H, located at 17° and 23°, correspond to the crystalline structures of cellulose Iα and Iβ, respectively. However, both of these peaks disappeared following the carbonisation and activation processes. The broad peaks of carbonized carbon lignin (CCL), CL-K-H, biochar (BCL), and BCL-K-H near 24° and 44° correspond to the graphite (002) and (100) planes, indicating the presence of an amorphous phase with a graphite structure33,34.
The surface charge characteristics of biochar are crucial for the adsorption of pollutant molecules, and its surface electrical properties were evaluated using the zeta potential test (Fig. 3d). The findings of this study suggest that the point of zero charge (pHpzc) of BCL and BCL-K-H is 5.36 and 5.24, respectively. Consequently, the surface of biochar is negatively charged when the ambient pH exceeds the pHpzc, while it becomes positively charged when the ambient pH is lower than the pHpzc. During the process of pollutant adsorption, positively charged biochar is electrostatically attracted to negatively charged pollutant molecules, thereby enhancing the efficacy of the adsorption process. This phenomenon is also critical for elucidating the underlying mechanisms governing the adsorption process35.
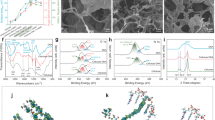
The carbon structure of the material and the elemental distribution on its surface were analyzed using Raman (Fig. 4a,b) and X-ray photoelectron spectroscopy (XPS) tests (Fig. 4c–e). The characteristic peaks of the material at approximately 1344 cm−1 (D-band) and 1592 cm−1 (G-band) correspond to the disordered amorphous structure and sp2 hybridized graphitic structure of the carbon material, respectively36. The intensity ratio (ID/IG) facilitates further analysis of the structural defects in the materials. The ID/IG ratios for the carbonized samples CCL and CCL-K-H were 4.80 and 4.92, respectively, which increased to 5.09 (BCL) and 5.20 (BCL-K-H) following activation. This increase suggests that the development of the amorphous structure in both biochars may enhance the pollutant adsorption process37. XPS results indicated that the biochars BCL and BCL-K-H were primarily composed of carbon (C), nitrogen (N), and oxygen (O). The relative nitrogen content of BCL-K-H decreased compared to BCL, likely due to the pretreatment process. The three peaks in the C1s spectrum of BCL and BCL-K-H corresponded to C–C/C=C (284.09 ± 0.1 eV), C–O–R (285.04 ± 0.4 eV), and C=O (288.13 ± 0.2 eV), while the binding energies in the O1s spectrum were 530.72 ± 0.2 eV, 531.58 ± 0.4 eV, 533.04 ± 0.1 eV, and 535.11 eV, corresponding to C–O, –OH, C=O, and COOH, respectively38,39,40. The abundance of active functional sites in BCL-K-H plays a significant role in the adsorption of pollutant molecules41,42.
The results of N₂ adsorption–desorption isotherms and pore size distribution of the materials (Fig. 5 and Table S1) show that the specific surface areas of CL-K and CL-K-H have increased by a factor of 1.72 and 3.15, respectively, in comparison with pristine CL. This advantage is still significant after carbonisation (CCL-SBET: 1.92 m2/g, CCL-K-H-SBET: 4.20 m2/g). This discrepancy is primarily ascribed to the synergistic effect of the alkali and acid treatments. Alkali pretreatment induces fiber swelling and structural deconstruction by partially dissolving lignin and disrupting hydrogen bonds between cellulose fibrils43. This results in a more porous and looser biomass matrix, which significantly enhances pore formation during subsequent carbonization44. Acid pretreatment primarily removes inorganic constituents, thereby reducing ash content, while simultaneously generating microcracks within the biomass structure45. These microcracks facilitate improved penetration of activating agents during further processing46. All biochars exhibited typical H4-type isotherms with hysteresis loops, indicating the presence of both micro- and mesopores in the samples, which is in agreement with the pore size distribution data47. The specific surface areas of BCL and BCL-K-H were 3052.12 m2/g and 3555.31 m2/g, with the total pore volumes of 1.94 cm3/g and 2.58 cm3/g. The specific surface areas of BCL-K-H was significantly higher than that of pristine biochar, indicating that the fibre swelling triggered by alkali pre-treatment and the microcracking produced by acid pre-treatment not only destroyed the compact lignofibre structure of straw, but also effectively enhanced the efficiency of the activator, which significantly contributed to the increase in the specific surface area and the generation of pore structure48.
Adsorption experiments
Adsorption kinetics experiment results
The mechanisms involved in the adsorption process were analysed through kinetic experiments of TH and RhB adsorption on biochar. The calculated parameters were further analysed by fitting the kinetic models of pseudo-first-order (PFO), pseudo-second-order (PSO) and intra-particle diffusion (IPD) to the adsorption data (Fig. 6, Tables S2, and S3). For the adsorption of TH on BCL, the rapid adsorption phase occurred in the first 5 min, followed by a decrease in the adsorption rate, and adsorption equilibrium was essentially reached at 120 min. The fitted parameters showed that both PFO and PSO exhibited high correlation coefficients (R2 = 0.9650–0.9924 and R2 = 0.9960–0.9993), suggesting that the adsorption process may be subjected to a combination of chemisorption and physical adsorption, with the presence of sufficient active sites on the surface of the adsorbent, and also that the adsorption process may include synergistic effects of molecular diffusion and interfacial reactions. In the case of adsorption of TH by BCL-K-H, the correlation coefficients of PSO were significantly higher than those of PFO (R2 = 0.9901–0.9994 and R2 = 0.9346–0.9886, respectively), suggesting that the adsorption process is more consistent with the chemisorption mechanism. Adsorption is mainly driven by chemical interactions between the active sites on the adsorbent surface and the adsorbate molecules, rather than a purely physical diffusion process49. The IPD model divides the adsorption process into three phases. The first phase occurs within the first 5 min and is characterised by rapid adsorption, during which TH molecules in solution undergo surface diffusion and rapidly bind to the adsorption sites of the biochar50. In the second phase, the availability of adsorption sites decreases gradually due to the diffusion of TH molecules from the surface to the internal pore structure, resulting in a consequent decrease in the adsorption rate. The third stage represents the adsorption equilibrium where the maximum adsorption capacity is reached and the adsorption and desorption rates are balanced51. In the second stage, the relatively low R2 values for BCL (0.2629–0.4998) and BCL-K-H (0.2553–0.8431) indicate that the adsorption process is not only affected by the intra-particle diffusion mechanism, which is in agreement with the previously discussed results. For RhB adsorption, the fast adsorption process also occurred in the first 5 min and almost reached adsorption equilibrium at 90 min. The kinetic modelling and fitting results showed that BCL and BCL-K-H adsorption of RhB had the highest correlation coefficients (R2 > 0.99) with respect to the PSO model, indicating that the adsorption process was mainly controlled by the chemisorption mechanism. This implies that the chemical interactions between the active sites on the adsorbent surface and the RhB molecules dominate the adsorption process, and the adsorption rate is mainly determined by the electron exchange or bonding between the adsorbent and the adsorbate.The correlation coefficients of the PSO model and the IPD model are relatively low, suggesting that the adsorption process is not limited to the inward diffusion step alone, but may be a result of the joint action of multiple factors.
(a) PFO, PSO, and (b) IPD adsorption kinetic plots of BCL for adsorption of TH. (c) PFO, PSO, and (d) IPD adsorption kinetic plots of BCL-K-H for adsorption of TH. (e) PFO, PSO, and (f) IPD adsorption kinetic plots of BCL for adsorption of RhB. (g) PFO, PSO, and (hd) IPD adsorption kinetic plots of BCL-K-H for adsorption of RhB.
Adsorption isotherm experiment results
The interaction mechanisms between pollutant molecules and biochar were analyzed by fitting the Langmuir and Freundlich models to adsorption isotherm data (Fig. 7, Tables S4 and S5). The results showed that the adsorption capacity of biochar increased significantly with the initial concentration of the pollutant solution, indicating strong concentration-dependent adsorption behavior. For TH and RhB, the correlation coefficients (R2 > 0.99) for BCL and BCL-K-H with the Freundlich model were notably higher than those for the Langmuir model (R2 = 0.86–0.89). The Langmuir model assumes uniform energy distribution on the adsorbent surface and monolayer adsorption, while the Freundlich model accounts for surface heterogeneity and multilayer adsorption. The high correlation with the Freundlich model indicates that the adsorption processes of BCL and BCL-K-H are strongly influenced by the heterogeneity of surface energy distribution. In particular, the modified BCL-K-H exhibited a higher adsorption capacity and Freundlich constant (KF), suggesting the presence of more high-energy active sites capable of forming strong interactions with pollutant molecules.The fitting results from both models suggest that the adsorption processes of BCL and BCL-K-H are governed by multiple mechanisms. During the initial adsorption phase, high-energy sites are preferentially occupied, resulting in behavior resembling monolayer adsorption. At higher concentrations, however, multilayer adsorption becomes dominant, facilitated by the uneven distribution of active sites and the porous structure of the biochar52. This dual adsorption mechanism underscores the significant role of both surface heterogeneity and porosity in enhancing pollutant adsorption.
Adsorption thermodynamics experiment
The effect of temperature on the adsorption of TH and RhB by biochar was analyzed comprehensively through thermodynamic experiments and calculated parameters (Fig. 8, Tables S6 and S7). The results revealed that the adsorption capacity of both BCL and BCL-K-H increased significantly with a rise in temperature from 293 to 313 K. For BCL, the adsorption capacity for TH increased from 1046.50 to 1123.21 mg/g, while for RhB, it rose from 1095.23 to 1195.44 mg/g. In comparison, BCL-K-H exhibited superior performance, with the adsorption capacity for TH increasing from 1222.75 to 1542.68 mg/g and for RhB from 1358.52 to 1513.89 mg/g. These findings suggest that higher temperatures enhance the adsorption capacity of biochar, likely due to increased kinetic energy of pollutant molecules, which accelerates their diffusion to the surface and internal pores of the adsorbent.
(a) Effect of temperature on the adsorption capacity of BCL and BCL-K-H for TH. (b) The plot of ln(Qe/Ce) versus T for adsorption of BCL and BCL-K-H for TH. (c) Effect of temperature on the adsorption capacity of BCL and BCL-K-H for RhB. (d) The plot of ln(Qe/Ce) versus T for adsorption of BCL and BCL-K-H for RhB.
Thermodynamic parameters further supported this observation. The negative values of Gibbs free energy (∆G) across the temperature range confirmed that the adsorption process was spontaneous. Moreover, the greater absolute ∆G values for BCL-K-H compared to BCL at the same temperatures indicated that the acid–base pretreated biochar possessed a stronger adsorption driving force. The positive enthalpy (∆H) values suggested an endothermic process, meaning heat absorption was necessary for adsorption to occur. Such endothermic processes are typically associated with chemisorption, implying the involvement of strong intermolecular forces such as hydrogen bond formation or electron exchange reactions53. The higher ∆H value for BCL-K-H likely stemmed from its richer high-energy sites and more developed pore structure, which provide additional active sites for adsorption. The positive entropy (∆S) values reflected an increase in disorder at the solid–liquid interface with rising temperature. This increase in entropy may result from pollutant molecules rearranging on the biochar surface or entering the pores, leading to a more disordered system. The relatively higher ∆S values for BCL-K-H suggested a more complex adsorption mechanism, possibly involving multimolecular layer adsorption behavior54. Overall, BCL-K-H consistently demonstrated higher adsorption capacity and stronger driving forces for adsorption across the temperature range, underscoring the critical role of modification treatments in enhancing adsorption performance.
The effect of pH
The effect of pH on the adsorption process was analyzed by examining the adsorption performance of biochar for the pollutants TH and RhB under varying pH conditions (Fig. 9). The results demonstrated that for TH adsorption, the maximum adsorption capacities of BCL and BCL-K-H were 1113.86 mg/g and 1436.56 mg/g, respectively, at pH 5. This can be attributed to the fact that TH exists in different ionic forms depending on the pH. At pH values below 3.30, the surfaces of BCL (pHpzc: 5.36) and BCL-K-H (pHpzc: 5.24) carried positive charges, while TH predominantly existed in its cationic form (TH+). This led to electrostatic repulsion, reducing the adsorption capacity55. When the pH increased to 5, the adsorbent surface remained positively charged, facilitating electrostatic attraction with the anionic form of TH−, there by significantly enhancing the adsorption performance. However, at pH 11, the adsorbent surface became negatively charged, resulting in electrostatic repulsion with TH− and TH2− in the solution, which reduced the adsorption capacity.
For RhB adsorption, the adsorption capacity of BCL and BCL-K-H increased progressively with rising pH, reaching 1176.97 mg/g and 1510.22 mg/g, respectively, at pH of 11. This phenomenon can be explained by the pH-dependent speciation of RhB. At low pH, RhB predominantly existed in its cationic form (RhB+), and the positively charged surfaces of BCL and BCL-K-H repelled RhB+, leading to low adsorption capacity. As the pH surpassed the pHpzc, the adsorbent surface became negatively charged, generating electrostatic attraction with RhB+, which enhanced the adsorption capacity. Furthermore, RhB molecules underwent gradual deprotonation at high pH, reducing surface electrostatic repulsion and further promoting adsorption. Additionally, high pH conditions increased the abundance of active sites (e.g., carboxyl and hydroxyl groups) on the biochar surface, enhancing RhB adsorption. Hydrophobic interactions under alkaline conditions may have further supported the adsorption process Fig. 10.
Fixed bed experiments
The adsorption process of the sample in the actual mobile phase was simulated by fixed-bed experiments, and the experimental results are shown in Fig. 11. The data of penetration curves showed that the penetration time and saturation time of BCL-K-H for pollutant adsorption were significantly improved compared with BCL. For TH adsorption, the penetration time was extended from 80 to 140 min and the saturation time was extended from 180 to 285 min; for RhB adsorption, the penetration time was extended from 48 to 60 min and the saturation time was extended from 160 to 260 min. This indicates that BCL-K-H has more efficient and stable performance in fixed-bed adsorption systems, which enhances the sustainability and economy of the system. The adsorption data were further fitted to the Yoon-Nelson model and the Adam-Bohart model to evaluate the adsorption process of the samples in the mobile phase.The fitted correlation coefficients (R2) of the Yoon-Nelson model were high (TH: 0.98–0.99, RhB: 0.96–0.98), indicating that mass transfer resistance such as internal and external diffusion is not a dominant factor in the adsorption process and that the adsorbent surface has a more uniform adsorption property56. The lower fit R2 of the Adam-Bohart model (TH: 0.67–0.78, RhB: 0.60–0.68) reflects that the adsorption process may be limited by the mass transfer process, especially the effect of external diffusion is more significant.The significant improvement of the adsorption performance of BCL-K-H on fixed bed was attributed to the pre-treatment process. enhancement was attributed to the optimisation of the structure and surface properties of the straw biochar by the pretreatment process. The modified samples exhibited higher adsorption capacity, longer penetration and saturation times, and higher adaptability to mass transfer resistance57. This modified biochar demonstrated excellent stability and efficiency in the treatment of pollutants, indicating its potential for practical application in fixed-bed adsorption systems.
Comparison with other materials
Comparative analysis with previously reported adsorbents (Table S8) was conducted to evaluate the practical potential of BCL and BCL-K-H for water pollution treatment. The results demonstrate that both BCL (Rhodamine B: 1113.86 mg/g; tetracycline: 1206.78 mg/g) and BCL-K-H (Rhodamine B: 1436.56 mg/g; tetracycline: 1505.47 mg/g) exhibit superior pollutant adsorption performance compared to most biochar materials reported in literature. Specifically, their adsorption capacities significantly exceed those of chicken bone-derived biochar (98.90 mg/g for RhB), sunflower seed shell biochar (673.00 mg/g for RhB), lignin-based biochar (1163.00 mg/g for RhB), as well as sugarcane pith-activated carbon (264.48 mg/g for TC), polymer-modified baker’s yeast biomass (267.94 mg/g for TC), and treated rice husk-based activated carbon (518.49 mg/g for TC). These outstanding performance metrics suggest their promising potential for wastewater treatment applications.
Cyclic regeneration capacity and possible adsorption mechanisms
The cyclic regeneration performance of adsorbents is an important indicator to evaluate their practical applications. We performed 10 regeneration cycles of BCL and BCL-K-H to evaluate their potential for wastewater treatment (Fig. 10)58. The results showed that the removal rates of BCL (RhB: 61.8, TH: 60.0) and BCL-K-H (RhB: 62.4, TH: 60.4) remained above 60% after 10 cycles, and the similar regeneration capacity of BCL and BCL-K-H cycles could be attributed to the fact that both biochars were derived from CL, and the pretreatment process did not completely disrupt their lignocellulosic fiber structure. Thermal regeneration was chosen to regenerate the biochar, in which the pollutant molecules were thermally degraded under the action of high temperature. The decrease in regeneration capacity may be due to the by-products of the thermal decomposition of pollutants, which may lead to pore clogging or pore structure collapse at high temperatures.The excellent regeneration performance of BCL-K-H makes it more advantageous for the treatment of pollutants in real water bodies.
The adsorption of TH and RhB by BCL and BCL-K-H involves several complex and interactive mechanisms, including pore filling, π–π interactions, electrostatic attraction and hydrogen bonding. Together, these mechanisms determine the adsorption performance of biochar for target pollutants. In the adsorption process, the surface and internal pore structures of biochar perform dual functions: they provide channels for the transport of contaminants to the active sites, and they provide numerous active sites for the binding of contaminant molecules, which significantly enhances the adsorption efficiency59. The molecular structures of BCL and BCL-K-H are rich in π-electrons, allowing π–π interactions with the aromatic rings of contaminants such as TH and RhB. This interaction enhances the selectivity of the adsorbent for aromatic pollutants and effectively increases its adsorption capacity60. Electrostatic attraction also plays an important role in the adsorption process. As the surface charge of biochar varies with pH, it can form strong electrostatic interactions with pollutants of different charges (e.g. TH⁺, TH− and RhB⁺), facilitating pollutant immobilisation and removal61. In addition, the biochar surface is rich in oxygen-containing functional groups (e.g., carboxyl and hydroxyl) that bind to contaminants through hydrogen bonding. This hydrogen bonding is particularly important in complex aqueous environments as it increases the selectivity and stability of adsorption62,63. In summary, the adsorption of TH and RhB by BCL and BCL-K-H results from the synergistic action of multiple mechanisms. The pore structure provides physical support, π-π interactions and electrostatic attraction contribute to chemical binding, and hydrogen bonding enhances the stability and strength of adsorption. This synergy highlights the potential of biochar materials for the removal of various pollutants.
Conclusions
In this study, the combined acid–base pretreatment technology was used to modify the garden waste (CL), and the high-performance biochar BCL-K-H was successfully prepared. The systematic characterization analysis (TGA, FTIR, XRD, Raman spectroscopy, and BET specific surface area test) confirmed that the pretreatment process effectively optimized the lignofibrous structure of the CL, and the resultant BCL-K-H possessed significantly enhanced physicochemical properties: the specific surface area was as high as 3555.31 m2/g, which was 16.5% higher than that of the untreated sample (3052.12 m2/g). The adsorption experiments showed that BCL-K-H exhibited excellent removal ability for tetracycline (TH) and rhodamine B (RhB), with the maximum adsorption capacity reaching 1436.56 mg/g and 1505.47 mg/g, which was significantly better than that of conventional biochar materials. After 10 adsorption–desorption cycles, the pollutant removal rate was still maintained at more than 60%, which demonstrated excellent regeneration performance. Mechanistic studies showed that the adsorption process conformed to the pseudo-secondary kinetic model and Freundlich isothermal adsorption model, revealing that the mechanism of action included multiple synergistic effects: microporous filling effect, π–π stacking interaction, electrostatic attraction, and hydrogen bonding. The combined acid–base pretreatment process does not require special equipment under harsh conditions, and the operating parameters (e.g., temperature, concentration, time, etc.) are easy to control and scale up. The acid–base reagents used in the pretreatment process are common industrial chemicals, which is favorable for mass production. Although the pretreatment step adds some chemical and labor costs, these inputs are fully compensated by significantly improving the adsorption performance of the biochar. At the same time, the excellent cyclic stability of the material further extends the service life. The feedstock is made from garden waste CL, which reduces the pressure on the disposal of organic solid waste and provides a new strategy for the development of other high value-added biochar products from garden waste.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Zhao, Y. et al. Anaerobic digestion and biochar/hydrochar enhancement of antibiotic-containing wastewater: Current situation, mechanism, and future prospects. Environ. Res. 264, 120087. https://doi.org/10.1016/j.envres.2024.120087 (2025).
Huang, R., Lu, X., Li, W., Xiong, J. & Yang, J. Progress on the adsorption characteristics of nZVI and other iron-modified biochar for phosphate adsorption in water bodies. Circ. Econ. 3(4), 100112. https://doi.org/10.1016/j.cec.2024.100112 (2024).
Zango, Z. U. et al. A systematic review on applications of biochar and activated carbon derived from biomass as adsorbents for sustainable remediation of antibiotics from pharmaceutical wastewater. J. Water Process Eng. 67, 106186. https://doi.org/10.1016/j.jwpe.2024.106186 (2024).
Rajendran, S. et al. Biochar from cashew nut shells: A sustainable reinforcement for enhanced mechanical performance in hemp fibre composites. Clean. Eng. Technol. 20, 100745. https://doi.org/10.1016/j.clet.2024.100745 (2024).
Mishra, R. K. & Mohanty, K. A review of the next-generation biochar production from waste biomass for material applications. Sci. Total Environ. 904, 167171. https://doi.org/10.1016/j.scitotenv.2023.167171 (2023).
Teng, B., Zhao, Z., Xia, L., Wu, J. & Wang, H. Progress on the removal of PFAS contamination in water by different forms of iron-modified biochar. Chemosphere 369, 143844. https://doi.org/10.1016/j.chemosphere.2024.143844 (2024).
Guo, W. et al. Recent advance on application of biochar in remediation of heavy metal contaminated soil: Emphasis on reaction factor, immobilization mechanism and functional modification. J. Environ. Manag. 371, 123212. https://doi.org/10.1016/j.jenvman.2024.123212 (2024).
Yang, Y. et al. Applicability analysis of algae biochar for anaerobic membrane bioreactors in wastewater treatment: A review from a sustainability assessment perspective. Sci. Total Environ. 957, 177609. https://doi.org/10.1016/j.scitotenv.2024.177609 (2024).
Saraugi, S. S. & Routray, W. Advances in sustainable production and applications of nano-biochar. Sci. Total Environ. 955, 176883. https://doi.org/10.1016/j.scitotenv.2024.176883 (2024).
Mishra, R., Shu, C.-M., Ong, H. C., Gollakota, A. R. K. & Kumar, S. Progress and development of biochar as a catalyst for hydrogen production. J. Clean. Prod. 477, 143853. https://doi.org/10.1016/j.jclepro.2024.143853 (2024).
Wu, C. et al. Adsorption of dye through hydrochar derived from co-hydrothermal carbonization of garden waste and sewage sludge: The adsorption enhancement mechanism of lignin component. J. Water Process Eng. 67, 106233. https://doi.org/10.1016/j.jwpe.2024.106233 (2024).
Adhikari, S., Mahmud, M. A. P., Moon, E. & Timms, W. Comprehensive life cycle assessment of garden organic waste valorisation: A case study in regional Australia. J. Clean. Prod. 472, 143496. https://doi.org/10.1016/j.jclepro.2024.143496 (2024).
Farmanbordar, S., Javid, A., Amiri, H., Denayer, J. F. M. & Karimi, K. Enhanced biobutanol production with sustainable co-substrates synergy from paper waste and garden waste with municipal biowaste. Biomass Bioenergy 186, 107262. https://doi.org/10.1016/j.biombioe.2024.107262 (2024).
Santhana Kumar, V. et al. Sustainable biodiesel production from microalgae Graesiella emersonii through valorization of garden wastes-based vermicompost. Sci. Total Environ. 807, 150995. https://doi.org/10.1016/j.scitotenv.2021.150995 (2022).
Noblet, C. et al. Emission factors and chemical characterization of particulate emissions from garden green waste burning. Sci. Total Environ. 798, 149367. https://doi.org/10.1016/j.scitotenv.2021.149367 (2021).
Gao, W. Porous biomass carbon derived from clivia miniata leaves via NaOH activation for removal of dye. Materials 15, 1285. https://doi.org/10.3390/ma15041285 (2022).
Zhang, M. et al. The structure and properties of lignin isolated from various lignocellulosic biomass by different treatment processes. Int. J. Biol. Macromol. 243, 125219. https://doi.org/10.1016/j.ijbiomac.2023.125219 (2023).
Kai, X. et al. Structure characteristics and gasification reactivity of co-pyrolysis char from lignocellulosic biomass and waste plastics: Effect of polyethylene. Int. J. Biol. Macromol. 279, 135185. https://doi.org/10.1016/j.ijbiomac.2024.135185 (2024).
Pandey, A. K. & Negi, S. Impact of surfactant assisted acid and alkali pretreatment on lignocellulosic structure of pine foliage and optimization of its saccharification parameters using response surface methodology. Bioresour. Technol. 192, 115–125. https://doi.org/10.1016/j.biortech.2015.04.054 (2015).
Chai, Y. et al. Upgrading lignocellulosic biomass to high-value products through the pretreatment driven by bio-based ethylene glycol solvent. Chem. Eng. J. 496, 153797. https://doi.org/10.1016/j.cej.2024.153797 (2024).
Shakeel, U. et al. Unraveling interplay between lignocellulosic structures caused by chemical pretreatments in enhancing enzymatic hydrolysis. Carbohydr. Polym. 334, 122037. https://doi.org/10.1016/j.carbpol.2024.122037 (2024).
Tian, H. et al. Optimizing the gasification reactivity of biochar: The composition, structure and kinetics of biochar derived from biomass lignocellulosic components and their interactions during gasification process. Fuel 324, 124709. https://doi.org/10.1016/j.fuel.2022.124709 (2022).
Gerbin, E. et al. Tuning the functional properties of lignocellulosic films by controlling the molecular and supramolecular structure of lignin. Int. J. Biol. Macromol. 181, 136–149. https://doi.org/10.1016/j.ijbiomac.2021.03.081 (2021).
Liu, B. et al. Application and prospect of organic acid pretreatment in lignocellulosic biomass separation: A review. Int. J. Biol. Macromol. 222, 1400–1413. https://doi.org/10.1016/j.ijbiomac.2022.09.270 (2022).
Shakeel, U., Zhang, Y., Liang, C., Wang, W. & Qi, W. Unrevealing the influence of reagent properties on disruption and digestibility of lignocellulosic biomass during alkaline pretreatment. Int. J. Biol. Macromol. 266, 131193. https://doi.org/10.1016/j.ijbiomac.2024.131193 (2024).
Deng, Y. et al. Phytic acid pre-modulated and Fe/N Co-doped biochar derived from ramie fiber to activate persulfate for efficient degradation of tetracycline via radical and non-radical pathways. Sep. Purif. Technol. 342, 126976. https://doi.org/10.1016/j.seppur.2024.126976 (2024).
Torboli, A., Foladori, P., Lu, M., Gialanella, S. & Maines, L. Spent coffee ground biochar for phosphate adsorption in water: Influence of pyrolysis temperature and iron-coating activation method. Clean. Eng. Technol. 20, 100839. https://doi.org/10.1016/j.clet.2024.100839 (2024).
Zhu, C., Zhang, J., Huang, G. & Zhu, D. Z. UV-modified biochar-bacillus subtilis composite: An effective method for enhancing Cd(II) adsorption from water. Biochem. Eng. J. 212, 109527. https://doi.org/10.1016/j.bej.2024.109527 (2024).
Ahmed, H. R., Kayani, K. F., Ealias, A. M. & George, G. Biochar as an eco-friendly adsorbent for ibuprofen removal via adsorption: A review. Inorg. Chem. Commun. 170(2), 113397. https://doi.org/10.1016/j.inoche.2024.113397 (2024).
Zhang, Y. et al. Relationship of CO₂ adsorption performance and physicochemical property of biochar prepared by different types of biomass waste. J. Environ. Chem. Eng. 12(6), 114571. https://doi.org/10.1016/j.jece.2024.114571 (2024).
Long, Y. et al. Competitive adsorption of H₂O and CO₂ on nitrogen-doped biochar with rich-oxygen functional groups. Sep. Purif. Technol. 359(1), 130476. https://doi.org/10.1016/j.seppur.2024.130476 (2025).
Wu, W. et al. Adsorption of CO₂ by sludge/bamboo shoot shell hybrid biochar prepared by a single-step K₂CO₃ activation. Fuel 381(C), 133555. https://doi.org/10.1016/j.fuel.2024.133555 (2025).
Kim, D. & Hadigheh, S. A. Oxidative pyrolysis for enhanced CO₂ adsorption capacity in biosolid-derived biochar. Biomass Bioenergy 190, 107407. https://doi.org/10.1016/j.biombioe.2024.107407 (2024).
Yin, Z.-X. et al. Quantitative mechanisms for the cd2⁺ adsorption increased by sewage-sludge biochar but decreased by rice-straw biochar after melamine modification. J. Environ. Chem. Eng. 12(6), 114430. https://doi.org/10.1016/j.jece.2024.114430 (2024).
Nand, S. et al. Arsenic removal using de-oiled mentha biomass biochar: adsorption kinetics and the role of iron modification. J. Clean. Prod. https://doi.org/10.1016/j.jclepro.2024.144247 (2024).
Xue, H. et al. Advanced biochar-based materials for specific antibiotics removal from hospital wastewater via adsorption and oxidative degradation. J. Environ. Chem. Eng. 12(6), 114275. https://doi.org/10.1016/j.jece.2024.114275 (2024).
Tang, G., Mo, H., Gao, L., Chen, Y. & Zhou, X. Adsorption of crystal violet from wastewater using alkaline-modified pomelo peel-derived biochar. J. Water Process Eng. 68, 106334. https://doi.org/10.1016/j.jwpe.2024.106334 (2024).
Li, Y. et al. Enriched extensin and cellulose for non-collapse biochar assembly to maximize carbon porosity and dye adsorption with high bioethanol production. Ind. Crops Prod. 222(4), 119924. https://doi.org/10.1016/j.indcrop.2024.119924 (2024).
Yin, Y. et al. Nanoconfinement of MgO in nitrogen pre-doped biochar for enhanced phosphate adsorption: Performance and mechanism. Bioresour. Technol. 414, 131613. https://doi.org/10.1016/j.biortech.2024.131613 (2024).
Liu, Q. et al. Adsorption desulphurization performance of biochar derived from eucalyptus waste. Powder Technol. 448, 120322. https://doi.org/10.1016/j.powtec.2024.120322 (2024).
Janu, R. et al. Biochar surface functional groups as affected by biomass feedstock, biochar composition and pyrolysis temperature. Carbon Resour. Conv. 4, 36–46. https://doi.org/10.1016/j.crcon.2021.01.003 (2021).
Ahmad, M. et al. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 99, 19–33. https://doi.org/10.1016/j.chemosphere.2013.10.071 (2014).
Jasmine, A. et al. Microwave-assisted alkali pre-treatment medium for fractionation of rice straw and catalytic conversion to value-added 5-hydroxymethyl furfural and lignin production. Int. J. Biol. Macromol.. 236, 123999. https://doi.org/10.1016/j.ijbiomac.2023.123999 (2023).
Lang, S. et al. Effect of hydrothermal and hydrothermal oxidation pretreatment on the physicochemical properties of biochar pellet and activated carbon prepared from biomass wastes. J. Anal. Appl. Pyrol. 188, 107700. https://doi.org/10.1016/j.jaap.2025.107007 (2025).
Hakeem, I. G., Rathnayake, N. & Surapaneni, A. S. K. Effects of mild acid pre-treatment on the co-pyrolysis behaviour of biosolids and wheat straw. Process Saf. Environ. 14, 375–391. https://doi.org/10.1016/j.psep.2024.03.022 (2024).
Zhang, Y., Zhang, X., Zhou, Z., Liu, G. & Wang, C. A review of the conversion of wood biomass into high-performance bulk biochar: Pretreatment, modification, characterization, and wastewater application. Sep. Purif. Technol. 361, 131448. https://doi.org/10.1016/j.seppur.2025.131448 (2025).
Han, R., Gao, Y., Jia, Y. & Wang, S. Heterogeneous precipitation behavior and mechanism during the adsorption of cationic heavy metals by biochar: Roles of inorganic components. J. Hazard. Mater. 480, 136322. https://doi.org/10.1016/j.jhazmat.2024.136322 (2024).
Huang, Z. et al. Co-adsorption performance and mechanism of ammonium and phosphate by iron-modified biochar in water. J. Water Process Eng. 67, 106209. https://doi.org/10.1016/j.jwpe.2024.106209 (2024).
Yu, Y.-L. et al. Development of modified MgO/biochar composite for chemical adsorption enhancement to cleanup fluoride-contaminated groundwater. J. Environ. Manag. 370, 123016. https://doi.org/10.1016/j.jenvman.2024.123016 (2024).
Chen, Q. et al. Electronic structure of biochar modified through self-doping with boron, nitrogen and sulfur atoms for rapid and efficient adsorption of COVID-19 drug residues. Sep. Purif. Technol. 359(1), 130480. https://doi.org/10.1016/j.seppur.2024.130480 (2025).
Jiang, H. & Hu, H. Sustainable synergistic adsorption of tetracycline in water by biochar and microplastics: Exploration of the mechanism of DFT. J. Water Process Eng. 66, 105998. https://doi.org/10.1016/j.jwpe.2024.105998 (2024).
Yu, D., He, Y., Zeng, S., Tian, H. & Ji, Z. A novel magnetic S/N Co-doped tea residue biochar applied to tetracycline adsorption in water environment. Colloids Surf. A Physicochem. Eng. Asp. 703(2), 135400. https://doi.org/10.1016/j.colsurfa.2024.135400 (2024).
Lu, Q. et al. Characteristics of chemical aged biochars and their adsorption behaviors for norfloxacin. J. Environ. Chem. Eng. 12(5), 113638. https://doi.org/10.1016/j.jece.2024.113638 (2024).
Babeker, T. M. A., Lv, S., Khalil, M. N., Hao, Z. & Chen, Q. Biochar modified by ammonium pyrrolidine dithiocarbamate for high selective adsorption of copper in wastewater. Sep. Purif. Technol. 354(7), 129436. https://doi.org/10.1016/j.seppur.2024.129436 (2025).
Myung, E., Kim, H., Choi, N. & Cho, K. The biochar derived from spirulina platensis for the adsorption of Pb and Zn and enhancing the soil physicochemical properties. Chemosphere 364, 143203. https://doi.org/10.1016/j.chemosphere.2024.143203 (2024).
Benedicto, D. F. C., Ferreira, G. M. D. & Thomasi, S. S. β-cyclodextrin-functionalized coffee husk biochar for surfactant adsorption. Colloids Surf. A Physicochem. Eng. Asp. 701, 134921. https://doi.org/10.1016/j.colsurfa.2024.134921 (2024).
Wang, D. et al. Interaction of biochar with extracellular polymers of resistant bacteria restrains Pb(II) adsorption onto their composite: Macro and micro scale investigations. Bioresour. Technol. 414, 131602. https://doi.org/10.1016/j.biortech.2024.131602 (2024).
Zhou, Z. et al. Assembly of 3D printed N-doped biochar as impeller with CaCO₃ as sacrificial pore generator for enhanced dye adsorption. Chem. Eng. J. 497, 154661. https://doi.org/10.1016/j.cej.2024.154661 (2024).
Pan, Z. et al. Lignin-based hierarchical porous biochar prepared from negative pressure pyrolysis enhanced CO₂ and VOCs adsorption. Sep. Purif. Technol. 345, 127398. https://doi.org/10.1016/j.seppur.2024.127398 (2024).
Liu, K. et al. High Fe utilization efficiency and low toxicity of Fe₃C@Fe⁰ loaded biochar for removing tetracycline hydrochloride in wastewater. J. Clean. Prod. 353, 131630. https://doi.org/10.1016/j.jclepro.2022.131630 (2022).
Zhang, M. et al. High-efficient co-removal of copper and zinc by modified biochar derived from tea stalk: Characteristics, adsorption behaviors, and mechanisms. J. Water Process Eng. 57, 104533. https://doi.org/10.1016/j.jwpe.2023.104533 (2024).
Loura, N., Rathee, K., Dhull, R., Singh, M. & Dhull, V. Carbon nanotubes for dye removal: A comprehensive study of batch and fixed-bed adsorption, toxicity, and functionalization approaches. J. Water Process Eng. 67, 106193. https://doi.org/10.1016/j.jwpe.2024.106193 (2024).
Asamoah, E. N., Liu, H. & Fan, X. Fixed-bed adsorption of methylene blue using granular NaX zeolite/attapulgite composite: Efficiency, mechanism and reusability of saturated G-NaX/A. Sep. Purif. Technol. 354, 128710. https://doi.org/10.1016/j.seppur.2024.128710 (2025).
Acknowledgements
This research was funded by Project of Jilin Provincial Department of Education (JJKH20210636KJ).
Author information
Authors and Affiliations
Contributions
S.J. Chen and W.Gao wrote the main manuscript text and Y. Shi Y. Zhou J. Jia prepared figures and texts. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gao, W., Shi, Y., Zhou, Y. et al. Biochar with further enhanced properties prepared by acid base combined pretreatment for removal of water pollutants. Sci Rep 15, 19432 (2025). https://doi.org/10.1038/s41598-025-03992-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-03992-8