Abstract
This study investigated cytokine profiles in the aqueous humor (AH) of 172 eyes with central serous chorioretinopathy (CSC, n = 65), pachychoroid neovasculopathy (PNV, n = 24), polypoidal choroidal vasculopathy (PCV, n = 43), and controls (n = 40). AH samples were analyzed using a multiplex bead assay for cytokines including vascular endothelial growth factor (VEGF), interleukin-6 (IL-6), IL-8, IL-10, IP-10, and MCP-1. Results showed no significant differences in IL-6 (P = 0.122) and MCP-1 (P = 0.054) levels among groups. However, VEGF levels were significantly higher in CSC, PNV, and PCV compared to controls (all P < 0.05). In CSC and PNV, only VEGF was elevated without significant changes in other inflammatory cytokines, suggesting that inflammation was not a dominant factor. In contrast, PCV exhibited significantly higher levels of VEGF, IL-10, and IP-10, indicating distinct cytokine profiles. These findings suggest that elevated angiogenic and inflammatory cytokines may contribute to the pathogenesis of PCV.
Similar content being viewed by others
Introduction
Pachychoroid disease spectrum, including pachychoroid pigment epitheliopathy (PPE), central serous chorioretinopathy (CSC), pachychoroid neovasculopathy (PNV), and polypoidal choroidal vasculopathy (PCV), has been widely described1. The clinical manifestations common to pachychoroid diseases include focal or diffuse choroidal thickening, dilated Haller’s layer vessels (pachyvessels) with thinning of the overlying inner choroid, and retinal pigment epithelium (RPE) abnormalities as well2. Although the pathophysiology of pachychoroid spectrum diseases remains controversial, abnormal choroidal changes with dilation of large choroidal vessels and increased permeability are recognized to play an important role3.
There is growing evidence to suggest an overlap between CSC and other pachychoroid disorders such as PNV and PCV4. For instance, both serous detachments and RPE abnormalities could be seen in CSC and PCV, making it difficult to distinguish the two pachychoroid phenotypes. And these formerly independent disease entities are considered to represent a disease continuum, in which CSC might stage into PNV, and then ultimately into PCV5,6. However, not all patients with CSC could progress to PNV, nor do all PNV patients eventually develop PCV. The molecular mechanism explaining the progressions of these distinct pachychoroid phenotypes was not yet fully elucidated. Studies have shown that CSC is frequently complicated by choroidal neovascularization (CNV)7,8. And CNV was reportedly present as well above the pachyvessels in PNV and PCV9,10,11. It is well-established that vascular endothelial growth factor (VEGF) and other angiogenic factors contribute to CNV formation.
To our knowledge, little is known about molecular pathological aspects of choroidal abnormality, which may be a major cause of CSC, PNV, and PCV. Measurement of cytokines in the aqueous humor (AH) may address the distinct molecular pathogenesis within the pachychoroid spectrum of macular disorders. In this study, we evaluated and compared cytokine profiles in the AH of CSC, PNV, and PCV patients. In addition, relationships between cytokine concentrations were analyzed among the three groups.
Results
Clinical features
Table 1 shows the demographic and clinical characteristics of patients with CSC, PNV, PCV, and normal controls. A total of 172 AH samples were collected from 132 patients in the study group and 40 controls. Significant differences in age (P < 0.001), best-corrected visual acuity (BCVA) (P < 0.001), central macular thickness (CMT) (P = 0.005), and subfoveal choroidal thickness (SFCT) (P < 0.001) among the CSC, PNV, and PCV groups were confirmed by the Kruskal–Wallis test. CMT was greater in the PCV group than in the CSC (P = 0.001) and PNV (P = 0.041) groups, whereas there were no differences in CMT between the CSC and PNV groups (P = 0.335). SFCT was greater in the CSC group (P < 0.001) and the PNV group (P < 0.001) compared to the PCV group, whereas there were no differences in SFCT between the CSC and PNV groups (P = 0.254). Sex was not different among the three groups (P = 0.170).
Concentrations of cytokines
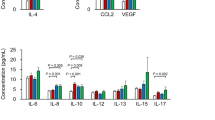
In this study, six cytokines (VEGF-A, IL-6, IL-8, IL-10, IP-10, and MCP-1) stably detected in all AH samples were used for statistical analysis (Table 2; Fig. 1). In the PCV group, levels of VEGF-A (P = 0.002), IL-10 (P < 0.001), and IP-10 (P < 0.001) were significantly higher than in controls. There were no significant differences in cytokine levels except for elevated VEGF-A levels between the CSC/PNV and control groups (P = 0.022 and P = 0.008, respectively).
Comparison of six cytokines in aqueous humor of patients with pachychoroid diseases and control subjects using violin plots. (A) The levels of VEGF were higher in pachychoroid entities than in controls. (B) There were no significant differences in IL-6 among the four groups. (C) For IL-8, there was a statistically significant difference overall among the four groups (P = 0.041), but the difference between groups was not statistically significant. (D) IL-10 levels were higher in PCV compared to controls. (E) IP-10 levels were higher in PCV than in controls. (F) There were no significant differences in the levels of MCP-1 among the pachychoroid and control groups. pg/ml, picogram per millilitre; VEGF-A, vascular endothelial growth factor-A; IL-6, interleukin 6; IL-8, interleukin 8; IL-10, interleukin 10; IP-10, interferon-inducible protein 10; MCP-1, monocyte chemotactic protein 1; CSC, central serous chorioretinopathy; PNV, pachychoroid neovasculopathy; PCV, polypoidal choroidal vasculopathy.
Table 3 showed the relationships between cytokine concentrations in the AH among the groups via Spearman’s rank correlation coefficients. The concentrations of VEGF-A were not correlated with any detected cytokines in the study among the CSC, PNV, and PCV groups.
Aqueous humor cytokines levels in CSC patients with different types of PED
Retinal pigment epithelial detachment (PED) is a classical biomarker of CSC imaged by SD-OCT. Based on differences in shape, size, and reflectivity, PED can be classified into two subtypes: focal PED and flat irregular PED (FIPED)12. Cytokine levels in the AH of 65 eyes with CSC, including 34 FIPED eyes and 31 focal PED eyes, and 40 control eyes were analyzed (Table 4). The VEGF-A levels in the FIPED group were 36.95 (27.84–48.25) pg/ml, higher than those in the focal PED group [30.20 (19.97–40.88) pg/ml, P = 0.018] and the control group [27.91 (20.71–35.42) pg/ml, P = 0.001] (Fig. 2). The levels of VEGF-A were similar in the focal PED and controls (P = 0.401, Fig. 2). No significant differences were noted in the levels of IL-6, IL-8, IL-10, IP-10, and MCP-1 among the FIPED, the focal PED, and controls (all P > 0.05).
Comparison of the VEGF levels in CSC patients with different PED types and controls. The VEGF levels in the FIPED group were higher than in the focal PED group and in the control group, and no difference was observed between the focal and control groups (P = 0.401). VEGF-A, vascular endothelial growth factor-A; FIPED, flat irregular pigment epithelial detachment; PED, pigment epithelial detachment.
Discussion
This study provides the first comprehensive analysis of intraocular cytokines of patients with pachychoroid spectrum diseases including CSC, PNV, and PCV. In CSC and PNV, VEGF was elevated without the elevation of proinflammatory cytokines. In the PCV, VEGF and proinflammatory cytokines IL-10 and IP-10 were significantly higher than those in controls.
Although a number of studies have described specific clinical and anatomical features of the four pachychoroid subtypes PPE, CSC, PNV, and PCV, their respective pathophysiology remains to be fully elucidated. The clinic manifestations common to the pachychoroid spectrum include choriocapillaris attenuation and abnormally dilated pachyvessels with choroidal vascular hyperpermeability1. The presence of choroidal morphologic changes as a key pathophysiologic mechanism was essential to diagnosing the pachychoroid spectrum of macular disorders. Cytokines were considered to be involved in ocular diseases that contributed to choroidal abnormalities13. This study aimed to investigate and compare various cytokine concentrations in the aqueous humor of patients with CSC, PNV, and PCV to better understand the respective molecular pathogenesis of these pachychoroid entities.
CSC was the archetypal disease of the pachychoroid spectrum, characterized by serous neurosensory retinal detachment at the posterior pole1. Choroidal congestion and choroidal hyperpermeability were believed to contribute to CSC1. Our study showed that the proangiogenic cytokine VEGF levels were distinctly upregulated in the CSC group than in the control group. Liu et al. have reported that the AH concentration of VEGF was significantly higher in various CNV diseases including CSC14. However, there was evidence that aqueous or plasma VEGF levels in patients with acute or chronic CSC were downregulated compared with the controls15,16. As the pathogenesis of CSC has not been fully elucidated, the true role of VEGF in CSC is unknown and warrants further investigation. No significant disparity was observed in other inflammatory factors between the CSC and control groups, suggesting that inflammatory mediators might play a minor role in CSC pathophysiology.
Pigment epithelial detachment (PED) is a well-recognized feature of CSC and was commonly observed in all stages of CSC. According to the different morphologies, PED can be classified into two different types: typical focal PED and flat irregular PED (FIPED)17. Clinical features of CSC may vary depending on the diverse type of PED. Hwang et al. found that CSC patients with FIPED involved more CNV and behaved with favorable anti-VEGF responsiveness in such cases17. It was unclear whether there was an association between the type of PED and cytokine levels. And the underlying molecular and cellular mechanisms of FIPED remain to be elucidated. We investigated and compared AH cytokine profiles according to PED type in CSC patients. In our study, the levels of VEGF were higher in eyes with FIPED than those in the focal PED and control groups, whereas no disparities were observed in IL-6, IL-8, IL-10, IP-10, and MCP-1 levels among the focal PED, FIPED and control groups. Our findings may explain why favorable treatment responses to anti-VEGF tend to occur in CSC patients with FIPED and the effect of inflammation mediators in CSC pathology was very minor. And this also showed that CSC was not an inflammatory disease.
PNV is a newly proposed clinical entity within a spectrum of “pachychoroid driven disease”, characterized by thick choroid, RPE abnormalities, and/or choroidal vascular hyperpermeability18. PNV is often misdiagnosed as neovascular age-related macular degeneration (nAMD) because neovascularization is similar both in PNV and AMD19. It is well known that the activation of proinflammatory cytokines and subsequent upregulation of angiogenic factors, especially vascular endothelial growth factor (VEGF), play a pivotal role in CNV formation. In PNV, ischemia in the choriocapillaris or RPE dysfunction, resulting from compression of expanded choroidal vessels, led to overexpression of angiogenic factors and neovascularization. It was rational that proangiogenic cytokine VEGF was elevated in AH of patients with PNV than in the controls in our study. Terao et al. compared cytokine profiles in AH of PNV and nAMD and found that VEGF-A levels were significantly lower in the PNV group than in the nAMD group but were no disparity compared with normal controls20. Hata et al. found that the mean VEGF concentration in the AH of patients with PNV was significantly lower than that in nAMD21. Their study suggested that PNV and nAMD may have different pathophysiology and CNV in the pachychoroid phenotype was triggered by a lower VEGF concentration threshold than in nAMD.
PCV, as one entity of the pachychoroid spectrum disease, is characterized by a branching vascular network with terminal aneurysmal (polypoidal) dilatations. It is increasingly thought to be a variant manifestation of type 1 neovascularization and may be closely correlated with CSC22,23. The etiology of PCV is still unclear. VEGF is considered a major stimulus for pathological angiogenesis in all eyes with CNV. Clinical studies have shown that intravitreal triamcinolone acetonide injection for the treatment of PCV was effective24,25, suggesting inflammation may play a contributing role in the pathogenesis of PCV. Other evidence indicated that proinflammatory factors such as IL-23, C-reactive protein, MCP-1, VEGF, and tumor necrosis factor-a were elevated in eyes with PCV26,27,28. In the present study, strong VEGF, IL-10, and IP-10 expression in AH was found in PCV eyes. We inferred that the disease continuum from CSC to PCV was also accompanied by the accumulation of inflammatory cytokines.
The age disparity among CSC, PNV, and PCV patients reflects the epidemiology of the diseases, as CSC predominantly affects young and middle-aged adults. Terao et al.29 found that age differences may have influenced the observed cytokine levels. Nevertheless, the changes in cytokines are likely also associated with the progression of pachychoroid spectrum diseases.
This study has several limitations. First, the AH samples may not be as valuable as vitreous fluid for detecting cytokine concentrations at the site of pathology in the retina and choroid. Second, there may be many other cytokines associated with pachychoroid spectrum diseases that were not included in this study, so more relevant new cytokines need to be explored in the future. Additionally, the enrolled CSC cases in this study exclusively had a disease duration exceeding 3 months. Further stratification of these cases into acute and chronic subgroups in future studies could facilitate a more nuanced comparison of cytokine profiles across pachychoroid spectrum diseases.
In summary, VEGF was elevated without the elevation of other inflammatory cytokines in CSC and PNV, indicating that inflammation was not the dominant factor on CSC and PNV diseases. PCV had significantly distinct cytokine profiles in the AH compared with controls. Elevated angiogenic and inflammatory cytokines, such as VEGF, IL-10, and IP-10, may contribute to the pathogenesis of PCV. In addition, higher VEGF levels in CSC patients with FIPED verified that VEGF contributed more to the pathogenesis of FIPED compared with focal PED.
Methods
This prospective, comparative, observational study was conducted in compliance with the tenets of the Declaration of Helsinki; the research was approved by the Institutional Review Board of Zhejiang Provincial People’s Hospital (IRB number#KY2022022). Written informed consent was obtained from the subjects after detailed explanation of the study protocol.
Study participants
We screened 145 patients over the age of 18 years with a diagnosis of CSC, PNV, and PCV, of whom 84 men and 48 women were finally included in this observational study. 65 eyes of patients with treatment-naïve CSC, 24 eyes had treatment-naïve PNV, and 43 eyes had treatment-naïve PCV were enrolled between January 2018 and June 2022 at Zhejiang Provincial People’s Hospital and Affiliated Eye Hospital of Wenzhou Medical University. And 40 eyes who were scheduled to receive cataract surgery were recruited as controls.
All patients received comprehensive ophthalmic examinations, including best-corrected visual acuity (BCVA) testing with the standard logarithmic visual acuity chart, slit-lamp biomicroscopy, color fundus photographs, fluorescein angiography (FA), indocyanine green angiography (ICGA), spectral-___domain optical coherence tomography (SD-OCT, Heidelberg Engineering, Heidelberg, Germany) and OCT angiography (AngioVue, RTVue XR Avanti SD-OCT, Optovue, Fremont, CA, USA).
Diagnosis of CSC, PNV, and PCV
The CSC diagnosis was based on4: (1) OCT demonstrating the presence of subretinal fluid and/or serous pigment epithelium detachment involving the fovea; (2) FA showing the typical leakage points at the level of the RPE in the macular region; (3) symptom duration exceeding 3 months.
PNV was diagnosed using the following criteria based on4: (1) type-1 CNV detected in either eye; (2) no or only non-extensive drusen or hard drusen in both eyes; (3) dilated choroidal vessels or thicking below type 1 CNV detected by ICGA and OCT; (4) choroidal vascular hyperpermeability detected in the late phase of ICGA; and (5) RPE abnormality independent of CNV lesions detected by FA or a history of CSC.
PCV was diagnosed based on early subretinal ICGA hyperfluorescence and at least one of the following diagnostic criteria4: (1) a sharply peaked pigment epithelium detachment (PED), subretinal pigment epithelium ring-like structures, double-layer sign, choroidal thickening with pachyvessels, and subretinal and subretinal pigment epithelium fluid on SD-OCT images. (2) nodular appearance of the polypoidal lesion on stereoscopic viewing, (3) hypofluorescent halo around the nodule, (4) abnormal vascular networks supplying the polypoidal lesion, (5) multiple aneurysmal staining or polypoidal lesions in the late phase of ICGA.
The exclusion criteria were: (1) patients with other systemic conditions, such as diabetes, cardiovascular diseases, cerebrovascular diseases, or malignancies; (2) history of glaucoma, uveitis, high myopia, and other retinal disorders; (3) previous anti-VEGF intravitreal injections or other intraocular procedures within the past 6 months. We excluded six subjects based on pre-existing diabetes mellitus, five subjects due to documented cardiovascular or cerebrovascular pathologies, and two subjects due to recent (< 6 months) retinal laser photocoagulation therapy.
Aqueous humor collection
AH samples (typically 0.1 ml) of eyes with CSC, PNV, or PCV were withdrawn before intravitreal conbercept (0.5 mg/0.05 ml; Kanghong Biotechnologies Co. Ltd, Chengdu, Sichuan Province, China) using a syringe with a 30-gauge needle (Nipro, Osaka, Japan) after topical anesthesia. AH samples from controls were aspirated before cataract surgery in the same manner. All samples were transferred to a sterile tube and stored at − 80 °C until cytokine analysis. The cytokines measured included VEGF-A, IL-6, IL-8, IL-10, IP-10, and MCP-1, and their concentrations were analyzed using Multi-Analyte Profiling (xMAP; Luminex200, Hercules, CA, USA). All procedures were undertaken in the dark at room temperature, avoiding light-induced contamination.
Image analysis
The central macula thickness (CMT) and subfoveal choroidal thickness (SFCT) were recorded using the caliper function of the Spectralis instrument. CMT was defined as the vertical distance from internal limiting membrane to the lower border of the RPE. Measuring SFCT from the hyperreflective line corresponding to Bruch’s membrane below the RPE to the inner surface of the sclera.
Statistical analysis
Statistical analysis was performed using SPSS 26.0 software (IBM SPSS Inc., Chicago, IL, USA). Continuous variables are presented as means ± standard deviation and categorical variables were assessed using Chi-squared tests. Quantitative variables were tested for normal distribution by the Shapiro–Wilk test. Data with a skewed distribution are expressed as the median and interquartile range (IQR). Intergroup comparisons of quantitative variables were made using the one-way analysis of variance (ANOVA) for normally distributed variables and the Kruskal–Wallis test for variables with nonnormal distribution. If the comparison among the groups yielded a statistically significant result (P < 0.05), post hoc pairwise comparisons were conducted with Bonferroni correction. Spearman’s rank correlation coefficients were used to evaluate the relationships between cytokine concentrations in the AH among the pachychoroid subtypes and control groups. P value < 0.05 was considered to be statistically significant.
Data availability
The data are not available for public access because of patient privacy concerns, but are available from the corresponding author upon reasonable request.
References
Hua, R., Duan, J. & Zhang, M. Pachychoroid spectrum disease: Underlying pathology, classification, and phenotypes. Curr. Eye Res. 46, 1437–1448. https://doi.org/10.1080/02713683.2021.1942073 (2021).
Mazzeo, T. J. M. M. et al. Pachychoroid disease spectrum: Review Article. Graefes Arch. Clin. Exp. Ophthalmol. 260, 723–735. https://doi.org/10.1007/s00417-021-05450-3 (2022).
Brown, B., Mohan, R., Chhablani, J. & S. & Pachychoroid spectrum disorders: An updated review. J. Ophthalmic Vis. Res. 18, 212–229. https://doi.org/10.18502/jovr.v18i2.13188 (2023).
Borooah, S. et al. Pachychoroid spectrum disease. Acta Ophthalmol. 99, 806–822. https://doi.org/10.1111/aos.14683 (2021).
Siedlecki, J., Schworm, B. & Priglinger, S. G. The pachychoroid disease spectrum-and the need for a uniform classification system. Ophthalmol. Retina 3, 1013–1015. https://doi.org/10.1016/j.oret.2019.08.002 (2019).
Siedlecki, J. et al. Progression of pachychoroid neovasculopathy into aneurysmal type 1 choroidal neovascularization or polypoidal choroidal vasculopathy. Ophthalmol. Retina 6, 807–813. https://doi.org/10.1016/j.oret.2022.04.004 (2022).
Shiragami, C. et al. Clinical features of central serous chorioretinopathy with type 1 choroidal neovascularization. Am. J. Ophthalmol. 193, 80–86. https://doi.org/10.1016/j.ajo.2018.06.009 (2018).
Savastano, M. C., Rispoli, M. & Lumbroso, B. The incidence of neovascularization in central serous chorioretinopathy by optical coherence tomography angiography. Retina 41, 302–308. https://doi.org/10.1097/IAE.0000000000002810 (2021).
Matsumoto, H., Kishi, S., Mukai, R. & Akiyama, H. Remodeling of macular vortex veins in pachychoroid neovasculopathy. Sci. Rep. 9, 14689. https://doi.org/10.1038/s41598-019-51268-9 (2019).
Altinisik, M., Kurt, E., Sonmezer, P., Kayikcioglu, O. & Ilker, S. S. A comparative study of type 1 neovascularization: neovascular age-related macular degeneration versus pachychoroid neovasculopathy. Eur. J. Ophthalmol. 32, 2404–2411. https://doi.org/10.1177/11206721211037828 (2022).
Sen, P., Manayath, G., Shroff, D., Salloju, V. & Dhar, P. Polypoidal choroidal vasculopathy: An update on diagnosis and treatment. Clin. Ophthalmol. 17, 53–70. https://doi.org/10.2147/OPTH.S385827 (2023).
Mao, J. et al. Predictors of anti-VEGF efficacy in chronic central serous chorioretinopathy based on intraocular cytokine levels and pigment epithelium detachment subtypes. Acta Ophthalmol. 100, 1385–1394. https://doi.org/10.1111/aos.15109 (2022).
Schellevis, R. L. et al. Role of the complement system in chronic central serous chorioretinopathy: A genome-wide association study. JAMA Ophthalmol. 136, 1128–1136. https://doi.org/10.1001/jamaophthalmol.2018.3190 (2018).
Liu, C. et al. Comparison of intraocular cytokine levels of choroidal neovascularization secondary to different retinopathies. Front. Med. (Lausanne) 8, 783178. https://doi.org/10.3389/fmed.2021.783178 (2021).
Karska-Basta, I. et al. Imbalance in the levels of angiogenic factors in patients with acute and chronic central serous chorioretinopathy. J. Clin. Med. 10, 1087. https://doi.org/10.3390/jcm10051087 (2021).
Karska-Basta, I. et al. Altered plasma cytokine levels in acute and chronic central serous chorioretinopathy. Acta Ophthalmol. 99, 222–231. https://doi.org/10.1111/aos.14547 (2021).
Hwang, H., Kim, J. Y., Kim, K. T., Chae, J. B. & Kim, D. Y. Flat irregular pigment epithelium detachment in central serous chorioretinopathy: A form of pachychoroid neovasculopathy? Retina 40, 1724–1733. https://doi.org/10.1097/IAE.0000000000002662 (2020).
Baba, T. et al. Association of IL-4 with pachychoroid neovasculopathy. Sci. Rep. 13, 1152. https://doi.org/10.1038/s41598-023-28108-y (2023).
Arf, S., Sayman Muslubas, I., Hocaoglu, M., Ersoz, M. G. & Karacorlu, M. Features of neovascularization in pachychoroid neovasculopathy compared with type 1 neovascular age-related macular degeneration on optical coherence tomography angiography. Jpn J. Ophthalmol. 64, 257–264. https://doi.org/10.1007/s10384-020-00730-7 (2020).
Terao, N. et al. Distinct aqueous humour cytokine profiles of patients with pachychoroid neovasculopathy and neovascular age-related macular degeneration. Sci. Rep. 8, 10520. https://doi.org/10.1038/s41598-018-28484-w (2018).
Hata, M. et al. Intraocular vascular endothelial growth factor levels in pachychoroid neovasculopathy and neovascular age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 58, 292–298. https://doi.org/10.1167/iovs.16-20967 (2017).
Dansingani, K. K., Gal-Or, O., Sadda, S. R., Yannuzzi, L. A. & Freund, K. B. Understanding aneurysmal type 1 neovascularization (polypoidal choroidal vasculopathy): A lesson in the taxonomy of ‘expanded spectra’—A review. Clin. Exp. Ophthalmol. 46, 189–200. https://doi.org/10.1111/ceo.13114 (2018).
Manayath, G. J., Shah, V. S., Saravanan, V. R. & Narendran, V. Polypoidal choroidal vasculopathy associated with central serous chorioretinopathy: Pachychoroid spectrum of diseases. Retina 38, 1195–1204. https://doi.org/10.1097/IAE.0000000000001665 (2018).
Liang, I. C., Lin, Y. R., Chien, H. W. & Liu, K. R. Vision preservation in eyes of polypoidal choroidal vasculopathy with low-dose intravitreal triamcinolone acetonide. J. Ocul. Pharmacol. Ther. 33, 42–49. https://doi.org/10.1089/jop.2015.0150 (2017).
Zhang, K. et al. Periocular triamcinolone acetonide injection for treating polypoidal choroidal vasculopathy concurrent with hemorrhagic retinal detachment. Medicine (Baltimore) 97, 12464. https://doi.org/10.1097/MD.0000000000012464 (2018).
Sasaki, S. et al. Associations of IL-23 with polypoidal choroidal vasculopathy. Invest. Ophthalmol. Vis. Sci. 53, 3424–3430. https://doi.org/10.1167/iovs.11-7913 (2012).
Zhou, H., Zhao, X., Yuan, M. & Chen, Y. Comparison of cytokine levels in the aqueous humor of polypoidal choroidal vasculopathy and neovascular age-related macular degeneration patients. BMC Ophthalmol. 20, 15. https://doi.org/10.1186/s12886-019-1278-8 (2020).
Hu, J. et al. The features of inflammation factors concentrations in aqueous humor of polypoidal choroidal vasculopathy. PLoS ONE 11, 0147346. https://doi.org/10.1371/journal.pone.0147346 (2016).
Terao, N. et al. Association of upregulated angiogenic cytokines with choroidal abnormalities in chronic central serous chorioretinopathy. Invest. Ophthalmol. Vis. Sci. 59, 5924–5931. https://doi.org/10.1167/iovs.18-25517 (2018).
Funding
This work was supported by the Zhejiang Key Laboratory of Precision Medicine for Eye Diseases, Zhejiang Provincial Natural Science Foundation of China under Grant No. LTGY23H120005, Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (No.23KY490), Zhejiang Province Traditional Chinese Medicine Science and Technology Project (No.2025ZL014), Shanghai Key Laboratory of Visual Impairment and Restoration (20DZ2271700), and National Key Research and Development Program of China (2022YFC2404305).
Author information
Authors and Affiliations
Contributions
C.Y.Z analyzed the data and wrote the original draft; J.B.M conceptualized and designed the study; Y.Z, S.A.Z, and Z.Y.X collected the data; L.J.S reviewed the draft and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, C., Zhao, Y., Zhang, S. et al. Analysis of cytokine profiles in aqueous humor based on optical coherence tomography in pachychoroid spectrum diseases. Sci Rep 15, 20639 (2025). https://doi.org/10.1038/s41598-025-04130-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-04130-0