Abstract
Functional constipation (FCon) is characterized by disrupted brain-gut communication, representing a core pathophysiological mechanism. Physical exercise, a safe and cost-effective intervention, has been proven efficacy in ameliorating constipation symptoms and is a vital self-management strategy. Tai Chi, a traditional Chinese exercise, has been demonstrated to benefit both the gastrointestinal tract and the brain. However, the potential mechanism underlying Tai Chi’s gastrointestinal regulatory effects through enhancement of brain-gut communication remains to be elucidated. This randomized controlled trial enrolled 80 FCon patients and 32 healthy subjects. Patients were randomized to either Tai Chi or aerobic exercise group. Both groups participated in four 60-min sessions per week for 8 weeks. Outcome measures included symptom severity, quality of life, autonomic neural function (assessed via heart rate variability), and brain activity. Both groups exhibited significant improvements in clinical symptoms, mood states, and heart rate variability post intervention. Tai Chi showed a marginal superiority over aerobic exercise in alleviating clinical symptoms. Notably, the neuroimaging findings suggested that Tai Chi effectively modulated the abnormal functional connectivity of the anterior insula within the central autonomic network in FCon patients, which was not observed in the aerobic exercise group. These modulations correlated with clinical symptoms and heart rate variability indices. Our study suggests that Tai Chi may facilitate the coordination between central and autonomic nervous functions by modulating functional connectivity of the anterior insula within the central autonomic network, and promoting sympathetic/parasympathetic balance, thereby enhancing the brain-gut communication. This may represent the underlying mechanism by which Tai Chi exerts gastrointestinal modulation to improve FCon symptoms.
Trial registration: ChiCTR1800019781.
Similar content being viewed by others
Introduction
Functional constipation (FCon) is a prevalent functional gastrointestinal disorder characterized by infrequent bowel movements and difficult defecation1.Globally, it affects 10.1–15.3% of the population2. FCon’s prolonged, recurring nature significantly impacts patients’ quality of life and poses a socio-economic burden3,4,5. Recent studies suggest that impaired brain-gut communication may be one of the core pathological mechanisms underlying FCon6,7. Specifically, an imbalance between the sympathetic and parasympathetic nervous systems in FCon patients significantly disrupts normal gut-brain communication8,9. Concurrently, significant structural and functional alterations have been observed in primary regions of the central autonomic network (CAN) in FCon patient, including insula (INS), anterior cingulate cortex (ACC), medial prefrontal cortex (mPFC) and amygdala10,11,12. Among these regions, the anterior insular cortex (aINS) stands out as a pivotal node12,13,14 due to its crucial roles in linking autonomic nervous activity15, processing visceral sensory signals16, and regulating visceral activities17. Our previous study also revealed disrupted patterns of dynamic functional connectivity in FCon patients compared with healthy subjects (HS), characterized by abnormal aINS-cortical coupling, the absence of connectivity from default network to cognitive control network, and atypical regional topological dynamics18.
Behavioral and emotional factors are intimately associated with the onset and progression of FCon. The interplay between a sedentary lifestyle and the psychological burden arising from chronic defecation disturbances constitutes a dual stressor affecting both physical and mental well-being. Exercise, a safe and cost-effective behavioral intervention, has demonstrated efficacy in addressing constipation and is a vital tool for patient self-management19,20,21,22. Tai Chi, a traditional health exercise integrating body relaxation, deep breathing, and meditation, originates from ancient China and demonstrates significant gut-brain regulatory effects23,24,25,26. It is reported that Tai Chi effectively alleviates constipation symptoms associated with irritable bowel syndrome27, modulates the gut microbiota of type 2 diabetes patients, thereby optimizing their metabolic status28. Furthermore, long-term practice of Tai Chi enhances functional coordination among brain regions in healthy elderly individuals, bolstering their self-regulatory bodily capabilities29, as well as significantly improves brain plasticity among university students30. These evidences confirm the benefits of Tai Chi from both intestinal and cerebral perspectives. However, whether Tai Chi can further enhance the communication along the brain-gut axis remains to be elucidated.
The autonomic nervous system and brain regions associated with CAN, particularly the aINS, are notably modulated by exercise. Research demonstrates that physical activity can markedly improve autonomic nerves system function in individuals with type 2 diabetes31, chronic kidney disease32, and breast cancer survivors33. Furthermore, compared to general individuals, long-term exercise significantly enhances functional connectivity within the CAN, especially in regions such as the aINS and dACC 34,35.
Given the crucial role of the aINS in modulating the autonomic nervous system and their significant involvement in brain-gut communication in FCon patients. The hypothesis of this study is that Tai Chi, by modulating resting-state functional connectivity (rs-FC) within the CAN, particularly focusing on the aINS, enhances brain-gut communication and fosters balance in autonomic nerve function, ultimately alleviating symptoms in FCon patient.
Therefore, this study designed a randomized controlled trial, using broadcast gymnastics, a common form of aerobic exercise, as the control group. The research objectives were: (a) to compare the impacts of Tai Chi and aerobic exercise on clinical symptoms and quality of life in FCon patients; (b) to assess their effects on autonomic nervous function in FCon patients; and (c) to examine their influence on resting-state functional connectivity (rs-FC) of the aINS within the CAN in FCon patients.
Methods
Study design
This is a randomized controlled trial recruited 80 FCon patients from December 2018 to January 2020 at the Affiliated Hospital of Chengdu University of Traditional Chinese Medicine and its campus. The study was registered with the Chinese Clinical Trial Registry in 28/11/2018 (registration number: ChiCTR1800019781).
Ethics approval and consent to participate
This study was approved by the Institutional Review Boards and Ethics Committees of the Affiliated Hospital of Chengdu University of Traditional Chinese Medicine, with ethical approval number 2018KL-047. Informed consent was obtained from all participants prior to their enrollment. The study adhered strictly to the relevant designated guidelines and regulations.
Participants
During the pilot phase, we employed the PAC-SYM scale to evaluate and compare the improvement in symptom severity between two groups of patients with FCon. Based on the analysis of pilot trial data and in accordance with the Minimal Clinically Important Difference (MCID) criteria of the PAC-SYM scale36, a conservative effect size of 0.7 was determined for the study. With the significance level (α) set at 0.05, statistical power (1-β) at 0.8, and standard deviation (SD) at 2.0, the required sample size was calculated to be 64 participants using the sample size calculation formula. Additionally, to account for potential bias, a 15% dropout rate was incorporated into the study design. Ultimately, a total of 80 FCon patients were recruited. Patients were randomly assigned in a 1:1 ratio to either the Tai Chi group (n = 40) or the aerobic exercise group (n = 40) using a computer-generated random number table. Within each group, an additional 18 subjects were randomly selected for functional magnetic resonance imaging (fMRI) scanning. Additionally, to further validate whether there are significant differences in brain activity between FCon patients and healthy subjects (HS), 32 right-handed HS aged 18–40 years were recruited through campus advertisements. These HS had no history of gastrointestinal, neurological, or psychiatric disorders and underwent comprehensive screenings, including medical histories, physical examinations, and laboratory tests, to exclude any underlying diseases. In this study, JW.C. generated the randomization sequence, YK.T. enrolled the participants, and YY.G. assigned the participants to their respective interventions.
FCon diagnostic criteria
Participants were diagnosed with FCon based on the Rome IV1 diagnostic criteria by two gastroenterologists. They also went to through conducted comprehensive physical and laboratory examinations.
FCon inclusion criteria
All patients enrolled in the study with FCon met the following inclusion criteria: they were aged between 18 and 40 years and right-handed; they had abstained from using any gastrointestinal prokinetics or laxatives for at least 15 days prior to enrollment; they had not participated in any clinical trials in the past 3 months; and they had provided their signed informed consent.
FCon exclusion criteria
All patients enrolled in the study with FCon did not exhibit any of the following exclusion criteria: they did not have organic or metabolic diseases; they did not have organic rectal abnormalities, such as masses or rectal stenosis, upon digital rectal examination; they did not have acute or chronic pain disorders, major depression, or anxiety; they did not have a history of head trauma, gastrointestinal surgery, or psychiatric or neurologic disorders; they were not pregnant, lactating, or planning to become pregnant within 6 months; and they did not have any contraindications to MRI scanning, such as the implantation of ferromagnetic metals or claustrophobia.
Intervention
Before formal intervention, patients were taught a 2-week Tai Chi/aerobic exercise instruction course after the enrollment assessment by certified instructors with more than 5 years of training experience. Both groups practiced for 8 weeks, with four session a week, each session lasting 60 min (10 min warm-up, 40 min exercise, and 10 min relaxation). If constipation interfered with daily life or bowel movements were less than twice a week, glycerin enemas were administered as needed. Details were documented in the case report form.
Symptom measurement
All patients were required to complete a 2-week stool diary to record weekly complete spontaneous bowel movements (cSBM). The Patient Assessment of Constipation Symptoms (PAC-SYM) was utilized as the primary outcome measure, with the Patient Assessment of Constipation Quality of Life (PAC-QoL) serving as the secondary outcome measure. Psychological status in all participants was assessed using the Self-Rating Anxiety Scale (SAS) and Self-Rating Depression Scale (SDS). Patients underwent evaluations at both baseline and the 8-week intervention endpoint.
Assessment of autonomic function
Heart rate variability (HRV) was employed to evaluate autonomic function in this study, as it provides a more accurate reflection of sympathetic and parasympathetic activity changes associated with colonic events37,38. Heart rate variability (HRV) data were collected using a dynamic electrocardiogram (ECG) recorder (CT-086 S device, BENE WARE, Hangzhou, China) with a sampling rate of 250 Hz.
All participants underwent ECG monitoring on campus, with data collection conducted within 1 week before and 1 week after the intervention. Participants were instructed to wear the device at 5:00 PM on the testing day and remove it at 5:30 PM the following day, ensuring continuous 24-h HRV recording. Throughout the testing period, participants were instructed to maintain their routine daily activities and refrain from consuming coffee, tea, alcohol, or other stimulants.
fMRI data acquisition
fMRI data were acquired at the Magnetic Resonance Image Research Center of the University of Electronic Science and Technology of China using a 3.0 T MRI scanner with an eight-channel phased-array head coil (GE Discovery MR750, GE HealthCare, Milwaukee, USA.). During the scan, participants were instructed to remain with their heads still and awake, keep their eyes closed, and have their ears plugged. Parameters of the high-resolution three‐dimensional T1‐weighted imaging were as follows: repetition time (TR)/echo time (TE) = 6.008 ms/1.7 ms, slice thickness = 1 mm, slice number = 156, matrix size = 256 × 256, and field of view = 256 × 256 mm2. The BOLD MRI data were acquired with the echo‐planar imaging: TR/TE = 2000 ms/30 ms, flip angle = 90°, slice number = 31, matrix size = 64 × 64, field of view = 240 × 240 mm2, slice thickness = 5 mm, total volume = 180.
Data analysis
Symptoms and HRV
-
(1)
HRV preprocessing: The raw ECG signals were processed using the Baihui Dynamic ECG Analysis Software V3.2 GUI (BENE WARE, Hangzhou, China), which integrates all preprocessing steps and algorithms. R-wave peaks were detected via the Pan-Tompkins algorithm to calculate RR intervals, forming a time series. Artifacts and ectopic beats were identified (deviations > 20% from the local mean) and corrected by linear interpolation. The RR intervals were detrended using a third-order polynomial fit, resampled at 4 Hz with cubic spline interpolation, and segmented into 5-min epochs. Stationarity was assessed via the Augmented Dickey-Fuller (ADF) test (p < 0.05), excluding non-stationary segments. Power spectral density (PSD) was estimated using FFT with a Hanning window, calculating power in VLF (0.003–0.04 Hz), LF (0.04–0.15 Hz), and HF (0.15–0.40 Hz) bands. The LF/HF ratio was derived, and data quality was ensured by excluding segments with > 10% abnormal RR intervals or non-stationarity (ADF p ≥ 0.05). For comprehensive analysis, 24-h average values for LF and HF were calculated by summing the hourly means of 5-min segment averages and dividing by 24 h.
-
(2)
Statistical analysis: Statistical analyses were conducted using SPSS version 25.0 (SPSS Inc., Chicago, IL, USA). For normally distributed data, intergroup comparisons were performed using independent t-tests, intragroup comparisons were conducted using paired t-tests, and correlation analyses were assessed using Pearson correlation coefficients. For non-normally distributed data, intergroup comparisons were analyzed using Mann-Whitney U tests, intragroup comparisons were evaluated using Wilcoxon signed-rank tests, and correlation analyses were examined using Spearman’s rank correlation coefficients. A two-tailed test was used for all statistical analyses, with a significance level set at 5%. Quantitative data are presented as median (IQR). p-values less than 0.05 were considered statistically significant.
Functional connectivity analysis based on aINS seed to CAN
fMRI data was processed using DPARSF4.1 (http://rfmri.org/DPARSF), SPM12 (http://www.fil.ion.ucl.ac.uk/spm), and MATLAB R2017b (Math Works, Inc., Natick, MA, USA). Initially, the first 10 time points were discarded to stabilize the signal. Subsequent steps included slice timing correction, adjustment for six head motion directions, and regression of white matter and cerebrospinal fluid signals. Following these corrections, the images were normalized to the Montreal Neurological Institute (MNI) space after correction. To ensure the reliability of rs-FC analysis and mitigate the impact of excessive head motion39,40, stringent inclusion criteria were applied: exclusion of participants with mean frame displacement41 > 0.2 mm, > 30% FD > 0.2 mm, or maximum displacement > one voxel size. Further preprocessing steps involved detrending, band-pass filtering (0.01 Hz to 0.08 Hz), and smoothing with a 4 mm Gaussian kernel. Based on previous research42,43, core brain regions of the CAN were masked using WFU_PickAtlas. Specifically, INS, amygdala, ACC, and ventromedial prefrontal cortex were identified (vMPFC), with left (BAN246 No. 167, MNI: x=-34, y=-18, z = 1) and right aINS (BAN246 No. 168, MNI: x = 36, y = 18, z = 1) serving as regions of interest (ROIs). The mean time courses between these ROIs and CAN mask voxels were calculated for each participant. Correlation coefficients between the ROIs and CAN mask voxels were computed, converted to z-scores for normality, and visualized in comparison images. The statistical analysis was a two-sample t-test to compare rs-FC between FCon patients and HS. Paired t-tests were employed for intra-group comparisons, while inter-group comparisons of changes were conducted using a two-sample t-test (using post-intervention minus pre-intervention). Significance thresholds were set at voxel-wise p < 0.005 and cluster-wise p < 0.05, corrected using Gaussian Random Field theory. To assess the role of aINS in FCon, correlation analyses were performed between the Fisher’s z-transformed values of ROI rs-FC differences within and between groups and clinical outcomes.
Results
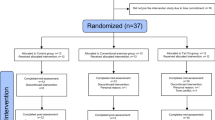
This study was conducted from December 2018 to January 2020. A total of 293 questionnaires were administered at Chengdu University of Traditional Chinese Medicine and its affiliated hospital, with 80 patients wit FCon meeting the inclusion criteria being enrolled in the study (Fig. 1). Seven patients were excluded due to incomplete study participation or excessive head movement. Additionally, 32 age- and sex-matched HS were recruited. One subject withdrew from the study during the observation period without reporting. Consequently, the final analysis involved 73 FCon patients and 31 HS.
Baseline characteristics
The demographic and clinical characteristics of the participants are shown in Table 1. No statistically significant differences were observed between the Tai Chi group (n = 37/17) and the aerobic exercise group (n = 36/17) in terms of gender, age, disease duration, cSBM, PAC-SYM, PAC-QOL, SAS, SDS, LF, HF, and LF/HF (p > 0.05). Also, there were no significant differences in age and gender between FCon patients and HS (p > 0.05). Although the SAS and SDS scores were higher in FCon patients than in HS (p < 0.05) yet failed meet the criteria for a diagnosis of anxiety or depression.
Clinical outcomes
Table 2 presents the clinical symptoms and emotional outcome indicators. Both Tai Chi group and aerobic exercise group demonstrated significant improvements in PAC-SYM, PAC-QoL, SAS and SDS compared to pre-intervention (p < 0.05). Except for a slight advantage demonstrated by the PAC-SYM in Tai Chi group compared to the aerobic exercise group (p = 0.02), no statistically significant differences were found between the two groups in the remaining outcome measures.
Automatic nervous evaluation
Table 3 presents the HRV indicators. Both Tai Chi group and aerobic exercise group demonstrated significant improvements in HF and LF/HF compared to pre-intervention (p < 0.05). There were no statistically significant differences between the two groups when compared.
aINS rs-FC results
FCon vs. HS
Figure 1; Table 4 illustrate the rs-FC of the aINS in FCon patients compared to HS, as well as the correlation between rs-FC and clinical symptoms in FCon patients. Despite controlling for the effects of the SAS and SDS by regression, FCon patients showed a significant reduction in rs-FC between the pINS and the left/right aINS, compared to HS (Table 4). As depicted in Fig. 2, Spearman correlation analysis revealed a moderate and statistically significant negative correlation between rs-FC levels of bilateral pINS with left aINS and PAC-SYM scores (left pINS: r = -0.46, p = 0.00; right pINS: r = -0.56, p = 0.00). Furthermore, a weak negative correlation was found between rs-FC levels of right pINS with right aINS and PAC-SYM scores (r = -0.35, p = 0.04).
Comparisons of left aINS/right aINS rs-FC between FCon and HS. (A) Resting-state functional connectivity based on the left anterior insula was compared between FCon patients and healthy subjects. (B): Resting-state functional connectivity based on the right anterior insula was compared between FCon patients and healthy subjects. Mask: 1= left insula; 2= right insula; 3 = left amygdala; 4 = right amygdala; 5 = anterior cingulate cortex; 6 = ventromedial prefrontal cortex. ** p <0.01. *p <0.05. aINS = anterior insula; L = left; pINS = posterior insula; PAC-SYM = patient assessment of constipation symptom questionnaire; R = right; rs-FC = resting-state functional connectivity.
Tai Chi group vs. aerobic exercise group.
After Tai Chi intervention, FCon patients exhibited increased rs-FC in the left aINS and left ACC, as well as bilateral pINS (Fig. 3; Table 5). Figure 3 illustrates that rs-FC between the left aINS and left ACC/bilateral pINS was negatively correlated with PAC-SYM (left ACC r = -0.41, p = 0.02; left pINS: r = -0.41, p = 0.01; right pINS: r = -0.41, p = 0.01), while rs-FC between the left aINS and left pINS was positively correlated with HF (r = 0.40, p = 0.02). No significant correlations were observed with LF or LF/HF.
Inter-group comparisons revealed that, compared to the aerobic exercise group, the Tai Chi group exhibited a greater increase in left aINS rs-FC after 8 weeks, primarily manifesting as an increase in rs-FC between the left aINS and the left ACC/left pINS /right aINS (Fig. 4). Furthermore, Fig. 4 demonstrates the increased rs-FC between the left aINS and left ACC/left pINS is negatively correlated with PAC-SYM (left ACC r = -0.51, p = 0.03; left pINS: r = -0.49, p = 0.04) ,while rs-FC between the left aINS and left pINS /right aINS was positively correlated with HF(r = 0.51, p = 0.03), and the left aINS and right aINS was negatively correlated with LF/HF (r = -0.66, p = 0.00). No significant correlations were observed with LF.
Comparisons of left aINS rs-FC between after Tai Chi intervention and baseline. *p < 0.05. ACC, anterior cingulate cortex; aINS, anterior insula; HF, high frequency; L, left; pINS, posterior insula; PAC-SYM, patient assessment of constipation symptom questionnaire; R, right; rs-FC, resting-state functional connectivity.
Discussion
To our knowledge, this is the first study to provide preliminary evidence suggesting that Tai Chi may improve gastrointestinal function. The findings further indicate that modulating the rs-FC of the aINS within the CAN to promote autonomic homeostasis could be a potential mechanism through which Tai Chi alleviates functional gastrointestinal symptoms.
Both Tai Chi and aerobic exercise can alleviate clinical symptoms in FCon patients.
After an 8-week exercise intervention, both Tai Chi and aerobic exercise significantly improved clinical symptoms and emotional states in FCon patients compared to baseline, with no adverse events reported. This further confirms the efficacy and safety of exercise in managing FCon. Lifestyle changes such as increased physical activity and dietary modifications are preferred self-management strategies for FCon patients22. Engaging in 30–60 min of moderate-intensity physical activity daily can improve stool consistency44. Additionally, regular exercise enhances intestinal motility patterns and rectal-sigmoid or overall colonic transit time in middle-aged patients with chronic constipation45,46. Notably, inter-group comparisons revealed that Tai Chi marginally outperformed aerobic exercise in improving clinical symptoms among FCon patients. Given previous studies demonstrating the effectiveness of pelvic floor exercise therapy in improving clinical symptoms of both obstructive and slow transit constipation47,48, we speculated that the continuous squatting movements in Tai Chi could exercise the pelvic floor and abdominal muscles more effectively them other aerobic exercise, thus enhancing their synergistic effects and yielding superior outcomes.
The regulation of the autonomic nervous system constitutes the common ground in improving functional constipation through Tai Chi and aerobic exercise.
The sympathetic and parasympathetic nervous systems, serving as the hubs of the autonomic nervous system, convey signals from the CAN and interact with the endogenous enteric nervous system to regulate gastrointestinal functions. Consequently, the homeostasis of the autonomic nervous system constitutes the foundation for maintaining normal visceral functional activities, such as body temperature, respiration, heart rate, digestion, excretion, and immune function49. A growing body of research suggests that role sympathetic/parasympathetic imbalance plays a pivotal role underlying the disruption of gut-brain communication in patients with functional gastrointestinal disorders including FCon8,9,50,51. Modulating this imbalance, particularly by augmenting parasympathetic tone, has been shown to effectively alleviate FCon symptoms52,53,−54. A systematic review reveals that endurance, coordination, and multimodal training exert positive effects on autonomic nervous activity in healthy elderly individuals55. Concurrently, exercise has been demonstrated to regulate parasympathetic activity in patients with type 2 diabetes by modifying the diversity of intestinal metabolites56. Furthermore, clinical studies have shown that a 3-month Qigong practice significantly improves HRV in anxious college students57. Our findings align with previous evidence, suggesting that both Tai Chi and aerobic exercise effectively regulate autonomic nervous activity, primarily by enhancing parasympathetic (HF) tone to promote balance between the sympathetic and parasympathetic (LF/HF) nervous systems.
The regulation of the local functional connectivity in the anterior insula by Tai Chi stands as a potential central mechanism for its gastrointestinal modulating effects.
As core regions of the CAN, the INS, ACC, amygdala, and prefrontal cortex collectively participate in regulating the balance between sympathetic and parasympathetic activity and the higher-order processing of visceral sensory information58,59. The aINS functions as a crucial node, primarily tasked with gathering and receiving afferent signals from the viscera, including the gastrointestinal tract60, and is recognized as a key visceral sensory cortex in processing interoceptive sensations61. This study focused on the bilateral aINS as ROIs to observe their functional activity patterns within the CAN. Our findings revealed abnormal functional connectivity of the aINS within the CAN in FCon patients compared with HS, as evidenced by reduced rs-FC of bilateral aINS and pINS. Correlation analysis further showed that the rs-FC between left aINS and bilateral pINS and was significantly negatively correlated with clinical symptoms, suggesting that the left aINS may play a crucial role in FCon. Therefore, we selected the left aINS as an ROI to further explore the central mechanism underlying the gastrointestinal regulatory effects of Tai Chi.
After intervention, the Tai Chi group demonstrated significant enhancement in rs-FC between the left aINS and the left ACC/left pINS and right aINS compared with the aerobic exercise group. Meanwhile, a significant negative correlation between increased rs-FC among the left aINS, left ACC/left pINS, and the severity of symptoms in FCon patients. Furthermore, rs-FC between the left aINS and left pINS/right aINS was positively correlated with parasympathetic tone, while rs-FC between the left and right aINS was negatively correlated with the sympatho-parasympathetic ratio. The aINS plays a pivotal role in integrating visceral and somatosensory information with autonomic nervous activity62,63,64, while the pINS, serving as a primary sensory cortex, is responsible for providing representations of the body’s physiological states65. The ACC, serving as a center for multisensory information integration, collaborates with the aINS and pINS in internal perception and visceral motor functions9,62. They participate in intestinal sensory perception, the maintenance of gastrointestinal homeostasis, and the coordination of multisensory integration16,66.
The regulatory effects of Tai Chi on them may be attributed to its integration of meditation, body relaxation, and deep breathing. Firstly, a mindfulness state directed at the present moment significantly activates regions such as the ACC and INS67, aiding the body in better processing stimuli. For instance, compared to non-meditators, long-term meditators exhibit lower unpleasantness ratings and increased activation in the aINS during pain stimulation68. Secondly, body relaxation during movement may generate a “self-touch” effect among limbs. Interestingly, the pINS display a somatotopic organization mediated by C-tactile afferents for the processing of gentle touch69. Lastly, deep breathing may also play a significant role. Recent research has elucidated a novel pathway extending from dACC neurons to GABAergic inhibitory neurons in the pontine reticular nucleus (PnCGABA), which subsequently innervates the ventrolateral medulla (VLM), playing a crucial role in coordinating slow breathing70. Furthermore, meditation, deep breathing, and body relaxation have been shown to regulate the balance between the sympathetic and parasympathetic nervous systems71,72,73.
These findings unveil the complexity and multifaceted nature of Tai Chi’s regulatory effects on gastrointestinal function. Externally, Tai Chi’s sustained and slow movement patterns stimulate spinal nerves, enhancing sensory information transmission and precise muscle control, while also promoting synergistic work among core muscle groups through exercise of the legs, hips, and abdomen. Internally, Tai Chi effectively modulates central and autonomic nervous system functions through deep breathing exercises and a focus on self-control and perception, fostering synergism between the two and enabling effective communication between the brain and gut.
This study has several limitations that should be acknowledged. First, the 8-week intervention period may not be sufficient to capture longer-term adaptations, and the durability of the observed effects remains uncertain. Second, the study design was not fully blinded, as participants were aware of their assigned interventions, which may introduce potential bias. Third, the sample size for fMRI analysis was relatively small (fewer than 20 participants), which may limit the generalizability and statistical power of the findings. These limitations highlight the need for future studies with longer follow-up durations, double-blind designs, and larger sample sizes to further validate and extend our results.
To further delve into the comprehensive impact of Tai Chi on gastrointestinal function, future studies could consider employing multimodal neuroimaging techniques to observe specific subpopulations (patients of different age groups, genders, and disease stages). Additionally, incorporating longitudinal follow-up studies is necessary to more comprehensively evaluate the long-term benefits of Tai Chi as an active health self-management approach for patients with functional gastrointestinal disorders.
Conclusion
In conclusion, the study findings indicate that Tai Chi may enhance the coordination between central and autonomic nervous functions by modulating functional connectivity of the aINS within the CAN and promoting the sympathetic/parasympathetic balance. This process enhances improved brain-gut communication, potentially representing the underlying mechanism through which Tai Chi exerts its gastrointestinal modulatory effects to alleviate symptoms associated with FCon.
Data availability
All relevant original data are as presented in this study. Further inquiries can be directed to the corresponding author.
References
Mearin, F. et al. Bowel disorders. Gastroenterology. https://doi.org/10.1053/j.gastro.2016.02.031 (2016).
Barberio, B., Judge, C., Savarino, E. V. & Ford, A. C. Global prevalence of functional constipation according to the Rome criteria: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 6, 638–648. https://doi.org/10.1016/s2468-1253(21)00111-4 (2021).
Arco, S. et al. Functional constipation in older adults: Prevalence, clinical symptoms and subtypes, association with frailty, and impact on quality of life. Gerontology 68, 397–406. https://doi.org/10.1159/000517212 (2022).
Nag, A. et al. The humanistic and economic burden of chronic idiopathic constipation in the USA: A systematic literature review. Clin. Exp. Gastroenterol. 13, 255–265. https://doi.org/10.2147/ceg.S239205 (2020).
Serra, J. et al. European society of neurogastroenterology and motility guidelines on functional constipation in adults. Neurogastroenterol. Motil. 32, e13762. https://doi.org/10.1111/nmo.13762 (2020).
Drossman, D. A. & Hasler, W. L. Rome IV-Functional GI disorders: Disorders of Gut-Brain interaction. Gastroenterology 150, 1257–1261. https://doi.org/10.1053/j.gastro.2016.03.035 (2016).
Mayer, E. A. et al. Role of brain imaging in disorders of brain-gut interaction: A Rome working team report. Gut 68, 1701–1715. https://doi.org/10.1136/gutjnl-2019-318308 (2019).
Carabotti, M., Scirocco, A., Maselli, M. A. & Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 28, 203–209 (2015).
Duan, H. et al. Regulation of the autonomic nervous system on intestine. Front. Physiol. 12, 700129. https://doi.org/10.3389/fphys.2021.700129 (2021).
Hu, C. et al. Cortical morphometry alterations in brain regions involved in emotional, motor-control and self-referential processing in patients with functional constipation. Brain Imaging Behav. 14, 1899–1907. https://doi.org/10.1007/s11682-019-00133-4 (2020).
Zhang, Z. et al. Functional constipation is associated with alterations in thalamo-limbic/parietal structural connectivity. Neurogastroenterol. Motil. 33, e13992. https://doi.org/10.1111/nmo.13992 (2021).
Zhu, Q. et al. Distinct resting-state brain activity in patients with functional constipation. Neurosci. Lett. 632, 141–146. https://doi.org/10.1016/j.neulet.2016.08.042 (2016).
Duan, S. et al. Altered functional connectivity within and between salience and sensorimotor networks in patients with functional constipation. Front. Neurosci. 15, 628880. https://doi.org/10.3389/fnins.2021.628880 (2021).
Jin, Q. et al. Sex-related differences in resting-state brain activity and connectivity in the orbital frontal cortex and Insula in patients with functional constipation. Neurogastroenterol. Motil. 31, e13566. https://doi.org/10.1111/nmo.13566 (2019).
Sonkusare, S. et al. Dynamic interactions between anterior Insula and anterior cingulate cortex link perceptual features and heart rate variability during movie viewing. Netw. Neurosci. 7, 557–577. https://doi.org/10.1162/netn_a_00295 (2023).
Mayer, E. A. Nat. Rev. Neurosci. 12, 453–466. https://doi.org/10.1038/nrn3071 (2011).
Rubio, A. et al. Uncertainty in anticipation of uncomfortable rectal distension is modulated by the autonomic nervous system–a fMRI study in healthy volunteers. Neuroimage 107, 10–22. https://doi.org/10.1016/j.neuroimage.2014.11.043 (2015).
Yin, T. et al. Aberrant functional brain network dynamics in patients with functional constipation. Hum. Brain Mapp. 42, 5985–5999. https://doi.org/10.1002/hbm.25663 (2021).
Costa, R. J. S., Snipe, R. M. J., Kitic, C. M. & Gibson, P. R. Systematic review: Exercise-induced Gastrointestinal syndrome-implications for health and intestinal disease. Aliment. Pharmacol. Ther. 46, 246–265. https://doi.org/10.1111/apt.14157 (2017).
Mearin, F. et al. Clinical practice guideline: Irritable bowel syndrome with constipation and functional constipation in the adult. Rev. Esp. Enferm Dig. 108, 332–363. https://doi.org/10.17235/reed.2016.4389/2016 (2016).
Silva, C. A. & Motta, M. E. The use of abdominal muscle training, breathing exercises and abdominal massage to treat paediatric chronic functional constipation. Colorectal Dis. 15, e250–255. https://doi.org/10.1111/codi.12160 (2013).
Krogh, K., Chiarioni, G. & Whitehead, W. Management of chronic constipation in adults. United Eur. Gastroenterol. J. 5, 465–472. https://doi.org/10.1177/2050640616663439 (2017).
Birdee, G. S. et al. T’ai Chi as exercise among middle-aged and elderly Chinese in urban China. J. Altern. Complement. Med. 19, 550–557. https://doi.org/10.1089/acm.2012.0223 (2013).
Silva, L. M., Cignolini, A., Warren, R., Budden, S. & Skowron-Gooch, A. Improvement in sensory impairment and social interaction in young children with autism following treatment with an original qigong massage methodology. Am. J. Chin. Med. 35, 393–406. https://doi.org/10.1142/s0192415x07004916 (2007).
Zeng, L. et al. Effects of Tai Chi on depression of middle-aged and older adults: An updated systematic review and meta-analysis. BMC Complement. Med. Ther. 23, 382. https://doi.org/10.1186/s12906-023-04207-1 (2023).
Yu, A. P. et al. Revealing the neural mechanisms underlying the beneficial effects of Tai Chi: A neuroimaging perspective. Am. J. Chin. Med. 46, 231–259. https://doi.org/10.1142/s0192415x18500131 (2018).
Staller, K. et al. Virtual Tai Chi program for patients with irritable bowel syndrome with constipation: Proof-of-concept feasibility trial. Neurogastroenterol. Motil. 34, e14429. https://doi.org/10.1111/nmo.14429 (2022).
Zhang, F. et al. Metabolic impairments associated with type 2 diabetes mellitus and the potential effects of exercise therapy: An exploratory randomized trial based on untargeted metabolomics. PLoS One 19, e0300593. https://doi.org/10.1371/journal.pone.0300593 (2024).
Xie, H. et al. Tai Chi Chuan exercise related change in brain function as assessed by functional near-infrared spectroscopy. Sci. Rep. 9, 13198. https://doi.org/10.1038/s41598-019-49401-9 (2019).
Cui, L. et al. Tai Chi Chuan vs general aerobic exercise in brain plasticity: A multimodal MRI study. Sci. Rep. 9, 17264. https://doi.org/10.1038/s41598-019-53731-z (2019).
Villafaina, S., Collado-Mateo, D., Fuentes, J. P., Merellano-Navarro, E. & Gusi, N. Physical exercise improves heart rate variability in patients with type 2 diabetes: A systematic review. Curr. Diab Rep. 17, 110. https://doi.org/10.1007/s11892-017-0941-9 (2017).
Jeong, J. et al. Exercise modulates sympathetic and vascular function in chronic kidney disease. JCI Insight 8 https://doi.org/10.1172/jci.insight.164221 (2023).
Toohey, K. et al. The impact of high-intensity interval training exercise on breast cancer survivors: A pilot study to explore fitness, cardiac regulation and biomarkers of the stress systems. BMC Cancer 20, 787. https://doi.org/10.1186/s12885-020-07295-1 (2020).
Sie, J. H. et al. Altered central autonomic network in baseball players: A resting-state fMRI study. Sci. Rep. 9, 110. https://doi.org/10.1038/s41598-018-36329-9 (2019).
de la Cruz, F. et al. Central autonomic network alterations in male endurance athletes. Sci. Rep. 12, 16743. https://doi.org/10.1038/s41598-022-20064-3 (2022).
Yiannakou, Y. et al. The PAC-SYM questionnaire for chronic constipation: Defining the minimal important difference. Aliment. Pharmacol. Ther. 46, 1103–1111. https://doi.org/10.1111/apt.14349 (2017).
Ali, M. K., Liu, L., Chen, J. H. & Huizinga, J. D. Optimizing autonomic function analysis via heart rate variability associated with motor activity of the human Colon. Front. Physiol. 12, 619722. https://doi.org/10.3389/fphys.2021.619722 (2021).
Yuan, Y. et al. Associations between colonic motor patterns and autonomic nervous system activity assessed by high-resolution manometry and concurrent heart rate variability. Front. Neurosci. 13, 1447. https://doi.org/10.3389/fnins.2019.01447 (2019).
Hutchison, R. M. et al. Dynamic functional connectivity: Promise, issues, and interpretations. Neuroimage 80, 360–378. https://doi.org/10.1016/j.neuroimage.2013.05.079 (2013).
Van Dijk, K. R., Sabuncu, M. R. & Buckner, R. L. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage 59, 431–438. https://doi.org/10.1016/j.neuroimage.2011.07.044 (2012).
Power, J. D., Barnes, K. A., Snyder, A. Z., Schlaggar, B. L. & Petersen, S. E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59, 2142–2154. https://doi.org/10.1016/j.neuroimage.2011.10.018 (2012).
de la Cruz, F. et al. The relationship between heart rate and functional connectivity of brain regions involved in autonomic control. Neuroimage 196, 318–328. https://doi.org/10.1016/j.neuroimage.2019.04.014 (2019).
Sklerov, M., Dayan, E. & Browner, N. Functional neuroimaging of the central autonomic network: Recent developments and clinical implications. Clin. Auton. Res. 29, 555–566. https://doi.org/10.1007/s10286-018-0577-0 (2019).
Oettlé, G. J. Effect of moderate exercise on bowel habit. Gut 32, 941–944. https://doi.org/10.1136/gut.32.8.941 (1991).
Schumann, D. et al. Effect of yoga in the therapy of irritable bowel syndrome: A systematic review. Clin. Gastroenterol. Hepatol. 14, 1720–1731. https://doi.org/10.1016/j.cgh.2016.04.026 (2016).
Zhou, C., Zhao, E., Li, Y., Jia, Y. & Li, F. Exercise therapy of patients with irritable bowel syndrome: A systematic review of randomized controlled trials. Neurogastroenterol. Motil. 31, e13461. https://doi.org/10.1111/nmo.13461 (2019).
Ba-Bai-Ke-Re, M. M. et al. Biofeedback-guided pelvic floor exercise therapy for obstructive defecation: An effective alternative. World J. Gastroenterol. 20, 9162–9169. https://doi.org/10.3748/wjg.v20.i27.9162 (2014).
Battaglia, E. et al. Long-term study on the effects of visual biofeedback and muscle training as a therapeutic modality in pelvic floor dyssynergia and slow-transit constipation. Dis. Colon Rectum 47, 90–95. https://doi.org/10.1007/s10350-003-0010-0 (2004).
Wehrwein, E. A., Orer, H. S. & Barman, S. M. Overview of the anatomy, physiology, and Pharmacology of the autonomic nervous system. Compr. Physiol. 6, 1239–1278. https://doi.org/10.1002/cphy.c150037 (2016).
Liu, L. et al. Diagnosis of colonic dysmotility associated with autonomic dysfunction in patients with chronic refractory constipation. Sci. Rep. 12, 12051. https://doi.org/10.1038/s41598-022-15945-6 (2022).
Liu, J. et al. Sleep deficiency is associated with exacerbation of symptoms and impairment of anorectal and autonomic functions in patients with functional constipation. Front. Neurosci. 16, 912442. https://doi.org/10.3389/fnins.2022.912442 (2022).
Wang, X., Yang, B., Yin, J., Wei, W. & Chen, J. D. Z. Electroacupuncture via chronically implanted electrodes improves Gastrointestinal motility by balancing sympathovagal activities in a rat model of constipation. Am. J. Physiol. Gastrointest. Liver Physiol. 316, G797–g805. https://doi.org/10.1152/ajpgi.00018.2018 (2019).
Jin, H., Liu, J., Foreman, R. D., Chen, J. D. & Yin, J. Electrical neuromodulation at acupoint ST36 normalizes impaired colonic motility induced by rectal distension in dogs. Am. J. Physiol. Gastrointest. Liver Physiol. 309, G368–376. https://doi.org/10.1152/ajpgi.00467.2014 (2015).
Chen, J. Neuromodulation and neurostimulation for the treatment of functional Gastrointestinal disorders. Gastroenterol. Hepatol. (N Y). 18, 47–49 (2022).
Grässler, B., Thielmann, B., Böckelmann, I. & Hökelmann, A. Effects of different exercise interventions on heart rate variability and cardiovascular health factors in older adults: A systematic review. Eur. Rev. Aging Phys. Act. 18 https://doi.org/10.1186/s11556-021-00278-6 (2021).
Bhati, P., Shenoy, S. & Hussain, M. E. Exercise training and cardiac autonomic function in type 2 diabetes mellitus: A systematic review. Diabetes Metab. Syndr. 12, 69–78. https://doi.org/10.1016/j.dsx.2017.08.015 (2018).
Sun, J. et al. Effects of 3-month qigong exercise on heart rate variability and respiration in anxious college students. Scand. J. Med. Sci. Sports. 34, e14521. https://doi.org/10.1111/sms.14521 (2024).
Benarroch, E. E. The central autonomic network: Functional organization, dysfunction, and perspective. Mayo Clin. Proc. 68, 988–1001. https://doi.org/10.1016/s0025-6196(12)62272-1 (1993).
Thayer, J. F. & Lane, R. D. A model of neurovisceral integration in emotion regulation and dysregulation. J. Affect. Disord. 61, 201–216. https://doi.org/10.1016/s0165-0327(00)00338-4 (2000).
Saper, C. B. The central autonomic nervous system: Conscious visceral perception and autonomic pattern generation. Annu. Rev. Neurosci. 25, 433–469. https://doi.org/10.1146/annurev.neuro.25.032502.111311 (2002).
Critchley, H. D. & Harrison, N. A. Visceral influences on brain and behavior. Neuron 77, 624–638. https://doi.org/10.1016/j.neuron.2013.02.008 (2013).
Singer, T., Critchley, H. D. & Preuschoff, K. A common role of Insula in feelings, empathy and uncertainty. Trends Cogn. Sci. 13, 334–340. https://doi.org/10.1016/j.tics.2009.05.001 (2009).
Garfinkel, S. N., Seth, A. K., Barrett, A. B., Suzuki, K. & Critchley, H. D. Knowing your own heart: Distinguishing interoceptive accuracy from interoceptive awareness. Biol. Psychol. 104, 65–74 (2015).
Karaivazoglou, K., Aggeletopoulou, I. & Triantos, C. Interoceptive processing in functional gastrointestinal disorders. Int. J. Mol. Sci. 25 https://doi.org/10.3390/ijms25147633 (2024).
Bauernfeind, A. L. et al. A volumetric comparison of the insular cortex and its subregions in primates. J. Hum. Evol. 64, 263–279. https://doi.org/10.1016/j.jhevol.2012.12.003 (2013).
Roy, H. A. & Green, A. L. The central autonomic network and regulation of bladder function. Front. Neurosci. 13, 535. https://doi.org/10.3389/fnins.2019.00535 (2019).
Ganesan, S. et al. Focused attention meditation in healthy adults: A systematic review and meta-analysis of cross-sectional functional MRI studies. Neurosci. Biobehav Rev. 141, 104846. https://doi.org/10.1016/j.neubiorev.2022.104846 (2022).
Lutz, A., McFarlin, D. R., Perlman, D. M., Salomons, T. V. & Davidson, R. J. Altered anterior Insula activation during anticipation and experience of painful stimuli in expert meditators. Neuroimage 64, 538–546. https://doi.org/10.1016/j.neuroimage.2012.09.030 (2013).
Björnsdotter, M., Löken, L., Olausson, H., Vallbo, A. & Wessberg, J. Somatotopic organization of gentle touch processing in the posterior insular cortex. J. Neurosci. 29, 9314–9320. https://doi.org/10.1523/jneurosci.0400-09.2009 (2009).
Jhang, J., Park, S., Liu, S., O’Keefe, D. D. & Han, S. A top-down slow breathing circuit that alleviates negative affect in mice. Nat. Neurosci. 27, 2455–2465. https://doi.org/10.1038/s41593-024-01799-w (2024).
Park, C., Youn, I. & Han, S. Single-lead ECG based autonomic nervous system assessment for meditation monitoring. Sci. Rep. 12, 22513. https://doi.org/10.1038/s41598-022-27121-x (2022).
Laborde, S. et al. Effects of voluntary slow breathing on heart rate and heart rate variability: A systematic review and a meta-analysis. Neurosci. Biobehav Rev. 138, 104711. https://doi.org/10.1016/j.neubiorev.2022.104711 (2022).
Cho, A., Park, S., Lee, H. & Whang, M. The physiological measurement and evaluation of empathy of video content. Sci. Rep. 13, 20190. https://doi.org/10.1038/s41598-023-47288-1 (2023).
Acknowledgements
F.Z. discloses support for the research of this work from the National Special Support Program for High-level Personnel Recruitment (No. W02020595). YK.T. discloses support for publication of this work from the National Natural Science Foundation of China (No. 82305362). FR.Z. discloses support for publication of this work from he National Natural Science Foundation of China (No. 82305360). We acknowledge the invaluable collaboration of multiple units and the dedication of numerous staff members who contributed to the success of this research. Special thanks go to: • The Affiliated Hospital of Chengdu University of Traditional Chinese Medicine for coordinating participant enrollment and clinical examinations. • The University of Electronic Science and Technology of China for facilitating fMRI scans of the participants. • SH.F. and ZQ.W. for their instruction and oversight of Tai Chi training. • J.F. and N.L. for their instruction and oversight of aerobic exercise training. • SR.C. for providing valuable suggestions on the manuscript. • All volunteers who participated in this study. The study was approved by the ethics committees of ChengduUniversity of Traditional Chinese Medicine (approval number:2018KL-047).
Author information
Authors and Affiliations
Contributions
F.Z., S.Y., and TY. L. designed the study. YK.T. and FR.Z. drafted the manuscript. F.Z., SY.T., ZJ.L., JW.C. and FR. Z. revised the manuscript. YK.T., JW.C., YY.G., RT.Y., YX.W., Y.L, HL.Z., KN.X. and S.Z. were involved in the study implementation and data collection. YK.T. was involved in the design of the diagrams in the manuscript. All authors have read and approved the publication of the final manuscript. YK.T., SY. T., JW.C., and FR.Z. contributed equally to this study and are also the co‐first authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Teng, Y., Tao, S., Chen, J. et al. Tai Chi’s synergistic modulation on autonomic nervous activity and central autonomic networks in functional constipation patients: a randomized controlled trial. Sci Rep 15, 23560 (2025). https://doi.org/10.1038/s41598-025-04088-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-04088-z