Abstract
The impact of glucocorticoid therapy on mortality and renal function recovery in patients with sepsis-associated acute kidney injury (SA-AKI) remains unclear. A multicenter retrospective study was conducted using data from the Medical Information Mart for Intensive Care (MIMIC)-IV and the electronic ICU Collaborative Research Database (eICU-CRD). Of 8,759 SA-AKI patients, 1,334 received glucocorticoids and 7,425 did not. After adjustment, glucocorticoid treatment was associated with an increased risk of ICU mortality (hazard ratio [HR] 1.23; 95% confidence interval [CI] 1.07–1.41; P = 0.004) and in-hospital mortality (HR 1.16; 95% CI 1.02–1.32; P = 0.021). However, no statistically significant association was found between glucocorticoid use and renal function recovery (odds ratio [OR] 0.98; 95% CI 0.85–1.13; P = 0.796). Subgroup analysis suggested that dexamethasone use was associated with improved renal function recovery (OR 1.55; 95% CI 1.02–2.35; P = 0.04). In critically ill patients with SA-AKI, glucocorticoid therapy was associated with increased ICU and in-hospital mortality, without significant benefits in overall renal function recovery. However, dexamethasone may be linked to better renal function recovery. Further validation through randomized controlled trials is warranted to clarify the potential benefits and risks of glucocorticoid therapy in SA-AKI.
Similar content being viewed by others
Background
Sepsis-associated acute kidney injury (SA-AKI) is a prevalent and potentially life-threatening complication that affects hospitalized and critically ill patients, with approximately one in three sepsis patients developing acute kidney injury (AKI)1. A comprehensive prospective observational study revealed that SA-AKI accounts for nearly half of all AKI cases2. SA-AKI is linked to an increased risk of mortality3and those patients who survive are at risk of developing chronic kidney disease4. In contrast to non-septic AKI, SA-AKI has a distinct pathophysiology, leading to significant differences in patient characteristics, responses to interventions, and clinical outcomes5. Treatment strategies for SA-AKI patients are currently controversial6.
Glucocorticoids, known for their anti-inflammatory and immunosuppressive effects, have been used as adjunctive therapy for septic shock since the 1960s7. The latest Surviving Sepsis Campaign Guidelines recommend intravenous corticosteroids for patients with septic shock requiring ongoing vasopressor therapy, albeit with only a weak recommendation8. It remains unclear whether glucocorticoid treatment can improve SA-AKI outcomes. Animal studies have suggested that glucocorticoids may alleviate SA-AKI9,10,11. A retrospective cohort study involving SA-AKI patients demonstrated that hydrocortisone reduces 28-day mortality rates12. However, large-scale real-world studies focusing on SA-AKI are still lacking. The effectiveness and safety of glucocorticoids as an adjunctive treatment for SA-AKI remain uncertain. Thus, the aim of the current study is to investigate the impact of glucocorticoids on both survival rates and kidney function in critically ill patients with SA-AKI.
Methods
Data source
We conducted a retrospective cohort study utilizing data from the Medical Information Mart for Intensive Care (MIMIC)-IV database and the electronic ICU Collaborative Research Database (eICU-CRD). The MIMIC-IV database is a comprehensive critical care database that stores patient data from the intensive care units (ICUs) of the Beth Israel Deaconess Medical Center in Boston, Massachusetts, covering the period from 2008 to 201913. The eICU-CRD version 2.0 is a multicenter database comprising information on ICU patients treated between 2014 and 2015 at 208 hospitals across the continental United States14. The MIMIC database was approved by the institutional review boards of the Beth Israel Deaconess Medical Center and the Massachusetts Institute of Technology. The eICU-CRD was released under the Health Insurance Portability and Accountability Act (HIPAA) safe harbor provision. Since the personal information of patients was de-identified, individual patient consent was not required. The reporting of this study adheres to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.
Study population
The study included septic patients who developed AKI within 48 h of ICU admission. Patients with a documented or suspected infection and an acute increase in the total Sequential Organ Failure Assessment (SOFA) score of at least 2 points were diagnosed with sepsis according to the Sepsis-3 criteria15. According to the Kidney Disease Improving Global Outcomes (KDIGO) criteria16AKI was diagnosed based on the following criteria: a rise in serum creatinine (SCr) levels of at least 1.5 times the baseline within the previous 7 days, an increase of at least 0.3 mg/dL in SCr levels within 48 h, or urine output less than 0.5 mL/kg/h for at least 6 h. SA-AKI was defined as the co-occurrence of sepsis (based on Sepsis-3 criteria for adults) and AKI (as defined by KDIGO criteria)17. We included only the first ICU admission for patients with multiple admissions in our analysis. Exclusion criteria were as follows: patients under 18 years of age, patients diagnosed with cancer, patients who were discharged or died within 48 h of ICU admission, patients who received local glucocorticoid administration (e.g., nasally inhaled or skin creams), and patients with missing covariate data.
Data collection and definitions
We extracted the following variables: age, gender, ethnicity, weight, vital signs and laboratory values on admission, comorbidities, the first 24-hour SOFA and the Glasgow Coma Scale (GCS) scores, and treatment information. Vital signs measured included heart rate, systolic blood pressure, and diastolic blood pressure. Laboratory parameters examined were white blood cell count, red blood cell count, hemoglobin, platelets, sodium, chloride, and potassium levels. Comorbidities included hypertension, congestive heart failure, myocardial infarction, diabetes mellitus, and chronic kidney disease. Treatment information encompassed mechanical ventilation, renal replacement therapy (RRT), and vasopressor administration. The group of vasopressors comprised norepinephrine, dopamine, epinephrine, vasopressin, phenylephrine, and dobutamine. Systemic glucocorticoids included dexamethasone, hydrocortisone, prednisone, prednisolone, and methylprednisolone administered orally, intravenously, or intramuscularly. Patients who received systemic glucocorticoids within 48 h after ICU admission were categorized as the glucocorticoid group, while the remaining patients were included in the non-glucocorticoid group. The Defined Daily Dose (DDD) was defined according to the World Health Organization’s (WHO)18. For glucocorticoid, 1 DDD was equivalent to 10 mg of prednisolone, 10 mg prednisone, 1.5 mg of dexamethasone, 30 mg of hydrocortisone, 7.5 mg of oral methylprednisolone, or 20 mg of intravenous or intra-muscular methylprednisolone.
Outcomes
The primary endpoint was ICU mortality and in-hospital mortality. Secondary outcome was recovery of renal function, which was defined as SCr level at ICU discharge below 1.5 times the baseline value and normal urine output (> 0.5mL/kg/h for 24 h upon discharge)19.
Statistical analyses
The Kolmogorov-Smirnov test and Levene’s test were used to assess normality and homogeneity of variances, respectively. Continuous variables with a normal distribution and equal variances were presented as means ± standard deviation, while skewed variables were expressed as medians with interquartile ranges. Depending on the results of the normality and variance homogeneity tests, either the t-test or the Kruskal–Wallis H test was applied for group comparisons. Categorical variables were expressed as numbers with proportions. Cox regression models were employed to estimate the associations between glucocorticoids and mortality in both univariate and multivariate analyses. Logistic regression models were used to determine the impact of glucocorticoids on the recovery of renal function. Multivariate model 1 was adjusted for gender, age, and ethnicity. Multivariate model 2 was further adjusted for additional factors, including weight, vital signs, laboratory data, comorbidities, SOFA score, GCS score, and treatment-related variables. For Cox regression, Schoenfeld residuals was used to test proportional hazards assumption. For logistic regression, multicollinearity was tested using the variance inflation factor (VIF) method, with a VIF ≥ 10 indicating the presence of multicollinearity. Hosmer–Lemeshow test was used to evaluate model fit of logistic regression which P > 0.05 means well model fit. Cumulative survival rates were compared using Kaplan-Meier survival curves and log-rank tests20. To assess the potential nonlinear relationship between the DDD of glucocorticoids and mortality, restricted cubic spline models with three knots and a nonlinear test were employed21. Subgroup analyses were conducted to investigate whether the relationship between glucocorticoid use and mortality or renal function recovery varied across different subgroups based on age, gender, race, SOFA score, GCS score, and vasopressor use. Log likelihood ratio test was used to test multiplicative interaction of subgroups. We performed additional subgroup analyses to compare the effects of different types of glucocorticoids (dexamethasone, hydrocortisone, methylprednisolone, and prednisolone) on SA-AKI outcomes. We evaluated the robustness of the observed association between glucocorticoid use and the primary outcome to potential unmeasured confounding by calculating E-values22. A larger E-value indicates that substantial unmeasured confounding would be required to fully explain away the observed association. A two-tailed P-value of less than 0.05 was considered statistically significant, indicating a significant association. For all statistical analyses, R software (ver. 3.4.3; R Foundation for Statistical Computing, Vienna, Austria) and Empower (X & Y Solutions, Inc., Boston, MA, USA) were utilized.
Results
Patients
A total of 6,727 and 2,032 SA-AKI patients were selected from the MIMIC-IV and eICU-CRD databases, respectively. Of these, 1,334 patients were in the glucocorticoid group, while 7,425 patients were in the non-glucocorticoid group (Fig. 1). Table 1 presents the baseline characteristics of the two groups in the MIMIC-IV and eICU-CRD databases and the overall population. Tables S1 and S2 present the results of the Kolmogorov–Smirnov and Levene’s tests, respectively, indicating that many clinical and laboratory parameters did not follow a normal distribution. Generally, patients who received glucocorticoids were younger, more likely to be female, had higher SOFA scores, and were more likely to receive mechanical ventilation and RRT compared to those in the non-glucocorticoid group.
Association between glucocorticoids and mortality
The proportional hazards assumptions were satisfied for ICU mortality (P for Schoenfeld test were 0.69 in MIMIC-IV cohort, 0.50 in eICU-CRD cohort, and 0.64 in overall population) and in-hospital mortality (P for Schoenfeld test were 0.14 in MIMIC-IV cohort, 0.78 in eICU-CRD cohort, and 0.21 in overall population). In the MIMIC-IV cohort, we found that glucocorticoid use was associated with a higher risk of ICU and in-hospital mortality in the unadjusted model (ICU mortality: hazard ratio [HR] 1.45; 95% confidence interval [CI] 1.24–1.69; P < 0.01; in-hospital mortality: HR 1.38; 95% CI 1.20–1.60; P < 0.01). After adjusting for potential confounders, these associations remained statistically significant (ICU mortality: HR 1.26; 95% CI 1.07–1.47; P < 0.01; in-hospital mortality: HR 1.20; 95% CI 1.03–1.38; P = 0.02). However, in the eICU-CRD cohort, the associations between glucocorticoid use and mortality were no longer statistically significant in the fully adjusted model (ICU mortality: HR 1.29; 95% CI 0.94–1.78; P = 0.12; in-hospital mortality: HR 1.21; 95% CI 0.93–1.57; P = 0.16) (Table 2; Fig. 2).
Association between glucocorticoid use and outcomes in the unadjusted and fully adjusted models. Abbreviations: CI, confidence interval; eICU-CRD, electronic ICU Collaborative Research Database; HR, hazard ratio; ICU, intensive care unit; MIMIC-IV, Medical Information Mart for Intensive Care IV; OR, odds ratio.
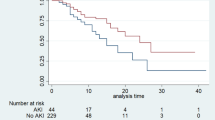
In the overall population combining both the MIMIC-IV and eICU-CRD databases, glucocorticoid use was associated with an increased risk of mortality in both the unadjusted and fully adjusted models (unadjusted model: ICU mortality HR 1.39; 95% CI 1.21–1.60; P < 0.01; in-hospital mortality HR 1.30; 95% CI 1.15–1.47; P < 0.01; fully adjusted model: ICU mortality HR 1.23; 95% CI 1.07–1.41; P < 0.01; in-hospital mortality HR 1.16; 95% CI 1.02–1.32; P = 0.02) (Table 2; Fig. 2). The Kaplan-Meier curve for in-hospital survival showed lower survival rates compared to the non-glucocorticoid group after adjustment for confounders, with statistical analysis confirming the significance of this difference (log-rank test, P < 0.001) (Fig. 3). A dose response curve demonstrated a positive association between the dose of glucocorticoid and the risk of mortality, suggesting that higher doses were associated with an increased likelihood of both ICU and in-hospital mortality (Fig. 4a and b).
Association between glucocorticoids and renal function recovery
Multicollinearity and model fit were evaluated in MIMIC-IV cohort, eICU-CRD cohort and overall population. There were no multicollinearity for all variables (VIFs for all variables were less than 10) and Hosmer-Lemeshow test showed that the models of renal function recovery were all had good degree of fit (P = 0.72 for MIMIC-IV cohort, P = 0.60 for eICU-CRD cohort, P = 0.92 for overall population). In the unadjusted model, glucocorticoid use was associated with poorer renal function recovery, with an odds ratio (OR) of 0.86 (95% CI 0.75–0.99, P = 0.03) in the MIMIC-IV cohort, 0.63 (95% CI 0.42–0.95, P = 0.03) in the eICU-CRD cohort, and 0.84 (95% CI 0.74–0.96, P < 0.01) in the combined MIMIC-IV + eICU-CRD cohort. However, in the fully adjusted model, no significant association was observed between glucocorticoid use and renal function recovery, with an OR of 1.04 (95% CI 0.89–1.21, P = 0.60) in the MIMIC-IV cohort, 0.64 (95% CI 0.41–1.00, P = 0.05) in the eICU-CRD cohort, and 0.98 (95% CI 0.85–1.13, P = 0.80) in the combined cohort (Table 2; Fig. 2).
Sensitivity analysis
The associations between glucocorticoid use and both in-hospital mortality and renal function recovery in various subgroups are illustrated in Figs. 5, 6 and 7. Notably, among SA-AKI patients with a SOFA score > 7, a GCS score ≥ 14, and vasopressor use, glucocorticoid use was significantly associated with higher ICU mortality and in-hospital mortality. Regarding renal function recovery, the interaction P-values across subgroups defined by age, gender, severity scores, and vasopressor use were not statistically significant. We conducted a subgroup analysis stratified by glucocorticoid type. The use of methylprednisolone or prednisone was not significantly associated with either mortality or renal function recovery. However, dexamethasone use was linked to improved renal function recovery, with an OR of 1.55 (95% CI 1.02–2.35, P = 0.04). In contrast, hydrocortisone use was associated with an increased risk of both ICU and in-hospital mortality (ICU mortality: HR 1.50; 95% CI 1.23–1.83; P < 0.01; in-hospital mortality: HR 1.39; 95% CI 1.15–1.67; P < 0.01) (Table 3). To evaluate the robustness of the observed associations to potential unmeasured confounding, we calculated E-values. The E-values for the associations between glucocorticoid use and ICU mortality and in-hospital mortality were 1.76 and 1.37, respectively, indicating that moderate unmeasured confounding would be needed to fully explain these observed effects. The association between dexamethasone use and renal recovery had an E-value of 1.80, suggesting a relatively stronger robustness to unmeasured confounding.
Discussion
In this retrospective study, which included both separate and combined analyses of two large cohorts, we observed that overall glucocorticoid use was associated with increased ICU and in-hospital mortality in patients with SA-AKI and showed no significant association with renal recovery. However, when analyzing specific glucocorticoid types, we found that hydrocortisone use was linked to a higher mortality rate, whereas dexamethasone use was associated with improved renal recovery and was not significantly associated with mortality risk.
The incidence rate of sepsis is increasing among critically ill patients worldwide, leading to high mortality and economic burden23. Sepsis is a well-known risk factor for AKI, and the treatment of AKI in sepsis remains reactive and nonspecific. Corticosteroids have been used as adjuvant therapy for sepsis for over half a century. Theoretically, during sepsis, inflammatory mechanisms contribute to infection clearance and tissue recovery on one hand, but also to organ injury on the other24. Glucocorticoids can inhibit the activation of nuclear factor kappa B (NF-κB) and the release of extensive inflammatory factors, thereby modulating the inflammatory response in sepsis25. However, glucocorticoids are associated with various side effects, including the potential to exacerbate infection26. Previous clinical trials and systematic reviews have evaluated the efficacy and safety of corticosteroids in septic patients, but the findings remain inconclusive27,28,29,30. Meta-analyses suggest that corticosteroid use may reduce mortality, and subgroup analyses have examined the effects of different corticosteroid types. The reported HRs for 28-day mortality were 0.53 (0.27–1.02) for dexamethasone, 0.95 (0.86–1.05) for hydrocortisone, 1.04 (0.66–1.63) for methylprednisolone, and 0.94 (0.66–1.35) for prednisolone27. These findings indicate that dexamethasone may offer the greatest potential benefit in reducing mortality risk. However, clinical studies specifically investigating the use of corticosteroids in patients with SA-AKI remain limited. A previous study found that hydrocortisone supplementation reduced the 28-day mortality of patients with SA-AKI12. In contrast, our study, which included a larger sample size, found that glucocorticoid use was associated with an increased risk of mortality in this population. Moreover, high-dose corticosteroid use was significantly associated with increased mortality. This finding is consistent with earlier evidence indicating that in patients with severe sepsis or septic shock and elevated serum creatinine, high-dose methylprednisolone therapy significantly increased 14-day mortality compared to placebo (59% [46 of 78] vs. 29% [17 of 58], P < 0.01)31. Further subgroup analyses revealed that hydrocortisone use was linked to higher mortality, whereas dexamethasone showed a trend toward a protective effect, although the association was not statistically significant (in-hospital mortality: HR 0.70 [0.41–1.18]; ICU mortality: HR 0.69 [0.37–1.29]).
Glucocorticoids have been extensively used in glomerular diseases, although their indications for different subtypes of glomerular diseases vary. In septic patients, studies evaluating the renal effects of glucocorticoids have yielded inconsistent results32,33,34. A study by Moreno et al. reported that septic shock patients randomized to receive hydrocortisone exhibited a faster resolution of renal failure compared to those receiving a placebo (60.5% vs. 44.3%)32. Similarly, Laviolle et al. investigated the effects of low-dose steroids in septic shock patients and found that while low-dose steroids increased urine output, they did not significantly impact creatinine clearance33. In contrast, a study by Gordon et al., which included septic shock patients without end-stage kidney disease, found no significant differences between the hydrocortisone and placebo groups in terms of renal function, incidence of renal failure, use of renal replacement therapy, or mortality34.
Survivors of SA-AKI are at an increased risk of developing chronic kidney disease or end-stage kidney disease6. As natural suppressors of proinflammatory cytokine secretion, glucocorticoids have been proposed as a potential therapeutic strategy to modulate immune homeostasis and treat sepsis-associated acute lung injury35. However, their routine use in SA-AKI remains uncertain and is not currently recommended by clinical guidelines6,36. The renal effects of glucocorticoids in SA-AKI remain unclear, as most studies have been conducted under experimental conditions. In a mouse model of septic AKI, dexamethasone administration was shown to decrease plasma creatinine and urea concentrations, consistent with findings from other studies9,10,11. However, recent evidence suggests that glucocorticoids may induce a maladaptive epithelial stress response, exacerbating AKI in various clinically relevant models, including myoglobinuric and gentamicin-induced AKI37.
Clinical observational studies have suggested a potential protective role of dexamethasone in AKI. Specifically, dexamethasone use has been associated with a reduced risk of AKI in critically ill COVID-19 patients admitted to the ICU38,39. Nevertheless, studies on the real-world effects of glucocorticoids in SA-AKI remain limited. A previous retrospective study involving 228 SA-AKI patients in China suggested that hydrocortisone supplementation improved renal function12. Although our overall findings indicated no significant association between glucocorticoid use and renal recovery, subgroup analyses revealed that dexamethasone may promote renal recovery (OR: 1.55 [1.02–2.35]). These findings suggest that, among septic patients, dexamethasone may provide greater benefits in terms of renal recovery and overall prognosis.
The current study has several strengths. It benefits from a relatively large sample size and multicenter data provided by MIMIC-IV and eICU-CRD. However, there are several limitations to consider. First, as an observational study, residual confounding factors cannot be completely eliminated. To strengthen the results, high-quality multicenter RCTs are necessary. Second, the long-term impact of glucocorticoids on renal function recovery could not be assessed due to the absence of follow-up SCr measurements after ICU discharge. Third, the study was not designed to determine the underlying mechanism linking glucocorticoids and SA-AKI. Therefore, the underlying pathophysiological mechanism cannot be speculated upon.
Conclusion
In this multicenter retrospective study, we found that glucocorticoid therapy in critically ill patients with SA-AKI was associated with increased ICU and in-hospital mortality, without a significant overall benefit in renal function recovery. However, dexamethasone may be associated with improved renal function recovery, highlighting potential differences in the effects of specific glucocorticoids. Given the retrospective nature of our study, residual confounding cannot be ruled out, and further prospective studies, particularly randomized controlled trials, are needed to confirm our findings and better elucidate the role of glucocorticoid therapy in SA-AKI management.
Data availability
The datasets are available in the physionet (https://physionet.org/content/mimiciv/2.2/and https://physionet.org/content/eicu-crd/2.0/).
References
Murugan, R. et al. Acute kidney injury in non-severe pneumonia is associated with an increased immune response and lower survival. Kidney Int. 77, 527–535. https://doi.org/10.1038/ki.2009.502 (2010).
Bagshaw, S. M. et al. Septic acute kidney injury in critically ill patients: clinical characteristics and outcomes. Clin. J. Am. Soc. Nephrol. 2, 431–439. https://doi.org/10.2215/cjn.03681106 (2007).
Kellum, J. A. et al. The effects of alternative resuscitation strategies on acute kidney injury in patients with septic shock. Am. J. Respir Crit. Care Med. 193, 281–287. https://doi.org/10.1164/rccm.201505-0995OC (2016).
Chawla, L. S., Amdur, R. L., Amodeo, S., Kimmel, P. L. & Palant, C. E. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int. 79, 1361–1369. https://doi.org/10.1038/ki.2011.42 (2011).
Wan, L., Bellomo, R., Di Giantomasso, D. & Ronco, C. The pathogenesis of septic acute renal failure. Curr. Opin. Crit. Care. 9, 496–502. https://doi.org/10.1097/00075198-200312000-00006 (2003).
Poston, J. T. & Koyner, J. L. Sepsis associated acute kidney injury. Bmj 364, k4891. https://doi.org/10.1136/bmj.k4891 (2019).
Bennett, I. et al. The effectiveness of hydrocortisone in the management of severe infections. JAMA: J. Am. Med. Association. 183, 462–465 (1963).
Evans, L. et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 47, 1181–1247. https://doi.org/10.1007/s00134-021-06506-y (2021).
Chen, Y. et al. Panaxadiol saponin and dexamethasone improve renal function in Lipopolysaccharide-Induced mouse model of acute kidney injury. PloS One. 10, e0134653. https://doi.org/10.1371/journal.pone.0134653 (2015).
Choi, H. M. et al. Glucocorticoids attenuate septic acute kidney injury. Biochem. Biophys. Res. Commun. 435, 678–684. https://doi.org/10.1016/j.bbrc.2013.05.042 (2013).
Johannes, T. et al. Low-dose dexamethasone-supplemented fluid resuscitation reverses endotoxin-induced acute renal failure and prevents cortical microvascular hypoxia. Shock (Augusta Ga). 31, 521–528. https://doi.org/10.1097/SHK.0b013e318188d198 (2009).
Ying, P., Yang, C., Wu, X., Cai, Q. & Xin, W. Effect of hydrocortisone on the 28-day mortality of patients with septic acute kidney injury. Ren. Fail. 41, 794–799. https://doi.org/10.1080/0886022x.2019.1658605 (2019).
Johnson, A. E. W. et al. MIMIC-IV, a freely accessible electronic health record dataset. Sci. Data. 10, 1. https://doi.org/10.1038/s41597-022-01899-x (2023).
Pollard, T. J. et al. The eICU collaborative research database, a freely available multi-center database for critical care research. Sci. Data. 5, 180178. https://doi.org/10.1038/sdata.2018.178 (2018).
Singer, M. et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA: J. Am. Med. Association. 315, 801–810 (2016).
Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pract. 120, c179–c184 (2012).
Zarbock, A. et al. Sepsis-associated acute kidney injury: consensus report of the 28th acute disease quality initiative workgroup. Nat. Rev. Nephrol. https://doi.org/10.1038/s41581-023-00683-3 (2023).
Methodology, W. C. C. f. D. S. Guidelines for ATC classification and DDD assignment. Oslo 165–175 (2013). (2012).
Zhao, G. et al. Association between Furosemide administration and outcomes in critically ill patients with acute kidney injury. Crit. Care. 24, 1–9 (2020).
Efron, B. Logistic regression, survival analysis, and the Kaplan-Meier curve. J. Am. Stat. Assoc. 83, 414–425 (1988).
Harrell, F. E. Jr., Lee, K. L. & Pollock, B. G. Regression models in clinical studies: determining relationships between predictors and response. J. Natl Cancer Inst. 80, 1198–1202. https://doi.org/10.1093/jnci/80.15.1198 (1988).
Haneuse, S., VanderWeele, T. J. & Arterburn, D. Using the E-Value to assess the potential effect of unmeasured confounding in observational studies. JAMA: J. Am. Med. Association. 321, 602–603. https://doi.org/10.1001/jama.2018.21554 (2019).
Organization, W. H. Global report on the epidemiology and burden of sepsis: current evidence, identifying gaps and future directions. (2020).
Angus, D. C. & van der Poll, T. Severe sepsis and septic shock. The New England journal of medicine 369, (2063). https://doi.org/10.1056/NEJMc1312359 (2013).
Heming, N., Sivanandamoorthy, S., Meng, P., Bounab, R. & Annane, D. Immune effects of corticosteroids in Sepsis. Front. Immunol. 9, 1736. https://doi.org/10.3389/fimmu.2018.01736 (2018).
Ponticelli, C. & Locatelli, F. Glucocorticoids in the treatment of glomerular diseases: pitfalls and pearls. Clin. J. Am. Soc. Nephrology: CJASN. 13, 815–822. https://doi.org/10.2215/CJN.12991117 (2018).
Fang, F. et al. Association of corticosteroid treatment with outcomes in adult patients with sepsis: A systematic review and Meta-analysis. JAMA Intern. Med. 179, 213–223. https://doi.org/10.1001/jamainternmed.2018.5849 (2019).
Annane, D. et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA: J. Am. Med. Association. 288, 862–871. https://doi.org/10.1001/jama.288.7.862 (2002).
Annane, D. et al. Hydrocortisone plus fludrocortisone for adults with septic shock. N. Engl. J. Med. 378, 809–818. https://doi.org/10.1056/NEJMoa1705716 (2018).
Venkatesh, B. et al. Adjunctive glucocorticoid therapy in patients with septic shock. N. Engl. J. Med. 378, 797–808. https://doi.org/10.1056/NEJMoa1705835 (2018).
Bone, R. C. et al. A controlled clinical trial of high-dose Methylprednisolone in the treatment of severe sepsis and septic shock. N. Engl. J. Med. 317, 653–658. https://doi.org/10.1056/nejm198709103171101 (1987).
Moreno, R. et al. Time course of organ failure in patients with septic shock treated with hydrocortisone: results of the corticus study. Intensive Care Med. 37, 1765–1772. https://doi.org/10.1007/s00134-011-2334-x (2011).
Laviolle, B., Annane, D., Fougerou, C. & Bellissant, E. Gluco- and mineralocorticoid biological effects of a 7-day treatment with low doses of hydrocortisone and fludrocortisone in septic shock. Intensive Care Med. 38, 1306–1314. https://doi.org/10.1007/s00134-012-2585-1 (2012).
Gordon, A. C. et al. Effect of early vasopressin vs norepinephrine on kidney failure in patients with septic shock: the VANISH randomized clinical trial. JAMA: J. Am. Med. Association. 316, 509–518. https://doi.org/10.1001/jama.2016.10485 (2016).
Thompson, B. T. Glucocorticoids and acute lung injury. Crit. Care Med. 31, 253–257. https://doi.org/10.1097/01.Ccm.0000057900.19201.55 (2003).
Zarbock, A., Koyner, J. L., Gomez, H., Pickkers, P. & Forni, L. Sepsis-associated acute kidney injury-treatment standard. Nephrol. Dial Transpl. 39, 26–35. https://doi.org/10.1093/ndt/gfad142 (2023).
Zhou, L. et al. Glucocorticoids induce a maladaptive epithelial stress response to aggravate acute kidney injury. Sci. Transl. Med. 16, eadk5005. https://doi.org/10.1126/scitranslmed.adk5005 (2024).
Rubin, S. et al. Impact of dexamethasone in severe COVID-19-induced acute kidney injury: a multicenter cohort study. Ann. Intensive Care. 14 https://doi.org/10.1186/s13613-024-01258-6 (2024).
Orieux, A. et al. Impact of dexamethasone use to prevent from severe COVID-19-induced acute kidney injury. Crit. Care. (London, England). 25, 249. https://doi.org/10.1186/s13054-021-03666-7 (2021).
Acknowledgements
The authors are particularly grateful to all the researchers who built and maintained the MIMIC database and eICU-CRD.
Funding
Funding sources: Supported by National Youth Science Foundation of China (No. 81600536), Provincial Natural Science Foundation of Hunan (No. 2021JJ40972), Changsha Municipal Natural Science Foundation (No. kq2014276).
Author information
Authors and Affiliations
Contributions
W.C designed the study. X.Z collected and analyzed data. R.M and Y.L helped with data analysis. W.C, J.X, and W.D contributed to writing this manuscript. QL.Z, Q.Z, S.Z, H.Xu and H.Xie guided the literature review.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The use of the databases was approved by the Institutional Review Boards of the Massachusetts Institute of Technology (MIT) and Beth Israel Deaconess Medical Center. As the database contains de-identified, standardized data, separate ethical approval and informed consent were not required, in accordance with the principles of the Declaration of Helsinki. Therefore, this study was exempt from the requirement for an additional ethics statement and informed consent.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhu, X., Xue, J., Maimaitituerxun, R. et al. Association of glucocorticoids with outcomes in critically ill patients with sepsis-associated acute kidney injury: a multicenter retrospective cohort study. Sci Rep 15, 20885 (2025). https://doi.org/10.1038/s41598-025-05515-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-05515-x