Abstract
Vortex-assisted liquid–liquid microextraction was used to extract and derivatize propylamine, butylamine, pentylamine, benzylamine, and sec-butylamine in various environmental water and refinery effluent samples. Herein, µL-volume of butyl chloroformate as a derivatization agent was added to 1,1,2-trichloroethane as an extraction solvent, allowing for simultaneous extraction and derivatization of the amines. The derivatization agent reacted with the amines in an alkaline medium under mild conditions to create their related carbamates. The carbamate derivatives obtained from primary amines performed well in gas chromatography system. Various affecting parameters of the method, such as the derivatization agent amount, type of extraction solvent, ionic strength of solution, pH, vortexing time, and centrifuging time and rate were carefully optimized. In the end, gas chromatography-flame ionization detector was used to separate, identify, and quantify the enriched derivatives. Under optimal conditions, enrichment factors varied from 440 to 515, while extraction recovery of the desired amines was between 88 and 103%. The limits of detection for amines ranged from 0.4 to 1.0 µg L−1, and inter- and intra-day precision were in the ranges of 2.2–5.8 and 1.0–3.3%, respectively. Also, relative recoveries were between 85 and 107%. This method proved to be valuable due to its simplicity, sensitivity, high precision, lack of need for special equipment, and high ability in the extraction and derivatization of the selected amines. The use of µL-volume of organic solvents made it economical and environmentally friendly.
Similar content being viewed by others
Introduction
There are increasing concerns regarding the expansion of chemical substances consumption in industries. These compounds as pollutants infiltrate the environment and pose risks to human health. Amines are an important category of these pollutants1,2. Amines are derivatives of ammonia in which one or more alkyl or aryl groups have replaced ammonia hydrogens. Primary aliphatic amines are obtained by substituting an aliphatic alkyl or aryl group for a hydrogen atom in ammonia3,4. Short-chain primary aliphatic amines are widely used in the industries of production of explosives, catalysts, paints, cosmetics, medicines, antioxidants, plastics, pesticides, detergents, surfactants, corrosion inhibitors, textiles, electroplating, and petrochemical industry. Amines enter the environment due to improper wastewater treatment in these industries2,5,6. Additionally, amines are produced from the decomposition of nitrogenous organic matter, primarily proteinaceous waste, and are distributed into the air, and underground and surface waters7,8. Aliphatic amines (C1–C8) are highly toxic to humans9. Amines have unpleasant odors and irritants that affect gastrointestinal system, skin, eyes, mucous membranes, and respiratory tract. Prolonged exposure to amine vapors can lead to pneumonia, bronchitis, tracheitis, and pulmonary edema. Moreover, amines can react with nitrosating agents to form carcinogenic compounds known as N-nitrosamines10,11,12,13,14,15. Identifying and measuring these dangerous pollutants in various sample types is crucial16. Gas chromatography (GC)17,18spectrofluorimetry19capillary electrophoresis20and liquid chromatography21,22 are essential analytical methods for amine analysis. However, a major challenge in their analysis is the attendance of interfering agents in sample matrix and their low concentration in the samples. Therefore, the extraction and preconcentration of them is a key step before analysis23. solid phase extraction (SPE)24,25 and Liquid-liquid extraction26 were used to extract various analytes, including amines, due to their simplicity. These methods are time-consuming or require the use of more organic solvents. Furthermore, SPE requires cartridge conditioning and may exhibit memory effects27. These methods show relatively limited efficacy in enriching and extracting amines. To overcome these challenges, liquid and solid phase microextraction (LPME and SPME) techniques were developed and employed in extracting amines12,23,28,29,30,31,32. Unfortunately, the fibers needed in SPME are fragile and expensive and this method demonstrates low extraction recovery (ER) in extracting analytes. The most important defect in single-drop microextraction (SDME) is the lack of repeatability and stability of extractant droplet volume. Hollow fiber-LPME (HF-LPME) solves this problem. However, both mentioned techniques are slow and have low ER27. Extraction happens instantaneously and efficiently in dispersive liquid-liquid microextraction (DLLME) because the extractant is completely dispersed into aqueous media33. However, the presence of a dispersion solvent in this method reduces partition coefficient of the analytes between aqueous phase and extraction solvent. In this method, a portion of volatile analytes is lost during the injection of the disperser and extractant mixture into aqueous phase and the formation of emulsion. Homogeneous liquid–liquid microextraction, in situ solvent formation microextraction, vortex-assisted liquid-liquid microextraction (VALLME), solvent-based microextraction, and ionic liquid-based extraction are recent developments of DLLME. These methods form a biphasic system consisting of an aqueous phase and an extraction solvent for the extraction process, unlike DLLME. These methods have high enrichment factor (EF) and extraction efficiency, are simple, economical, and eco-friendly34,35,36,37,38,39,40,41. VALLME uses vortex to disperse the extractant into sample solution. It eliminates the need for the disperser solvent, is low-cost, and reduces the problem of wasting volatile analytes. Using minimal organic solvent and simple extraction enhance the value of this method. This method also has higher ER than most LPME and SPME methods. It overcomes the non-reproducibility of extraction phase volume that occurs in SDME. Vortex mixing as a mild emulsification step solves the lack of uniformity in the distribution of analytes in DLLME. Also, VALLME is faster than HF-LPME, SPME, and SDME41,42,43,44. Amines are challenging to extract from aqueous media due to their high polarity and solubility in water. On the other hand, in GC, the interaction between the amines and stationary phase leads to tailing peaks. Derivatization of amines to less polar compounds is necessary to improve extraction efficiency, precision, and sensitivity45. Various derivatization reactions including acylation, permethylation, dinitrophenylation, silylation, and formation of phosphonamides, sulfonamides, Schiff bases, and carbamates have been used before the analysis of amines8,12,26,46,47,48. Carbamate derivatives have shown favorable chromatography behaviors in GC. Alkyl chloroformates are commonly used as the reagents to produce carbamate derivatives. Amines react efficiently with this reagent under mild conditions in alkaline aqueous solution8. Butyl chloroformate (BCF) was selected as the derivatization reagent in this study due to its high reactivity with specific functional groups, e.g., carboxylic acids, amines, its ability to form stable derivatives, and its compatibility with specific analysis methods, e.g., GC, HPLC. Recent studies have demonstrated BCF’s effectiveness in derivatizing compounds such as amphetamine, methamphetamine, ammonia, aliphatic amines, morphine, and oxymorphone, enhancing the sensitivity and selectivity of the analytical method, achieving low detection limits and high repeatability. These properties make it particularly suitable for the analysis of target compounds in water samples23,49,50,51.
For the first time in this work, a mixture of 1,1,2-trichloroethane (1,1,2-TCE) and BCF was used for the extraction and derivatization of some primary aliphatic amines in water samples. The mixing of them allows the integration of the extraction and derivatization of amines in one step. Also, this work significantly increases the efficiency of the method. It provides very high extraction efficiencies (nearly complete) with the use of µL-volumes of solvent, which is especially valuable for short-chain amine compounds. The EF and enhancement factors (EnF) of the method reduce the limit of detection (LOD) of the method, which is of particular importance in the measurement of these amines in different samples. Also, the good repeatability and wide linear range (LR) demonstrate the value of the method. The resulting carbamate derivatives with less polarity than the corresponding amines, were successfully extracted and analyzed by GC-flame ionization detector (FID).
Materials and methods
Chemicals and solutions
Propylamine (PrA, 99%) and butylamine (BuA, 98%) were purchased from Fluka (Buchs, Switzerland). Other target amines including pentylamine (PeA, 99%), benzylamine (BeA, 98%), and sec-butylamine (sec-BuA, 98%), were bought from Merck (Darmstadt, Germany). 1,1,1-Trichloroethane (1,1,1-TCE, 99.7%) and chloroform (98%) from Merck and 1,1,2-TCE (99.5%) and 1,2-dibromoethane (1,2-DBE, 98%) from Janssen (Beerse, Belgium) were used as extraction solvents. Sodium hydroxide (99%), boric acid (99%), acetic acid (98%, w/w), and sodium dihydrogen phosphate (99%) were used in preparation of Briton-Robinson buffer; “salting-out” agents (sodium sulfate (99%), potassium chloride (99.9%), and sodium chloride (99%)), and BCF (95%) as a derivatization agent, all of which were obtained from Merck. Also, disodium salt of ethylenediaminetetraacetic acid (EDTA) was purchased from Merck. Daily working solutions were prepared by diluting the stock solution with deionized water was prepared from Ghazi Co. (Tabriz, Iran). Methanol (from Merck) was used for preparation of a stock solution at a concentration of 500 mg L− 1 of each amine.
Samples
Various environmental water samples (river, well, aqueduct, and rain waters) and refinery effluent were prepared from Tabriz (Iran). The water samples and refinery effluent were left undisturbed to settle coarse solid particles. Subsequently, filtration through filter paper was performed to remove fine suspended particles. All samples (without dilution) were subjected to the developed process for simultaneous derivatization and extraction of amines. EDTA (10 mg) was added to 5 mL of the samples before adjusting pH at 10 to prevent precipitation of existing cations.
Apparatus
A Shimadzu GC-FID (model 2014) instrument equipped with a split/splitless inlet system (Kyoto, Japan) was utilized to identify and quantify derivatives of amines. They were separated using an RTX-5 capillary column (Restek, Centre, PA, USA) with a 0.25 μm film thickness (95% dimethyl-5% diphenyl polysiloxane), 0.25 mm inner diameter, and 30 m length. The temperature programming for the column oven was as follows: first, the temperature was held for 2 min at 60 °C, then, the temperature was elevated with a ramp of 10 °C min− 1 to 200 °C. In the end, the temperature was held for 2 min at 200 °C to clean up the column. The temperature of the injection port and FID was adjusted at 300 °C. Helium (99.999%, Gulf Cryo, Dubai, United Arab Emirates) was utilized as the carrier gas (linear velocity of 30 cm s− 1) and makeup gas (flow rate of 40 mL min− 1). An OPGU 1500 S Shimadzu hydrogen generator was utilized to produce hydrogen gas as the fuel for FID (flow rate of 30 mL min− 1). Air was used as the FID oxidant (flow rate of 300 mL min− 1). pH of Briton-Robinson buffer was adjusted using a 654-pH meter (Herisau, Switzerland). An L46 vortex (Labinco, Breda, the Netherlands) and a D-7200 Hettich centrifuge (Kirchlengern, Germany) were utilized in the simultaneous extraction and derivatization procedures.
Simultaneous derivatization and extraction procedure
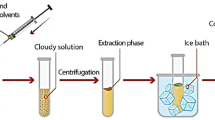
Initially, an aliquot of 5.0 mL of deionized water containing 500 µg L− 1 of each amine or sample solution was poured into a conical bottom glass tube with a cap. Subsequently, the solution pH was adjusted at 10.0 using 1.0 mL of Briton-Robinson buffer. Sodium chloride (375 mg) was dissolved in it. A mixture of 1,1,2-TCE (20 µL) and BCF (5.0 µL) was provided and slowly added by a 50-µL micro syringe into the solution. Then the tube was sealed by a cap and the mixture vortexed for 5.0 min to perform the extraction and derivatization procedure. Centrifugation for 3 min at 5000 rpm was employed to form two discernable phases and settle the extraction phase. In the end, 1.0 µL of the sedimented phase (10 ± 0.5 µL) was injected into GC-FID. The derivatization reaction and schematic of the method are shown in Fig. 1.
EF, ER, enf, and relative recovery (RR)
EF demonstrates the method’s ability to enrich the analytes. Equation (1) shows this notion52,53. ER indicates the fraction of the analyte extracted into the organic phase from the initial amount. This concept is calculated according to Eq. (2). EnF reflects the enhancement of the FID signal resulting from the analyte’s derivatization and its enrichment into an organic phase. This notion calculated according to Eq. (3).
.
In these equations, Csed, nsed, and Vsed are analyte concentration and amount in the final extracted phase, and settled phase volume, respectively. Also, Co, n₀, and Vo are the initial concentration of the analyte and its amount in sample solution, and sample volume, respectively. Also, m₀ and m1 are slopes of the calibration graphs obtained from direct injection of standard solutions and after performing the derivatization/microextraction procedure, respectively.
RR is a concept to evaluate the established methodology’s efficacy in real samples, calculated using Eq. 4.
.
In this equation, Creal, Cadd, and Ctotal refer to the concentrations of the analyte present in real sample, defined quantity of the analyte spiked into the real sample, and cumulative concentration of the analyte in spiked sample, respectively.
Results and discussion
Optimization of VALLME effective parameters
Derivatization agent volume
In the absence of an appropriate derivatization agent, weak analytical signals and tailing peaks were observed for amines. However, the reaction of amines with BCF significantly improved the analytical signals and led to considerably sharp peaks. These results suggest that BCF is suitable for the derivatization process. The optimal volume of BCF should be determined for the desirable derivatization of amines. Different volumes of BCF (2.0, 5.0, 7.0, 10.0, and 13.0 µL) were added to the extraction solvent for derivatization. The comparison of the results showed that adding 5 µL of BCF into the extractant is the best choice (Fig. 2). Two microliters of BCF were not adequate for the derivatization of all target amines. In the cases where BCF volumes higher than 5.0 µL were used, due to the decreasing solution pH (decomposition of BCF in water produces HCl), derivatization was not complete. On the other hand, increasing the BCF amount increased volume of the settled organic phase, which reduced analytical signals owing to dilution effect. Therefore, in the next steps, 5.0 µL of BCF was utilized for the derivatization process.
Optimization of derivatization agent. Procedure conditions: aqueous solution, 5 mL of deionized water containing 500 µg L−1 of each analyte (pH 8.0); derivatization agent, BCF; extraction solvent, 1,2-DBE; vortexing time, 5.0 min; and centrifuging time (rate), 5.0 min (7000 rpm). Error bars were used to illustrate the minimum and maximum values obtained from three repeated determinations.
pH of sample
In this study, pH emerges as a critical parameter due to its direct influence on the analytes and the derivatization process. Aliphatic amines are converted into ammonium forms in acidic media, which cannot be derivatized well with BCF. Therefore, only neutral and alkaline pHs (7.0, 8.0, 9.0, 10.0, 11.0, and 12.0) were studied to assess their impact on extraction efficiency and derivatization (pH was modified by Britton-Robinson buffer). According to Fig. 3, pH 10.0 is the most suitable pH for the simultaneous extraction and derivatization of target amines. Also, protonation of amines and incomplete derivatization have happened at pHs 7.0, 8.0, and 9.0 because target aliphatic amines possess pKa values ranging from 10.0 to 11.0, except benzylamine, which has pKa = 9.33. In more alkaline pHs (11.0 and 12.0), hydrolysis and decomposition of the BCF occur. Following this, the pH of the aqueous phases was adjusted at 10.0 by the used buffer.
Effect of pH on the extraction efficiency and derivatization reaction. Procedure conditions: are the same as those used in Fig. 2, except 5.0 µL BCF was used as the derivatization agent.
Addition of salt
Owing to that partition coefficients of the analytes between extraction solvent and the aqueous phase depend on ionic strength of aqueous media, therefore, the effect of salt addition should be studied. One popular approach for this purpose is addition of certain inorganic salts to the aqueous phase. Adding salt can produce two effects on ER: “salting-out” and “salting-in” effects. In the first impact, adding salt enhances the medium’s ionic strength, decreases the solubility of analytes in the aqueous phase, and increases ERs. In the next impact, salt addition increases the aqueous phase viscosity, making it difficult to transfer the analytes into the extractant and decrease ERs. In light of the above, the impact of this parameter on the efficiency of the process was studied using solution without salt and different aqueous solutions containing 1.0 M of Na2SO4, KCl, or NaCl. Figure 4a authenticated that NaCl yields the highest ERs for all studied amines. Hence, the solutions containing various NaCl concentrations (0, 2.5, 5.0, 7.5, 10.0, 12.5, and 15.0%, w/v) were evaluated. Based on the results, 7.5%, w/v, NaCl was chosen as the optimal concentration (Fig. 4b). The “salting-out” effect cannot be successfully implemented in the lower NaCl concentrations. Higher NaCl concentrations enhanced the solution’s viscosity (“salting-in” effect) and decreased ERs. Therefore, 7.5%, w/v, NaCl was optimized and employed in the next steps.
Study of salt effect in the extraction of amines. (a) Salt type. Procedure conditions: are the same as those used in Fig. 3, except solution pH is adjusted at 10.0. (b) Salt concentration. Procedure conditions: are the same as those used in (a), except NaCl was added to the sample solution.
Extraction solvent type and volume
The chosen extraction solvent should be water-immiscible, compatible with BCF, exhibit a density differential from water, and demonstrate high solubility for amine derivatives. To determine the most effective extractant, various extraction solvents (16 µL of 1,2-DBE, 18 µL of 1,1,1-TCE, 28 µL of chloroform, and 20 µL of 1,1,2-TCE) were assessed. Different volumes of the extraction solvents were used due to their varying solubility in water and the necessity to achieve a specific settled phase volume (10 ± 0.5 µL). According to Fig. 5a, 1,1,2-TCE proves to be a good extractant for the desired analytes. The other three solvents have less ability to extract the amine derivatives. This can be attributed to several synergistic factors. First, the relatively high solubility of the carbamate derivatives in this halogenated solvent, due to its moderate polarity and strong dipole–dipole interactions, facilitates effective extraction from the aqueous phase. Second, the presence of bulky nonpolar groups (e.g., n-butyl moiety) in the derivatives allows favorable van der Waals interactions with the chlorinated hydrocarbon chain of the solvent. Additionally, the high density of 1,1,2-trichloroethane promotes clear phase separation, minimizing emulsion formation and enhancing the physical extraction process. Finally, the chemical stability of 1,1,2-trichloroethane under the extraction conditions prevents any potential degradation of the analytes, resulting in higher recovery and reproducibility compared to the solvents like chloroform or 1,1,1-trichloroethane. Regarding the two isomers of TCE, it should be said that the difference in their polarity causes a difference in the efficiency of these two solvents. The suitable volume of 1,1,2-TCE was determined by testing different volumes (20, 25, 30, and 35 µL). The final settled phase volumes were 10, 15, 21, and 28 µL, respectively. Figure 5b demonstrates that increasing volume of the extractant decreases EFs and analytical signals. This investigation concludes that 20 µL of 1,1,2-TCE is the most effective choice for extracting target amine derivatives.
Study of extraction solvent type (a) and volume (b). (a) Procedure conditions: are the same as those used in Fig. 4b, except that 7.5%, w/v, NaCl was added to the aqueous solution. (b) Procedure conditions: are the same as those used in (a), except 1,1,2-TCE was used as the extractant.
Vortexing time
The selection of an optimal vortexing duration is crucial in this methodology because the mixture of derivatization agent and extraction solvent is dispersed within the aqueous media through the vortexing. Vortexing enhances the interface between the analytes and derivatization-extractant mixture, thereby markedly enhancing method efficiency. However, long vortexing times may have no favorable effect because of evaporation of organic phase and reverse extraction of analytes. The effectiveness of this parameter was examined by applying various vortexing times (1.0–9.0 min at 2.0-min intervals). The highest ERs were observed at 5.0 min of vortexing (Fig. 6).
Effect of vortexing time on simultaneous derivatization and extraction procedure. Procedure conditions: are the same as those used in Fig. 5b, except 20 µL of 1,1,2-TCE was used.
Centrifuging time and rate
Centrifugation was employed to appropriate separation and collection of the organic phase droplets containing the desired analytes from the aqueous phase. The effect of centrifugation on the ERs can be examined by varying the duration of centrifugation and its rate. To evaluate the effects of centrifugation time in the method, the resulting cloudy solution was subjected to centrifugation for different lengths of time (from 3.0 to 9.0 min in intervals of 2.0-min). This parameter did not have much effect on the efficiency (data not shown here). The reduction in ERs occurred only at long times (7.0 and 9.0 min) and could be attributed to the rise in temperature which causes some parts of the organic phase to lose. Also, the centrifugation rates of 4000 to 7000 rpm with the intervals of 1000-rpm were evaluated. This parameter did not have much effect on ERs (data not shown here). As a result, the centrifuge was employed for 3.0 min at 5000 rpm in the subsequent steps.
Validation of the method
To evaluate the effectiveness of the method for the preconcentration and derivatization of the target amines, the quantitative validity factors of the method were determined and listed in Table 1.
ER, EF, and EnF
EF and ER are the most main factors in determining the value of a microextraction procedure.
In this study, 1 µL of standard solution containing a known concentration of derivatized amines was injected into the GC system, and the peak areas corresponding to each analyte were obtained. Then, the proposed method was applied on 5 mL of deionized water containing a known concentration of amines. 1 µL of the extract (10 ± 0.5 µL) was injected into the GC, and the peak areas were recorded. By comparing the peak areas in the standard solution and sample, concentrations of the analytes in the extractive phase were determined, and the EFs were calculated using Eq. (1). Then, using the EFs and the specified volumes of the aqueous and extractive phases, the ERs were calculated using Eq. (2). ERs were obtained for all amines between 88 and 103%. EFs were in the range of 440–515 in this work.
Moreover, when simultaneous derivatization and microextraction are employed, calculating EnF becomes essential to compare it with EF and thereby assess efficiency of the derivatization process. The high EnF values obtained according to Eq. 3 (~ 20000), along with their comparison to the calculated EFs, indicate the success of the derivatization method. Figure 7a–c illustrate the effectiveness of the proposed method for the simultaneous extraction and derivatization of the primary aliphatic amines.
GC-FID chromatograms: (a) Direct injection of a standard solution of derivatized analytes in methanol (at a concentration of 250 mg L−1 for each analyte). (b) Direct injection of a standard solution of underivatized analytes in methanol (at a concentration of 5000 mg L−1 for each analyte). (c) Deionized water, containing 500 µg L−1 of each analyte. (d) River water sample. The developed method was applied to obtain two chromatograms (c, d). Peaks identification: (1) derivatized PrA, (2) derivatized sec-BuA, (3) derivatized BuA, (4) derivatized PeA, (5) derivatized BeA, (6) PrA, (7) sec-BuA, (8) BuA, (9) PeA, and (10) BeA.
Linearity
In microextraction techniques, selecting LR is particularly important in detecting trace amounts of analytes in samples. LR denotes the interval of analyte concentrations within which the response of the instrument’s signal demonstrates a linear and proportional correlation with the concentration of the analyte. Calibration curve evaluates the linearity and LR of the method. In this study, the method’s linear range (LR) is defined from the LOQ up to the concentration at which a 5% deviation from linearity is observed. Standard solutions containing the compounds of interest were prepared in 5 mL deionized water at the concentrations ranging from limit of quantification (LOQ) to 104 µg L− 1. Hereafter, these solutions were analyzed under optimized conditions. Linearity was assessed by graphing peak areas against analyte concentrations. The process displayed good linearity, as evidenced by the coefficient of determination (r2) being greater than 0.99 for all analytes. Additionally, a wide linear range (LR) was achieved for all analytes in the proposed methodology.
LOQ and LOD
LOD and LOQ are key indicators of the method and are essential for evaluating its ability to detect trace amounts of target compounds. LOD is the lowest concentration of an analyte that can be reliably detected but not necessarily measured accurately. Also, it is worth noting that the LOQ refers to the lowest concentration of an analyte that can be measured with an acceptable level of confidence. In this work, a concentration with a signal-to-noise ratio of 3 was defined as LOD, while LOQ was a concentration with a mentioned ratio of 10. The LOQ values obtained for amines ranged from 1.2 to 3.4 µg L− 1. The LODs for the desired amines were lower than or equal to 1.0 µg L− 1.
Precision
The precision of a method indicates the repeatability of repeated analyses performed under the same conditions, by the same person, and in the same laboratory. Intra-day precision assesses the consistency of the results obtained within one day under the same conditions, whereas inter-day precision evaluates how reliably the method performs when repeated over several days, highlighting its repeatability and consistency of results in the long term. The intra- and inter-day precisions of the presented method for extracting and derivatizing amines were determined by calculating relative standard deviation (RSD) for similar solutions at the concentrations of 50 and 250 µg L− 1 of each target amine. Intra-day (n = 6) precisions were less than or equal to 2.6 and 3.3% at the concentrations of 250 and 50 µg L− 1, respectively. Also, inter-day (n = 3) precisions (9 measurements in 3 successive days, 3 measurements in each day) were less than or equal to 4.5 and 5.8% for all analytes at the concentrations of 250 and 50 µg L− 1, respectively.
Real samples analysis
Real samples (river, well, aqueduct, and rain waters, and refinery effluent) as well as deionized water were spiked with the concentrations of 50 and 100 µg L− 1 of each primary aliphatic amine. Subsequently, simultaneous extraction and derivatization were conducted on the solutions using the development method. The RRs were calculated and documented in Table 2. The resulting RRs (85–107%) show that the matrices of the studied samples do not significantly affect the extraction and derivatization of the corresponding amines. Therefore, this method is reliable for determining amines in the samples. These results indicated that none of the amines studied were present in the real samples at concentrations equal to or higher than the method’s LOD (The chromatogram corresponding to one of the real samples (river water) after applying the proposed method is shown in Fig. 7d).
Comparison to reported similar methods
According to Table 3, the development method provides improved linearity, wider LRs, and better ERs and EFs than the other methods with similar goals. This method is very repeatable and its RSD values of this process are lower than or comparable with the mentioned procedures. The time required for successful derivatization and extraction of the target amines is either less or comparable to the other methods. LODs and LOQs are less than or similar to the amounts established by most of mentioned analytical procedures. The used derivatizing agent (BCF) reacts with amines very efficiently, quickly, and without special conditions. The produced product has good chromatographic properties in GC.
Extraction mechanism
Based on the success of the extraction, it is important to discuss the probable interactions between the extraction solvent and derivative of amines (Fig. 1).
-
1.
Dipole–dipole interaction, as an electrostatic attraction between two molecules that each have permanent dipoles, between the carbonyl oxygen of a carbamate (permanent dipole donor) and the partially positive carbon of a C–Cl bond in 1,1,2-TCE, facilitates solvation and extraction in microextraction system.
-
2.
Dipole-induced dipole interaction occurs when a polar molecule (dipole) distorts the electron cloud of a nonpolar molecule, inducing a temporary dipole in it. This results in weak electrostatic attraction between them.
Derivative of amines have permanent dipoles (e.g., C = O or N–H groups). Although 1,1,2-TCE has polar C–Cl bond, it behaves mostly like a low-polarity solvent and can act as an induced dipole partner:
The N–H bond from the carbamate creates an electric field due to its dipole. This field perturbs the electron cloud around the Cl atom of 1,1,2-TCE. The electron cloud in Cl shifts slightly, creating a temporary dipole in 1,1,2-TCE.
Electrostatic attraction occurs between the δ⁺ hydrogen of the NH group and the induced δ⁻ region near the Cl atom on 1,1,2-TCE.
Dipole-induced dipole interaction enhances solubility/extraction efficiency in microextraction.
-
3.
Van der Waals force happens between –OBu group of the amine derivatives and hydrocarbon chain of 1,1,2-TCE. This phenomenon helps to further extraction of the analytes into the extractive phase.
-
4.
Hydrogen bond between NH group of derivatives of the amines and Cl atom of 1,1,2-TCE.
Analytical eco-scale (AES) assessment
In this study, the environmental impact and greenness of the proposed method were evaluated using the AES (analytical eco-scale) tool. This assessment system assigns penalty points to aspects of the analytical procedure that deviate from the principles of an ideal green method. Penalties are applied based on factors such as reagent quantity, hazards of chemicals and solvents, energy consumption, and waste production. A perfectly green method receives a score of 100, with the final score calculated by subtracting the total penalties from this maximum. According to the AES scale, scores above 75 reflect excellent greenness, scores between 50 and 75 indicate acceptable greenness, and scores below 50 are considered inadequate. As shown in Table 4, the proposed method achieved an AES score of 80, indicating that it meets the criteria for acceptable green analysis and is therefore well-suited for environmentally friendly applications.
Conclusions
A simple and fast VALLME procedure was used to simultaneously extract and derivate primary aliphatic amines, including PrA, sec-BuA, BuA, PeA, and BeA. BCF was used as a selective derivatization agent that reacted with the corresponding amines in an alkaline medium, forming carbamate derivatives. GC-FID was successfully used in the analysis of carbamates. This method was efficient and environmentally friendly because it required µL-volumes of organic solvents (derivatization agent and extraction solvent). The method demonstrated broad LRs (3.4–104 µg L− 1), low LODs (0.4–1.0 µg L− 1) and LOQs (1.2–3.4 µg L− 1), high EFs (440–515), EnF (19524–24419), notable r2 values (0.9974–0.9997), and excellent ERs (88–103%). The present process was reliable for extracting and in situ derivatizing some amino compounds in environmental water and refinery effluent samples (with RRs ranging from 85 to 107%).
Data availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- VALLME:
-
Vortex-assisted liquid-liquid microextraction
- GC:
-
Gas chromatography
- FID:
-
Flame ionization detector
- EF:
-
Enrichment factor
- EnF:
-
Enhancement factor
- ER:
-
Extraction recovery
- RSD:
-
Relative standard deviation
- LR:
-
Linear range
- LOD:
-
Limit of detection
- LOQ:
-
Limit of quantification
- RR:
-
Relative recovery
- PrA:
-
Propylamine
- sec-BuA:
-
sec-Butylamine
- BuA:
-
Butylamine
- PeA:
-
Pentylamine
- BeA:
-
Benzylamine
- BCF:
-
Butyl chloroformate
References
Bhat, A. P. & Gogate, P. R. Degradation of nitrogen-containing hazardous compounds using advanced oxidation processes: A review on aliphatic and aromatic amines, dyes, and pesticides. J. Hazard. Mater. 403, 123657 (2021).
Kaykhaii, M., Nazari, S. & Chamsaz, M. Determination of aliphatic amines in water by gas chromatography using headspace solvent Microextraction. Talanta 65, 223–228 (2005).
Fitzsimons, M. F., Tilley, M. & Cree, C. H. The determination of volatile amines in aquatic marine systems: A review. Anal. Chim. Acta. 1241, 340707 (2023).
Jang, J. K. Amines as occupational hazards for visual disturbance. Ind. Health. 54, 101–115 (2016).
Ulrich, S. et al. Electrospun colourimetric sensors for detecting volatile amines. Sens. Actuators B Chem. 322, 128570 (2020).
Malinowski, S., Wróbel, M. & Woszuk, A. Quantum chemical analysis of the corrosion Inhibition potential by aliphatic amines. Materials 14, 6197 (2021).
Llop, A., Borrull, F. & Pocurull, E. Pressurised hot water extraction followed by simultaneous derivatization and headspace solid-phase Microextraction and gas chromatography-tandem mass spectrometry for the determination of aliphatic primary amines in sewage sludge. Anal. Chim. Acta. 665, 231–236 (2010).
Kataoka, H. Derivatization reactions for the determination of amines by gas chromatography and their applications in environmental analysis. J. Chromatogr. A. 733, 19–34 (1996).
Xie, J. et al. Simultaneous determination of four aliphatic amines in aquatic products by ultrasound-assisted dispersive liquid–liquid Microextraction coupled with high performance capillary electrophoresis. Anal. Methods. 6, 5140–5146 (2014).
Verdú-Andrés, J., Campins-Falco, P. & Herráez-Hernández, R. Determination of aliphatic amines in water by liquid chromatography using solid-phase extraction cartridges for preconcentration and derivatization. Analyst 126, 1683–1688 (2001).
Greim, H., Bury, D., Klimisch, H. J., Oeben-Negele, M. & Ziegler-Skylakakis, K. Toxicity of aliphatic amines: structure-activity relationship. Chemosphere 36, 271–295 (1998).
Deng, C., Li, N., Wang, L. & Zhang, X. Development of gas chromatography-mass spectrometry following headspace single-drop Microextraction and simultaneous derivatization for fast determination of short-chain aliphatic amines in water samples. J. Chromatogr. A. 1131, 45–50 (2006).
Lijinsky, W., Reuber, M. D., Saavedra, J. E. & Singer, G. M. Carcinogenesis in F344 rats by N-nitrosomethyl-n-propylamine derivatives. J. Natl Cancer Inst. 70, 959–963 (1983).
Ábalos, M., Bayona, J. M. & Ventura, F. Development of a solid-phase Microextraction GC-NPD procedure for the determination of free volatile amines in wastewater and sewage-polluted waters. Anal. Chem. 71, 3531–3537 (1999).
Gagnaire, F. et al. Nasal irritation and pulmonary toxicity of aliphatic amines in mice. J. Appl. Toxicol. 9, 301–304 (1989).
Feng-Xian, L. I. U. et al. Determination of amines associated with particles by gas chromatography-mass spectrometry. Chin. J. Anal. Chem. 45, 477–482 (2017).
Huang, J. et al. Determination of aliphatic amines in food by on-fiber derivatization solid-phase Microextraction with a novel zeolitic imidazolate framework 8-coated stainless steel fiber. Talanta 165, 326–331 (2017).
Choi, N. R., Lee, J. Y., Ahn, Y. G. & Kim, Y. P. Determination of atmospheric amines at seoul, South Korea via gas chromatography/tandem mass spectrometry. Chemosphere 258, 127367 (2020).
Cao, L. W., Wang, H., Liu, X. & Zhang, H. S. Spectrofluorimetric determination of aliphatic amines using a new fluorigenic reagent: 2, 6-dimethylquinoline-4-(N-succinimidyl)-formate. Talanta 59, 973–979 (2003).
Deng, Y., Wang, H., Zhong, L. & Zhang, H. Trace determination of short-chain aliphatic amines in biological samples by micellar electrokinetic capillary chromatography with laser-induced fluorescence detection. Talanta 77, 1337–1342 (2009).
Fei, J. et al. Synthesis of 2-methyl-6-methoxy-4-quinolinecarboxylic acid N-hydroxysuccinimide ester (MMQC-OSu) for streamlined and effective HPLC-based fluorescence detection of aliphatic amines in environmental samples. J. Chromatogr. B. 1248, 124348 (2024).
Gil, R. L., Amorim, C. G., Montenegro, M. C. & Araújo, A. N. HPLC-potentiometric method for determination of biogenic amines in alcoholic beverages: A reliable approach for food quality control. Food Chem. 372, 131288 (2022).
Farajzadeh, M. A. & Nouri, N. Simultaneous derivatization and air-assisted liquid-liquid Microextraction of some aliphatic amines in different aqueous samples followed by gas chromatography-flame ionization detection. Anal. Chim. Acta. 775, 50–57 (2013).
Mishra, S., Singh, V., Jain, A. & Verma, K. K. Simultaneous determination of ammonia, aliphatic amines, aromatic amines and phenols at µg L– 1 levels in environmental waters by solid-phase extraction of their benzoyl derivatives and gas chromatography-mass spectrometry. Analyst 126, 1663–1668 (2001).
Kusch, P., Knupp, G., Hergarten, M., Kozupa, M. & Majchrzak, M. Solid-phase extraction-gas chromatography and solid-phase extraction-gas chromatography-mass spectrometry determination of corrosion inhibiting long-chain primary alkyl amines in chemical treatment of boiler water in water-steam systems of power plants. J. Chromatogr. A. 1113, 198–205 (2006).
Sacher, F., Lenz, S. & Brauch, H. J. Analysis of primary and secondary aliphatic amines in waste water and surface water by gas chromatography-mass spectrometry after derivatization with 2, 4-dinitrofluorobenzene or benzene sulfonyl chloride. J. Chromatogr. A. 764, 85–93 (1997).
Farajzadeh, M. A., Mohammad Mehri, S. & Mogaddam, M. R. A. Application of core-shell magnetic metal-organic framework in developing dispersive micro solid phase extraction combined with dispersive liquid–liquid Microextraction for the extraction and enrichment of some pesticides in orange blossom, Aloysia citrodora, and fennel herbal infusions. J. Chromatogr. A. 1741, 465608 (2025).
Zhao, Y. Y. et al. Determination of aliphatic amines using N-succinimidyl benzoate as a new derivatization reagent in gas chromatography combined with solid-phase Microextraction. J. Chromatogr. A. 1021, 175–181 (2003).
Singh, D. K., Sanghi, S. K., Gowri, S., Chandra, N. & Sanghi, S. B. Determination of aliphatic amines by gas chromatography-mass spectrometry after in-syringe derivatization with pentafluorobenzoyl chloride. J. Chromatogr. A. 1218, 5683–5687 (2011).
Lorenzo-Parodi, N., Kaziur-Cegla, W., Gjelstad, A. & Schmidt, T. C. Liquid-phase Microextraction of aromatic amines: Hollow fiber–liquid-phase Microextraction and parallel artificial liquid membrane extraction comparison. Anal. Bioanal. Chem. 415, 1765–1776 (2023).
He, Y. & Lee, H. K. Liquid-phase Microextraction in a single drop of organic solvent by using a conventional microsyringe. Anal. Chem. 69, 4634–4640 (1997).
Kamarei, F., Ebrahimzadeh, H. & Asgharinezhad, A. A. Optimization of simultaneous derivatization and extraction of aliphatic amines in water samples with dispersive liquid-liquid Microextraction followed by HPLC. J. Sep. Sci. 34, 2719–2725 (2011).
Farajzadeh, M. A., Ebrahimi, S., Pezhhanfar, S. & Afshar Mogaddam, M. R. Dispersive micro solid phase extraction of three phthalate esters and bis-(2-ethylhexyl) adipate from the plastic packaged soda and beverage samples using MIL-96 (Al). Microchem. J. 211, 113030 (2025).
Hosseini, M., Dalali, N. & Nejad, S. M. A new mode of homogeneous liquid–liquid Microextraction (HLLME) based on ionic liquids: in situ solvent formation Microextraction (ISFME) for determination of lead. J. Chin. Chem. Soc. 59, 872–878 (2012).
Hosseini, M., Khoshfetrat, S. M., Panahimehr, M. & Rezaei, A. ISFME extraction of as species from some real water samples using an imidazolium-based task-specific ionic liquid (TSIL): synthesis and characterization. Sep. Sci. Technol. 59, 580–591 (2024).
Hosseini, M. A high-performance ionic liquid-based Microextraction (ILBME) method for the trace determination of Paroxetine as a pharmaceutical pollutant in environmental and biological samples. Anal. Methods. 16, 8457–8470 (2024).
Hosseini, M., Rezaei, A. & Soleymani, M. Homogeneous solvent-based Microextraction method (HSBME) using a task-specific ionic liquid and its application to the spectrophotometric determination of Fluoxetine as pharmaceutical pollutant in real water and urine samples. Chem. Pap. 78, 8195–8210 (2024).
Hosseini, M., Castillo, R. & Soleymani, M. A novel magnetic-assisted ionic liquid-based Microextraction method (MA-ILBME): specific design system for sensitive spectrophotometric analysis of Paracetamol as a pharmaceutical pollutant in environmental samples. Talanta 286, 127486 (2025).
Hosseini, M. & Khoshfetrat, S. M. Sensitive spectrophotometric determination of U (VI) ion at trace level in water samples: a simple and rapid homogenous solvent-based/in-situ solvent formation Microextraction based on synthesized/characterized task-specific ionic liquid. J. Solution Chem. 53, 1443–1461 (2024).
Hosseini, M. & Rezaei, A. Synthesis, characterization, and application of 1-methyl-2-hexylthioimidazolium chloride liquid for solvent-based Microextraction (SBME) of hg in water samples. Anal. Bioanalytical Chem. Res. 11, 423–433 (2024).
Bai, B. et al. Determination of flavonoid compounds in shanxi aged vinegars based on hydrophobic deep eutectic solvent VALLME-HPLC method: assassment of the enviromental impact of the developed method. Molecules. 28, 5619 (2023)
Chang, W., Wang, C., Jan, J., Lo, Y. & Wu, C. Vortex-assisted liquid–liquid Microextraction coupled with derivatization for the fluorometric determination of aliphatic amines. J. Chromatogr. A. 1248, 41–47 (2012).
Wang, C. Y. et al. Sensitivity enhancement in the fluorometric determination of aliphatic amines using naphthalene-2,3-carboxaldehyde derivatization followed by vortex-assisted liquid–liquid Microextraction. Talanta 152, 475–481 (2016).
Kakalejčíková, S. & Baze, Y. A combination of vortex-assisted liquid–liquid Microextraction with fluorescence detection: an innovative approach for a green and highly sensitive determination of sodium Dodecyl sulfate in water samples and pharmaceuticals. Microchem. J. 199, 110226 (2024).
Lorenzo-Parodi, N., Moebus, S. & Schmidt, T. C. Analysis of aromatic amines in human urine using comprehensive multi-dimensional gas chromatography-mass spectrometry (GCxGC-MS). Int. J. Hyg. Environ Health. 257, 114343 (2024).
Ngim, K. K., Ebeler, S. E., Lew, M. E., Crosby, D. G. & Wong, J. W. Optimized procedures for analyzing primary alkylamines in wines by pentafluorobenzaldehyde derivatization and GC-MS. J. Agric. Food Chem. 48, 3311–3316 (2000).
Terashi, A., Hanada, Y., Kido, A. & Shinohara, R. Determination of primary and secondary aliphatic amines in the environment as sulphonamide derivatives by gas chromatography-mass spectrometry. J. Chromatogr. A. 503, 369–375 (1990).
Zhao, Y. Y., Jing, Z. Z., Wang, H., Zhang, H. S. & Yu, J. X. N-hydroxysuccinimidyl phenylacetate as a novel derivatizing reagent for aliphatic amines in gas chromatography. Anal. Chim. Acta. 468, 255–261 (2002).
Golsanamlou, M., Nemati, M., Afshar Mogaddam, M. A. & Farajzadeh, M. A. Simultaneous derivatization and extraction of amphetamine and methamphetamine using dispersive liquid–liquid Microextraction prior to their analysis using GC-FID in creatine supplements. Anal. Methods. 15, 6482–6491 (2023).
Katilie, C. J., Simon, A. G. & DeGreeff, L. E. Quantitative analysis of vaporous ammonia by online derivatization with gas chromatography-mass spectrometry with applications to ammonium nitrate-based explosives. Talanta 193, 87–92 (2019).
Norouzi, F. et al. Determination of morphine and oxymorphone in exhaled breath condensate samples: application of microwave enhanced three–component deep eutectic solvent-based air–assisted liquid–liquid Microextraction and derivatization prior to gas chromatography–mass spectrometry. J. Chromatogr. B. 1152, 122256 (2020).
Quigley, A., Cummins, W. & Connolly, D. Dispersive liquid-liquid Microextraction in the analysis of milk and dairy products: A review. J. Chem. 2016, 4040165 (2016).
Yan, H., Cheng, X. & Liu, B. Simultaneous determination of six phthalate esters in bottled milks using ultrasound-assisted dispersive liquid–liquid Microextraction coupled with gas chromatography. J. Chromatogr. B. 879, 2507–2512 (2011).
Gionfriddo, E., Passarini, A. & Pawliszyn, J. A facile and fully automated on-fiber derivatization protocol for direct analysis of short-chain aliphatic amines using a matrix-compatible solid-phase Microextraction coating. J. Chromatogr. A. 1457, 22–28 (2016).
Pietsch, J., Hampel, S., Schmidt, W., Brauch, H. J. & Worch, E. Determination of aliphatic and alicyclic amines in water by gas and liquid chromatography after derivatization by chloroformates. Fresenius’ J. Anal. Chem. 355, 164–173 (1996).
Acknowledgements
The authors thank the University of Tabriz for the financial support. M.A.F. (Analytical methodology and edited the manuscript). S.E. (Analytical analysis and methodology, data analysis, software applications, and manuscript writing). S.M.M. (Analytical analysis, data analysis, and edited the manuscript). M.R.A.M. (Edited the manuscript).
Author information
Authors and Affiliations
Contributions
M.A.F. Performed the analytical methodology and edited the manuscript. S.E. Performed analytical analysis and methodology, data analysis, software applications, and manuscript writing. S.M.M. Performed analytical analysis, and data analysis, and edited the manuscript. M.R.A.M. Edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Farajzadeh, M.A., Ebrahimi, S., Mehri, S.M. et al. Vortex-assisted liquid–liquid microextraction for the simultaneous derivatization and extraction of five primary aliphatic amines in water sample. Sci Rep 15, 19142 (2025). https://doi.org/10.1038/s41598-025-04155-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-04155-5