Abstract
While Oregon and Washington contribute approximately 60% of the total production of blueberries in the United States, there is still potential for growth on the east side of the Cascade Mountain Range in both states. The region is well suited to organic production of blueberries, but the soils tend to be sandy and low in organic matter. Currently, organic inputs used in blueberry systems are expensive and difficult to source in the region. Therefore, easily available alternatives that can foster productivity at a lesser cost are needed. An organic blueberry field trial was established in 2021 at Oregon State University’s Hermiston Agricultural Research and Extension Center to evaluate four organic amendments, including biochar, grape pomace compost, grape pomace co-composted with biochar, and woodchips (grower standard), each of which were either incorporated to a depth of 0.2 m or, with the exception of woodchips, banded on the soil surface. Soil samples were collected in September 2021 and 2022 and analyzed for a suite of soil health indicators and soil microbial community structure, including Soil pH, electrical conductivity, Total N, NH4-N, NO3-N, available P and K, soil organic matter, extractable organic carbon, active carbon, microbial biomass carbon, β-glucosidase enzyme activity, microbial respiration, and bacterial and fungal community structure and abundance. Results showed that incorporated compost resulted in higher soil organic matter, increased nutrient availability, and greater microbial activity than the other treatments. However, none of the treatments had any effect on the structure of the bacterial community, which by the end of the second year of the study was largely dominated by Acidobacteria, or on the structure of the fungal community, which in both years was dominated by Ascomycota and Mortierellomycota. Data collected from this study will help us understand the suitability of organic inputs to enhance soil health indices while improving resource use efficiency.
Similar content being viewed by others
Introduction
Blueberries (Vaccinium corymbosum) are a member of the Ericaceae family and are known to have rich nutritional value1,2. The production of blueberries has increased rapidly over the past two decades, mainly due to profitability and high consumer demand for healthy fruit3. Currently, around 32,000 acres of blueberries are cultivated in Oregon and Washington, contributing to 35% of the national harvested acreage and 62% of the total production4. Over a third of this acreage is located east of the Cascade Mountain Range in the U.S. Pacific Northwest, where the conditions are dry and favorable for organic production of blueberry. Currently, more than half of the organic blueberries produced in the United States come from this region5.
Blueberry plants require high soil organic matter (SOM) to support their growth and development6. Additionally, an acidic soil pH of 4.5–5.5 is essential for nutrient uptake in blueberries7. Soils east of the Cascade Range are primarily calcareous and coarse-textured and typically exhibit high pH and very low content of clay and SOM8. To meet the soil requirements for growing blueberries in this region, growers commonly amend the soil with organic materials such as woodchips prior to planting. Once applied, these amendments tend to improve soil health by enhancing microbial communities, which act as decomposers and transform nutrients in the SOM into plant-usable forms9. The microbes also help to improve soil aggregation, which is important for water infiltration, drainage, and physical protection of SOM10,11.
Presently, the organic amendments used for blueberries are costly, and alternatives are needed that are less expensive, locally available, and perhaps more productive. One potential option includes grape (Vitis vinifera) pomace compost. Grape pomace is a by-product of the wine-making process, in which grape skins, seeds, pulp, stems, and leaves are left behind after the juice is extracted12. These components account for approximately 20–30% of the total grape weight, thereby creating abundant waste that needs management13. Grape pomace is acidic, has a high carbon: nitrogen (C: N) ratio, and contains a high content of nutrients, including K, P, and Mg, and biologically active compounds, such as polyphenols14. Once composted, grape pomace has been reported to enhance soil water retention, soil C accumulation, and gradual release of nutrients15. Therefore, grape pomace compost could serve as a viable amendment for blueberry systems, especially since it is readily available and low-cost due to the ubiquitous wine industry of eastern Oregon and Washington.
Biochar is another potential alternative amendment for blueberry. Like compost, biochar improves SOM due to its rich porosity, numerous surface functional groups, high levels of stable C, and strong adsorption capacity16,17,18. Studies report that biochar increases growth and yield of various crops by enhancing aeration, water holding capacity, nutrient availability, and structure of the soil18,19. Recently, biochar has been shown to improve both growth and fruit production of highbush blueberry in western Oregon20. However, there have also been studies where biochar did not show any positive effects on crop production21,22. Therefore, evaluating biochar as an amendment for blueberry warrants investigation east of the Cascade Range. There is also rising interest in evaluating co-composted biochar for enhancing soil health because as a compost additive, biochar enhances the composting process, increases microbial diversity, and immobilizes toxic metals and pollutants23. As a soil amendment, past studies have reported that co-composted biochar has shown great promise in enhancing soil health and crop production24,25,26. When biochar is co-composted with another feedstock such as grape pomace, it can help overcome the low nutrient content of the biochar, regulate nutrient release from the compost27,28and prevent leaching of nutrients29.
Despite knowing the benefits of amending soils with grape pomace compost and biochar alone or in concert, little information is available on how these amendments will influence the soil conditions for blueberry production in calcareous and low SOM soils east of the Cascade Range. We hypothesize that the organic amendments will increase soil nutrient availability, and support biological health indicators and microbial community structure in an organically managed blueberry field. Therefore, the objectives of this study were: (i) to investigate the role of these amendments on soil health indicators of permeable soils under blueberries, and (ii) to evaluate the impacts of various organic inputs on soil microbial community dynamics. The results from this study will be harmonized with information on the effects on production to develop a better understanding of the organic blueberry production system east of the Cascade Range by striking a balance between grower profitability and soil sustainability.
Materials and methods
Study sites
The study was conducted in a new organic planting of ‘Draper’ northern highbush blueberry that was established at the Oregon State University’s Hermiston Agricultural Research and Extension Center, Hermiston, OR (lat. 45°48′ N, long. 119°17′ W) in April 2021. We obtained permission and consent to establish this trial at the center. The soil at the site is classified as Atkins fine sandy loam (fine-loamy, mixed mesic Fluvaquentic Endoaquepts). The site was acidified to adjust soil pH to recommended levels for blueberries though a split application of 1687 kg ha–1 of elemental sulfur (Tiger 85 CR organic; Tiger-Sul, Sheldon, CT) in August and November 2020. The planting was established following standard grower practices and included rows of raised beds covered with 0.7 mm-thick woven polypropylene landscape fabric (Weed Barrier 20 Year, DeWitt, Sikeston, MO) for weed suppression. Each row was 68-m long by 1.30 m wide on raised beds approximately 15 cm tall. Prior to planting, holes were cut in the center of the landscape fabric every 0.91 m. Two-year-old ‘Draper’ planting stock was obtained from a commercial nursery (Fall Creek Farm and Nursery, Lowell, OR) and transplanted in each hole ___location. Irrigation water was applied using two lines of drip tubing per row (Netafim, Fresno, CA; specifications: 16 mm diameter, 2.0 L·h-1, 207 kPa pressure-compensating emitters every 0.3 m) placed beneath the landscape fabric and ≈ 0.1 m from each side of the crown of the plants. The irrigation system was equipped with a sulfur burner (CTC waterworks sulfur burner, Haley Manufacturing, Yakima, WA) to acidify the water and a sand filter (Flowguard series 1100 media filter, Model 215, Fresno Valves and Castings Inc., Selma, CA) to remove sediments, algae, and other biological contaminants. The blueberry plants were irrigated between April and November based on weekly estimates of evapotranspiration obtained from an AgriMet weather station (USBR, Cooperative Agricultural Weather Network) located 375-m away from the planting. The plants were fertigated through the drip system using a passive fertilizer injector (D14MZ2–14GPM, Dosatron, Clearwater, FL) and fertilized with a liquid pea-protein hydrolysate (Explorer 16, Ferticell, Tempe, AZ) at a total rate of 10 kg ha–1 N in 2021 and 22.4 kg ha–1 N in 2022. Please refer30 for more information on establishment and management of the study.
Experimental design
Treatments were arranged in a randomized complete block design with five replicates and included grape pomace compost, biochar, or biochar co-composted with grape pomace, each of which were either banded on the soil surface (1-m wide band for each row) or incorporated to a depth of 0.2 m prior to shaping the beds (the treatments are abbreviated throughout the text as C band, B band, B + C band, C incorp, B incorp, and B + C incorp, respectively). The biochar was prepared using mixed coniferous wood feedstock pyrolyzed at 750 °C at Biomass One in White City, OR. The grape pomace was obtained from a commercial farm (Wyckoff Farms, Grandview, WA) and then composted indoors for six weeks. The compost pile was turned every other day, and volumetric moisture content of 50–60% and a temperature of 50 °C was maintained throughout the process. Biochar was co-composted with grape pomace (1:4 by weight) under similar conditions as the grape pomace compost pile for six weeks. Each compost and biochar treatment were applied at a rate of ≈ 10% by soil volume. Apple woodchips were also included as a control and incorporated (10% by volume) to a depth of 0.2 m prior to shaping the beds, which is a standard practice in the region. All treatments were applied in April 2021, additional details are listed in Table 1.
Soil sampling and analysis
Soil samples were collected to a depth of 0–30 cm from each treatment plot using a 2.5-cm-diameter handheld probe (AMS, American Falls, ID) in September 2021 and 2022. The samples were pulled from 10 to 12 locations and mixed to obtain a composite sample in each plot. A subsample of each was then shipped in coolers with ice packs within 5 days to the Oregon State University’s Center for Qualitative Life Sciences for DNA extraction and sequencing analyses. The remaining sample was passed through a 2-mm sieve and analyzed for the following chemical and biological soil health indicators. Soil pH was determined using 1:1 soil: water suspension31and soil EC was determined using 1:2 soil: water suspension32. Soil ammonium-N (NH4-N) and nitrate-N (NO3-N) were extracted using 2 M potassium chloride solution33 and determined using automated colorimetric methods. Available P and K were analyzed using the Mehlich III method34. Total N and SOM were determined using the dry combustion method35. Extractable organic C was extracted using 0.5 M potassium sulfate solution and microbial biomass C was analyzed using chloroform fumigation method36. Extracellular enzyme activity of β-glucosidase enzyme was analyzed using assay incubation of fresh soil samples, followed by a colorimetric method37. Active C was determined using 0.02 M potassium permanganate solution38,39. Briefly, 20 ml of potassium permanganate solution was added to 2.5 g of soil. The solution was shaken at 120 rpm for 2 min, and the supernatant was diluted 100 times followed by measuring the absorbance at 550 nm using a plate reader. Microbial respiration was measured on moist soil following 24-hr of incubation and analyzed using an infrared gas analyzer40. The bacterial and fungal community structure was identified using amplicon sequencing of the V3-V4 region of 16 S rRNA and ITS genes, respectively41. The DNA extraction from soil samples was done using the DNeasy PowerSoil Pro Kit® (QIAGEN), following the manufacturer’s suggested protocol. For bacterial community, the extracted DNA was amplified using S-D-Bact-0341-b-S-17/S-D-Bact-0785-a-A-21 primers42. The Polymerase Chain Reaction (PCR) conditions included an initial denaturation at 95 °C for 3 min, followed by 25 cycles each at 95 °C for 30 s (denaturation), 55 °C for 30 s (annealing), 72 °C for 30 s (elongation), and 72 °C for 5 min (final elongation). The fungal DNA was amplified using ITS86F/ITS4R primers43. The PCR conditions for ITS included an initial denaturation at 94 °C for 5 min, followed by 30 cycles each at 94 °C for 15 s (denaturation), 55 °C for 90 s (annealing), 72 °C for 90 s (elongation), and 72 °C for 5 min (final elongation). Library preparation and sequencing was performed at Oregon State University’s Center for Qualitative Life Sciences. Both the bacterial and fungal communities were assessed till Genus’s level. Bioinformatics analyses was conducted using Divisive Amplicon Denoising Algorithm 2 in RStudio44. The sequences were filtered using the “filter and trim” function, after which the sequences were deduplicated, denoised, and merged, chimeras were removed, and Amplicon Sequence Variants (ASVs) were inferred at > 99% similarity. The sequences are submitted to the National Center for Biotechnology Information Sequence Read Archive with accession number PRJNA1229086 and PRJNA1227142 for bacterial and fungal sequences, respectively.
Statistical analyses
The effects of different amendments on soil health indicators were analyzed using SAS v. 9.4 (SAS Institute Inc., 2002). Posthoc comparisons for elucidating the effects of different organic inputs on SOM, nutrients, and biological indicators were performed using PROC GLIMMIX in SAS. Means were separated using Tukey’s HSD test and considered different at P ≤ 0.05. For all data analysis, organic amendments were treated as fixed effects. Data normality was checked using the Shapiro-Wilk test. Diversity indices, including evenness, Simpson–1, and Shannon’s, were determined for bacterial and fungal communities in the soil sampled from each treatment using the “vegan” package in RStudio. Evenness refers to how numerically close each species in an environment, while Shannon’s and Simpson–1 are sensitive to changes in rare and highly abundant species in an environment, respectively. Redundancy analysis was also conducted using the “vegan” package to investigate the relationship between soil characteristics, including pH, EC, total N, NH4-N, NO3-N, and available P and K, and the bacterial and fungal communities. A Monte Carlo permutation test was conducted to compute statistical significance between bacterial and fungal communities with soil properties.
Results
Soil organic matter and nutrient availability
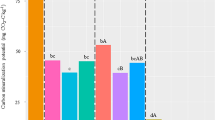
Soil organic matter content at the site was 10 (± 0.6) g kg–1 at the start of experiment (Apr. 2021) and was significantly influenced by the organic amendments following the first 2 years after planting in Fall 2021 (P = 0.03) and 2022 (P = 0.0003) (Fig. 1). In year 1, SOM was lowest with C band and highest with C incorp, followed by B band, B + C band, B + C incorp, B incorp, and woodchips. By year 2, SOM continued to remain the lowest in C band and highest in C incorp, followed by B + C band, woodchips, B + C incorp, B band, and B incorp.
Effect of organic amendments on soil organic matter in a new organic planting of ‘Draper’ blueberry in eastern Oregon over two sampling periods in 2021 and 2022. Each bar represents the mean of five replicates. Subsamples for the analyses were collected from a composite of 10-12 soil samples from each plot over five replicates (n=35). Means with a different letter within a sampling date are statistically different at P ≤ 0.05.
Soil pH and EC were within the recommended range for northern highbush blueberry (pH 4.5–5.5; EC < 2 dS m–1) but were unaffected by the organic amendments in 2021 or 2022 (Table 2). However, soil nutrients, including total N, NH4-N, NO3-N, and available P and K, were affected by the treatments (Table 2). In most cases, the concentration of these nutrients was higher with C incorp than with the woodchip control during both years of the study. Several nutrients were also higher with B + C band and/or B + C incorp than with woodchips, including NH4-N, NO3-N, and P in 2021 and K in 2021 and 2022. Total N, on the other hand, was lower with B band than with woodchips in 2021.
Soil biological indicators
Like soil nutrients, several soil biological indicators were affected by the treatments and higher with C incorp than with woodchips during both years of the study, including extractable organic C, microbial biomass C, active C, and microbial respiration (Table 3). Extractable organic C and microbial respiration were also higher with B + C band and/or B + C incorp than with woodchips in 2022, while β-glucosidase and active C were lower with B band and/or B incorp during the second or both years of the study (Table 3). No differences were observed in microbial biomass C.
Microbial community structure
To understand treatment impacts on bacterial and fungal community structures, soils were profiled, and the diversity indices were assessed for both bacterial and fungal communities including evenness, Shannon’s and Simpson–1. There were no differences among the treatments for any of the bacterial or fungal diversity indices, including evenness, Simpson–1, or Shannon’s, during either year of the study (Table 4). In general, relative abundance of the bacteria phyla in the soil differed between years and was dominated by Acidobacteria, Actinobacteria, Armatimonadetes, Chloroflexi, Cyanobacteria, Firmicutes, Gemmatimonadetes, Patescibacteria, Planctomycetes, and Proteobacteria; however, their relative abundances varied with time (Fig. 2). Even though we did not see any significant differences between treatments for each phylum, we did observe that distribution of certain phyla changed considerably over time. In the first year, there was a high abundance of Acidobacteria, Actinobacteria, Planctomycetes, Proteobacteria, and Patescibacteria. However, by the second year, Acidobacteria was dominant, while all other phyla decreased considerably. In contrast, the fungal community was similar between years and dominated by Ascomycota, Basidiomycota, Chytridiomycota, Mortierellomycota, Mucoromycota, Rozellomycota, and other unidentified fungi (Fig. 3). The relative abundances of these phyla did not seem different in both years, and we did not see any significant differences in the relative abundance of phyla among the treatments as well. The fungal community remained relatively stable in response to the treatments and the time of sampling.
Redundancy analysis was conducted to investigate the relationship between microbial community and soil characteristics (Fig. 4). Based on the redundancy analysis, we observed a strong association between numerous soil characteristics and the bacterial phyla found at the site, including with soil EC, total N, NH4-N, NO3-N, P, and K in Planctomycetes, Proteobacteria, Firmicutes, and Acidobacteria at the end of the first year, and with total N, NH4-N, P, and K in Acidobacteria, Firmicutes, Chloroflexi, Actinobacteria, and Armatimonadetes at the end of the second year (Fig. 4). The Monte Carlo permutation tests showed that NO3-N (R2 = 0.06) and P (R2 = 0.12) had the largest explanatory power to bacterial communities in 2021 (Table 5) while in 2022, EC explained 14% of the bacterial communities (Table 5). We observed strong associations between fungal community and soil characteristics, including with soil EC, total N, P, and K in Ascomycota and with soil pH in Chytridiomycota and Rozellomycota at the end of the first year, and with P and K in Mucoromycota and with soil pH and NH4-N in Mortierellomycota and unidentified fungi at the end of the second year (Fig. 5). The Monte Carlo permutation tests showed that P (R2 = 0.15) was the largest explanatory variable for fungal communities in 2021 (Table 6) while in 2022, soil pH (R2 = 0.16) and NO3-N (R2 = 0.10) had the largest explanatory power to fungal communities (Table 6).
Discussion
Blueberry plants thrive in acidic and high SOM soils, and in this study, we evaluated a suite of organic amendments that help in increasing SOM. Our finding of higher SOM in C incorp treatment (Fig. 1) can plausibly be explained by the increased contact surface of the soil and compost through incorporation, which enhances the microbe-mediated mineralization as compared to when the compost is surface applied45. Also, compost is more easily decomposable due to which the effect of compost was faster to be seen as compared to biochar which is more stable46,47.
Several of our findings are consistent with previous studies48,49,50. For example, soil pH was not affected by organic amendments, likely because the field was irrigated with acidified water30. Like SOM, there was an elevation in available nutrients, including total N, NH4-N, NO3-N, and available P and K, in the C incorp treatment (Table 2), which was also reported by others51,52,53. An increase in SOM is often correlated to higher nutrient retention54. It is important to note that availability of NH4- and NO3-N are strongly influenced by soil pH, and since each plot was irrigated with acidified water, any differences observed in these nutrients were probably solely influenced by organic amendments. The results highlight the importance of compost application in blueberry systems, not only in nutrient availability, but also in enhancing the resource use efficiency of waste. We observed high concentrations of NO3-N in the soil in Fall 2021, especially in treatments with incorporated compost, which could potentially lead to unwanted N leaching; however, this threat may be alleviated by incorporating biochar with compost55. In the second year, a reduction in NO3-N was observed in treatments with co-composted biochar, thus relieving concerns of N leaching.
In addition to nutrients, a suite of soil biological health indicators was also analyzed, and per our expectations, C incorp resulted in some of the highest levels of extractable organic carbon, active carbon, β-glucosidase activity, and microbial respiration among the treatments (Table 3). Even though no differences were observed in microbial biomass carbon among the treatments, there was a clear trend in carbon fractions, as observed by extractable organic and active carbon. Both these fractions are labile fractions of soil carbon, and both were highest with C incorp, which agrees with our SOM results. Higher mineralization of incorporated compost could contribute to a higher presence of extractable organic and active carbon. Similarly, high microbial activity was observed with C incorp, as evidenced by microbial respiration and β-glucosidase activity. Enzyme activity regulates SOM and nutrient cycling, and their activity is enhanced in treatments with higher amounts of carbon substrate. Consistent with carbon fractions and SOM results, microbial activity was also higher in treatment with higher SOM availability56. In most cases, we observed incorporated amendments showed better results than band applied treatments, especially in the case of compost.
In addition to soil health indicators, the response of the plants to these organic amendments was also assessed in a related study at the same research site30. In this case, the results showed that there were no differences in plant height, fruit set, yield, or fruit quality among the treatments. This suggests that the response may be long term, and more data may be needed to determine the impacts of these amendments and their method of application on growth and fruit production of organic blueberry plants grown in a sandy soil.
Microbial diversity is critical for soil processes and sustainability57. Therefore, it is crucial to understand how bacterial and fungal communities harbored in soils are influenced by various organic inputs. Typically, higher diversity confers higher ecosystem resilience in response to any kind of disturbance than lower diversity. In this study, we did not observe any differences in bacterial and fungal diversity indices, including evenness, Shannon’s, and Simpson–1. Even though we observed significant differences in nutrient availability, microbial activity, and C fractions, we did not see any significant differences in relative abundances of bacterial phyla in both years of soil sampling (Fig. 2). However, at the end of the first year, the relative abundance of bacterial phyla was dominated by Acidobacteria, Actinobacteria, Planctomycetes, Proteobacteria, and Patescibacteria, while by the end of second year, the relative abundance decreased in all phyla except Acidobacteria, which increased considerably. This could be due to acidic soil pH in all the treatments that was maintained by the acidified irrigation for blueberry production58. Looking at the redundancy analysis (Fig. 4), dominant phyla were strongly associated with nutrient availability in both years, which is in agreement with previous findings54. However, the relative abundance of fungal phyla was similar between years (Fig. 3). It is evident that fungi are resistant to change59,60 and therefore, it may take longer for a fungal community to respond to changes in soil conditions. It could be because fungi have filamentous structure that facilitates more access and utilization of substrates (organic amendments in this case), regardless of the application technique (banded versus incorporated). In both years, the relative abundances of the dominant fungal phyla looked similar. Our finding of less to no differences in bacterial and fungal community could also be attributed to the fact that this trial was established in April 2021, and soil was first sampled in Fall 2021, which is only few months after the trial established. The second sampling was conducted in Fall 2022, which was 16 months after the trial was established. Given that soil microbes are resilient to any kind of disturbances, it could take longer for these organic amendments to alter the structure of the bacterial and fungal communities61. The organic inputs also included biochar, which is highly stable and takes years to decompose and show any effect on soil health indicators and microbial community structure. Therefore, more data needs to be collected to investigate the long-term effects of these organic amendments on soil health and microbiome in perennial blueberry systems.
Conclusion
This present study was conducted to elucidate the effects of various organic amendments on soil nutrient availability, biological health indicators, and microbial community structure in an organically managed blueberry field in eastern Oregon. Our results showed that grape pomace compost resulted in higher soil nutrient availability and soil biological characteristics than other amendments, including biochar and co-composted biochar. Furthermore, compost showed better results when it was incorporated in the soil than when it was banded on the soil surface. As biochar is known to increase carbon sequestration and improve soil health over the long term, more data needs to be collected to assess its impact on soil health indicators.
Data availability
All data generated or analyzed during this study can be obtained upon request by contacting the corresponding author or Dr. Scott B. Lukas. The sequences are submitted to the National Center for Biotechnology Information Sequence Read Archive with accession number PRJNA1229086 and PRJNA1227142 for bacterial and fungal sequences, respectively.
References
Delpino, F. M., Figueiredo, L. M., da Silva, T. G. & Flores, T. R. Effects of blueberry and cranberry on type 2 diabetes parameters in individuals with or without diabetes: a systematic review and meta-analysis of randomized clinical trials. Nutr. Metabolism Cardiovasc. Dis. 32, 1093–1109 (2022).
Basu, A. et al. Dietary blueberry and soluble fiber supplementation reduces risk of gestational diabetes in women with obesity in a randomized controlled trial. J. Nutr. 151, 1128–1138 (2021).
Strik, B. In X International Symposium on Vaccinium and Other Superfruits 1017. 257–267 (2025).
USDA-ERS. Fruit and tree nuts yearbook Table (2021).
Fernandez-Salvador, J. A., Strik, B. & Lev, L. Changes in the organic blueberry industry in Oregon: 2015 and 2016 results of in-person, on-site interviews with growers (2018).
Retamales, J. B. E. A. Blueberries. Crop Production Science in Horticulture 2 (CABI, 2018).
Chen, J. et al. Effect of lou soil pH change on selenium forms and availability. Northwest. Geol. 53, 254–260 (2020).
Mutuo, P. K., Shepherd, K. D., Albrecht, A. & Cadisch, G. Prediction of carbon mineralization rates from different soil physical fractions using diffuse reflectance spectroscopy. Soil Biol. Biochem. 38, 1658–1664 (2006).
Seppey, C. V. et al. Distribution patterns of soil microbial eukaryotes suggests widespread algivory by phagotrophic protists as an alternative pathway for nutrient cycling. Soil Biol. Biochem. 112, 68–76 (2017).
Bach, E. M., Ramirez, K. S., Fraser, T. D. & Wall, D. H. Soil biodiversity integrates solutions for a sustainable future. Sustainability 12, 2662 (2020).
Fierer, N. Embracing the unknown: disentangling the complexities of the soil Microbiome. Nat. Rev. Microbiol. 15, 579–590 (2017).
Balbinoti, T. C. V. et al. Addition of grape pomace in the hydration step of parboiling increases the antioxidant properties of rice. Int. J. Food Sci. Technol. 55, 2370–2380 (2020).
Oliveira, M. & Duarte, E. Integrated approach to winery waste: waste generation and data consolidation. Front. Environ. Sci. Eng. 10, 168–176 (2016).
Sousa, E. C. et al. Chemical composition and bioactive compounds of grape pomace (Vitis vinifera L.), benitaka variety, grown in the semiarid region of Northeast Brazil. Food Sci. Technol. 34, 135–142 (2014).
Shuval, H., Jodice, R., Consiglio, M., Spaggiarri, G. & Spigoni, C. Control of enteric micro-organisms by aerobic–thermophilic co-composting of wastewater sludge and agro-industry wastes. Water Sci. Technol. 24, 401–405 (1991).
Lian, F. & Xing, B. Black carbon (biochar) in water/soil environments: molecular structure, sorption, stability, and potential risk. Environ. Sci. Technol. 51, 13517–13532 (2017).
Xiao, X., Chen, B., Chen, Z., Zhu, L. & Schnoor, J. L. Insight into multiple and multilevel structures of biochars and their potential environmental applications: a critical review. Environ. Sci. Technol. 52, 5027–5047 (2018).
Zheng, H. et al. Enhanced growth of halophyte plants in biochar-amended coastal soil: roles of nutrient availability and rhizosphere microbial modulation. Plant. Cell. Environ. 41, 517–532 (2018).
Yu, H. et al. Biochar amendment improves crop production in problem soils: a review. J. Environ. Manage. 232, 8–21 (2019).
Sales, B. K. et al. Biochar as an alternative soil amendment for establishment of Northern highbush blueberry. HortScience 57, 277–285 (2022).
Borchard, N., Siemens, J., Ladd, B., Möller, A. & Amelung, W. Application of biochars to sandy and silty soil failed to increase maize yield under common agricultural practice. Soil Tillage. Res. 144, 184–194 (2014).
Sigua, G., Novak, J., Watts, D., Johnson, M. & Spokas, K. Efficacies of designer biochars in improving biomass and nutrient uptake of winter wheat grown in a hard setting subsoil layer. Chemosphere 142, 176–183 (2016).
Guo, X., Liu, H. & Zhang, J. The role of Biochar in organic waste composting and soil improvement: a review. Waste Manage. 102, 884–899 (2020).
Awasthi, M. K. et al. Evaluation of Biochar amended biosolids co-composting to improve the nutrient transformation and its correlation as a function for the production of nutrient-rich compost. Bioresour. Technol. 237, 156–166 (2017).
Oldfield, T. L. et al. Biochar, compost and biochar-compost blend as options to recover nutrients and sequester carbon. J. Environ. Manage. 218, 465–476 (2018).
Wang, Y., Villamil, M. B., Davidson, P. C. & Akdeniz, N. A quantitative Understanding of the role of co-composted Biochar in plant growth using meta-analysis. Sci. Total Environ. 685, 741–752 (2019).
Luo, X. et al. Use of biochar-compost to improve properties and productivity of the degraded coastal soil in the yellow river delta, China. J. Soils Sediments. 17, 780–789 (2017).
Schulz, H., Dunst, G. & Glaser, B. No effect level of co-composted Biochar on plant growth and soil properties in a greenhouse experiment. Agronomy 4, 34–51 (2014).
Iqbal, H., Garcia-Perez, M. & Flury, M. Effect of Biochar on leaching of organic carbon, nitrogen, and phosphorus from compost in bioretention systems. Sci. Total Environ. 521, 37–45 (2015).
Retano, A. Evaluating alternative soil amendments for organic northern highbush blueberry cultivation in sub-optimal soils (2023).
Thomas, G. W. Soil pH and soil acidity. Methods Soil. Analysis: Part. 3 Chem. Methods. 5, 475–490 (1996).
Rhoades, J. Salinity Electrical conductivity and total dissolved solids. Methods Soil. Analysis: Part. 3 Chem. Methods. 5, 417–435 (1996).
Mulvaney, R. Nitrogen inorganic forms. Methods Soil. Anal. Part 3, 526 (1996).
Moebius-Clune, B. N. Comprehensive Assessment of Soil Health: the Cornell Framework Manual (Cornell University, 2016).
Nelson, D. W. & Sommers, L. E. Total carbon, organic carbon, and organic matter. Methods Soil. Analysis: Part. 3 Chem. Methods. 5, 961–1010 (1996).
Vance, E. D., Brookes, P. C. & Jenkinson, D. S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 19, 703–707 (1987).
Tabatabai, M. Soil enzymes. Methods Soil. Anal. Part. 2 Microbiol. Biochem. Prop. 5, 775–833 (1994).
Weil, R. R., Islam, K. R., Stine, M. A., Gruver, J. B. & Samson-Liebig, S. E. Estimating active carbon for soil quality assessment: a simplified method for laboratory and field use. Am. J. Altern. Agric. 18, 3–17 (2003).
Culman, S. W. et al. Permanganate oxidizable carbon reflects a processed soil fraction that is sensitive to management. Soil Sci. Soc. Am. J. 76, 494–504 (2012).
Franzluebbers, A., Haney, R., Hons, F. & Zuberer, D. Determination of microbial biomass and nitrogen mineralization following rewetting of dried soil. Soil Sci. Soc. Am. J. 60, 1133–1139 (1996).
Thompson, L. R. et al. A communal catalogue reveals earth’s multiscale microbial diversity. Nature 551, 457–463 (2017).
Klindworth, A. et al. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 41, e1–e1 (2013).
White, T. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications (Academic Press, Inc, 1990).
Team, R. Others R: A Language and environment for statistical computing. GBIF: Cph. Denmark (2013).
Kranz, C. N., McLaughlin, R. A., Johnson, A., Miller, G. & Heitman, J. L. The effects of compost incorporation on soil physical properties in urban soils–A concise review. J. Environ. Manage. 261, 110209 (2020).
Agegnehu, G., Bass, A. M., Nelson, P. N. & Bird, M. I. Benefits of biochar, compost and biochar–compost for soil quality, maize yield and greenhouse gas emissions in a tropical agricultural soil. Sci. Total Environ. 543, 295–306 (2016).
Steiner, C., Das, K., Melear, N. & Lakly, D. Reducing nitrogen loss during poultry litter composting using Biochar. J. Environ. Qual. 39, 1236–1242 (2010).
Costello, R. C., Sullivan, D. M., Bryla, D. R., Strik, B. C. & Owen, J. S. Compost feedstock and compost acidification affect growth and mineral nutrition in Northern highbush blueberry. HortScience 54, 1067–1076 (2019).
Kuzyakov, Y. Factors affecting rhizosphere priming effects. J. Plant Nutr. Soil Sci. 165, 382–396 (2002).
Sullivan, D., Strik, B. & Bryla, D. In II International Symposium on Organic Matter Management and Compost Use in Horticulture 1076 171–178 (2015).
Chalhoub, M. et al. Increased nitrogen availability in soil after repeated compost applications: use of the PASTIS model to separate short and long-term effects. Soil Biol. Biochem. 65, 144–157 (2013).
Feng, X. et al. The effects of green waste compost on soil N, P, K, and organic matter fractions in forestry soils: elemental analysis evaluation. RSC Adv. 11, 31983–31991 (2021).
Cogger, C., Hummel, R., Hart, J. & Bary, A. Soil and redosier Dogwood response to incorporated and surface-applied compost. HortScience 43, 2143–2150 (2008).
Singh, S. et al. Long-term agro-management strategies shape soil bacterial community structure in dryland wheat systems. Sci. Rep. 13, 13929 (2023).
Hagemann, N., Kammann, C. I., Schmidt, H. P., Kappler, A. & Behrens, S. Nitrate capture and slow release in Biochar amended compost and soil. PloS One. 12, e0171214 (2017).
Singh, S. et al. Does increased cropping intensity translate into better soil health in dryland wheat systems? Appl. Soil. Ecol. 204, 105728 (2024).
Zhao, J. et al. Effects of organic–inorganic compound fertilizer with reduced chemical fertilizer application on crop yields, soil biological activity and bacterial community structure in a rice–wheat cropping system. Appl. Soil. Ecol. 99, 1–12 (2016).
Dedysh, S. N. & Damsté, J. S. S. Acidobacteria eLS 1–10 (2018).
Singh, S. et al. Soil organic carbon cycling in response to simulated soil moisture variation under field conditions. Sci. Rep. 11, 10841 (2021).
Singh, S., Mayes, M. A., Kivlin, S. N. & Jagadamma, S. How the Birch effect differs in mechanisms and magnitudes due to soil texture. Soil Biol. Biochem. 179, 108973 (2023).
Allison, S. D. & Martiny, J. B. Resistance, resilience, and redundancy in microbial communities. Proc. Natl. Acad. Sci. 105, 11512–11519 (2008).
Acknowledgements
This study was a part of the USDA NIFA Organic Transitions Program which looked at management techniques to optimize soil pH and nutrient availability in organic highbush blueberry (grant no. 2019-51106-30194). USDA NIFA National Needs Graduate Fellowship Program (grant no. 2021-38420-34064) provided Andrea Retano’s stipend/fellowship. Part of the analyses for this study was supported by Oregon State University, Agricultural Research Foundation (grant ID: ARF#22082A) and Washington Blueberry Commission (No Award Number).
Author information
Authors and Affiliations
Contributions
SS, SL, and DB conceptualized the idea of the manuscript. SS and SL helped in experimental setup. SS and AR carried out field sampling. SS performed the data analysis and wrote the first draft. SL and DB revised and provided insightful suggestions on the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Singh, S., Lukas, S.B., Retano, A. et al. Evaluating locally available organic amendments to enhance soil health indicators for highbush blueberry production east of the Cascades in the U.S. Pacific Northwest. Sci Rep 15, 20933 (2025). https://doi.org/10.1038/s41598-025-05761-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-05761-z