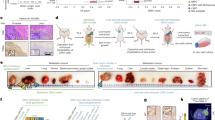
Abstract
Prostate-specific membrane antigen (PSMA)-targeted theranostics have profoundly reshaped prostate cancer (PCa) management. However, patterns of PSMA expression heterogeneity and the identification of alternative targets for PSMA-negative cases remain insufficiently understood. To address this gap, this study investigates PSMA expression heterogeneity in 127 primary lesions (PL) from hormone-sensitive PCa (HSPC) cohort and 76 bone metastatic lesions (BML) from metastatic castration-resistant PCa (mCRPC) cohort through immunohistochemical analysis, including 27 matched PL-BML samples. Notable inter-patient variability in PSMA expression was observed, with H-scores ranging from 1.42 to 197.16 overall. Among matched samples, six cases exhibited HSPC-/mCRPC + and seven cases showed HSPC+/mCRPC- PSMA expression patterns. Intra-tumoral heterogeneity was significant, with 67.7% of PL and 30.1% of BML showing high variability in PSMA staining intensity. Membranous PSMA expression and the normalized membrane ratio were significantly higher in PL compared to BML (both p < 0.001). PSMA-negative cases (membranous staining ≤ 20) were found in 15.0% of HSPC and 36.8% of mCRPC cases. PSMA expression levels differed significantly among androgen receptor (AR) expression groups (p < 0.001), with lower PSMA expression associated with lower AR expression levels. Alternative tumor-associated antigens (TAAs) were identified in PSMA-negative cases: B7H3 and TROP2 expression were prominent in HSPC, and STEAP1 and B7H3 expression dominated in mCRPC. These findings highlight the dynamic nature of PSMA expression and support the rationale for exploring alternative theranostic strategies in PSMA-negative PCa.
Similar content being viewed by others
Introduction
Prostate-specific membrane antigen (PSMA), a type II transmembrane protein encoded by the FOLH1 gene, is highly expressed in prostate cancer (PCa) and has become a widely used target for molecular theranostics of PCa. The advent of PSMA-PET imaging and 177Lu-PSMA-617 radioligand therapy have profoundly reshaped the landscape of clinical practice for the management of lethal metastatic castration-resistant PCa (mCRPC)1,2. However, a subset of patients derive limited benefit from PSMA-targeted therapies, presenting a significant clinical challenge3,4. This may be in part attributed to the heterogeneous or absent expression of PSMA leading to suboptimal responses to PSMA-targeted strategy in some cases5. The heterogeneity of PSMA expression in both primary hormone-sensitive prostate cancer (HSPC) and mCRPC remains insufficiently explored in the current literature.
Several studies have reported membranous-cytoplasmic co-staining of PSMA in immunohistochemistry (IHC), with some research suggesting that membranous expression is the clinically actionable target, while cytoplasmic expression is considered as a potential interference5. In contrast, other studies propose that cytoplasmic expression reflects the internalization activity of membrane receptors6and integrating both membranous and cytoplasmic expression may offer a better prediction of prognosis and therapeutic efficacy7.
Many studies relied on nuclear medicine techniques to evaluate PSMA expression8which, while informative and mainstream, do not provide detailed information on cellular or subcellular localization of PSMA expression. In comparison, IHC offers both qualitative and subcellular-quantitative data, and is commonly applied in clinical practice. While discrepancies between nuclear imaging and IHC results have been reported9low PSMA expression in IHC often corresponds to limited uptake of PSMA-targeted radiopharmaceuticals5.
Precision treatment strategies targeting cell surface tumor-associated antigens (TAAs) have become prominent approaches in oncology, with antibody-drug conjugates (ADCs) leading the field in development. ADCs combine specific antibodies with cytotoxic agents10,11. The response to ADCs can be influenced by various factors, including antibody specificity, cytotoxic payload, linker chemistry, and conjugation methods, with the expression level of TAAs being of paramount importance12,13,14. Although controversial, higher TAA expression levels are generally believed to enhance ADC efficacy theoretically11. As of early 2024, fifteen ADCs targeting eleven TAAs have been approved for both solid and hematologic tumors. Among these, four targets—human epidermal growth factor receptor 2 (HER2), nectin cell adhesion molecule 4 (NECTIN4), tumor-associated calcium signal transducer 2 (TROP2), and tissue factor (TF)—have been FDA-approved for use in solid tumors11. In the field of PCa, relatively well-established ADC targets further include six-transmembrane epithelial antigen of the prostate 1 (STEAP1) and B7-homolog 3/CD276 (B7H3)15,16.
The aim of this study was to systematically characterize the heterogeneity of PSMA expression in both HSPC and mCRPC, and to head-to-head explore the expression profiles of these alternative well-established TAAs in PSMA-negative cases.
Results
A total of 127 primary lesions (PL) from HSPC cohort and 76 bone metastatic lesions (BML) from mCRPC cohort, including 27 pairs of matched PL-BML samples were finally included, Supplementary Fig. 1 showed the flowchart of sample selection process. Of note, although the mCRPC cohort predominantly consisted of castration-resistant cases (69/76, 90.8%), it also included a small proportion of metastatic HSPC (mHSPC) cases (7/76, 9.2%).
The baseline characteristics of patents included were summarized in Table 1 and Supplementary Table 1.
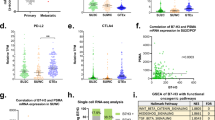
The PSMA mambranous H-score (MHscore) of PL samples did not show a significant correlation with age or tPSA levels (Fig. 1A, B), the PSMA MHscore was significantly higher in the ISUP = 2 HSPC group compared to the ISUP = 5 HSPC group (p = 0.026), while no significant differences were observed across the remaining subgroups (Fig. 1C and Supplementary Table 2). Figure 1D illustrates both the inter-patient and intra-tumoral heterogeneity of Membranous PSMA expression. The Shannon’s diversity index (SDI) reflects the heterogeneity abundance within each sample. SDI greater than 1, indicating relatively high intra-tumoral heterogeneity of PSMA expression, was observed in 86 (67.7%) PL samples. Twenty (15.7%) PL samples had SDI between 0.5 and 1, indicating moderate intra-tumoral heterogeneity. PSMA-negative was identified in 19 cases(15.0%) within the HSPC cohort. In the PSMA-negative HSPC cases, B7H3 and TROP2 demonstrated significantly better complementarity to PSMA and exhibited higher MHscore levels compared to other TAAs (Fig. 1E, F and Supplementary Table 3). HER2 was consistently negative in the PSMA-negative HSPC cases.
Membranous PSMA expression of PL is heterogeneous in clinical samples obtained from patients with HSPC at initial diagnosis of PCa. (A) Scatter plot of PSMA MHscore and age, showing no correlation was observed. (B) Scatter plot of PSMA MHscore and log(tPSA + 1). Due to the wide distribution of tPSA, a mathematical transformation was applied. However, no significant correlation was observed before or after the transformation. (C) Box plot of PSMA MHscore distribution across ISUP groups. Significant differences across subgroups are marked in the plot. (D) Membranous PSMA expression at the initial HSPC status. Expression of membranous PSMA quantified by H-score and presented in order of increasing MHscore. Degree of PSMA heterogeneity was measured by Shannon’s diversity index (SDI) and depicted as heat map ranging from low heterogeneity (light green) to high heterogeneity (dark green). PSMA-negative cases are indicated with a dashed line in the plot with the definition as MHscore ≤ 20. (E) Head-to-head comparison bar plots showing the expression profiles of other well-established TAAs in PSMA-negative HSPC cases. (F) Box plot showing the comparison of MHscore for well-established TAAs in PSMA-negative HSPC cases. Significant differences across subgroups are marked in the plot, *represents p < 0.05, **represents p < 0.01, ***represents p < 0.001.
The heterogeneity of membranous PSMA expression in mCRPC cohort was exhibited in Fig. 2A. SDI greater than 1 was observed in 23 (30.1%) BML samples, twenty-five (32.9%) BML had SDI between 0.5 and 1. PSMA-negative was identified in 28 cases (36.8%) within the mCRPC cohort. PSMA MHscore did not show any differences across the various bone-metastatic sites (Fig. 2B). Both STEAP1 and B7H3 exhibited significantly higher complementarily to PSMA, with higher MHscore levels in PSMA-negative mCRPC cases. Additionally, HER2 MHscore levels greater than 50 were observed in 28.6% of cases (Fig. 2C, D and Supplementary Table 4). TF emerged as the lowest expressing TAA in PSMA-negative mCRPC cases. The normalized membrane ratio (NMR) analysis revealed that STEAP1 and B7H3 displayed consistently high NMR expression patterns, while HER2 showed relatively heterogeneous NMR pattern, with 21.4% of PSMA-negative mCRPC cases showing a cytoplasmic-dominant HER2 expression pattern (Fig. 2E).
Fourteen mCRPC cases were identified in which neuroendocrine differentiation (NE) markers (Syn/CgA/NSE/CD56) had been assessed from the clinical pathology system of our center. Among these, three cases (21.4%) were classified as neuroendocrine prostate cancer (NEPC). Details of NE marker staining and PSMA expression level data of these 14 cases were presented in Supplementary Table 5. All the 3 NEPC cases were PSMA-negative cases. Using the Wilcoxon rank-sum test, a trend toward lower PSMA membrane expression levels in NEPC compared to non-NEPC BML was observed (p = 0.088). The NMR of PSMA expression was found to be significantly lower in NEPC than in non-NEPC BML (p = 0.035), indicating that PSMA may be predominantly localized in the cytoplasm to some extent rather than on the membrane in NEPC.
A total of 55 mCRPC cases had complete clinicopathological records of AR IHC staining. Of these 55 cases, 6 were classified as AR-negative, 9 as low expression, 11 as intermediate expression, and 29 as high expression. Membranous PSMA expression levels differed significantly across AR expression groups (overall p < 0.001). A positive correlation was observed between membranous PSMA expression and AR status, with the AR-negative group exhibiting significantly lower PSMA MHscores than other AR groups (p < 0.05), as shown in Supplementary Fig. 2 and Supplementary Table 6. These findings suggest that negative or low AR expression may be associated with reduced PSMA expression in BML. All six AR-negative mCRPC cases showed PSMA- negative expression, and the expression profiles of other actionable TAAs in these BML samples are detailed in Supplementary Table 7.
Membranous PSMA expression of BML is heterogeneous in clinical samples obtained from patients with mCRPC. (A) Membranous PSMA expression in mCRPC status. Expression of membranous PSMA quantified by H-score and presented in order of increasing MHscore. Degree of heterogeneity in mPSMA was measured by SDI and depicted as heat map ranging from low heterogeneity (light green) to high heterogeneity (dark green). PSMA-negative cases are indicated with a dashed line in the plot with the definition as MHscore ≤ 20. (B) Box plot of PSMA MHscore distribution across bone metastatic sites, with no significant difference was observed. (C) Head-to-head comparison bar plots showing the expression profiles of other well-established TAAs in PSMA-negative mCRPC cases. (D) Box plot showing the comparison of MHscore for well-established TAAs in PSMA-negative mCRPC cases. Significant differences across subgroups are marked in the plot, *represents p < 0.05, **represents p < 0.01, ***represents p < 0.001. (E) Heatmap of NMR classification across TAAs in PSMA-negative mCRPC cases. NMR > 50% indicates a membranous dominant expression pattern, while NMR < 50% indicates a cytoplasmic dominant expression pattern.
Overall, membranous PSMA expression in PL is significantly higher than in BML (p<0.001) (Fig. 3A). Meanwhile, the NMR levels in PL are also significantly higher than in BML(p<0.001) (Fig. 3B). In matched samples, although some patients exhibited increase in membranous PSMA expression of BML compared to the initial PL, 63.0% of patients showed decrease in membranous PSMA expression of BML. The treatment history of these patients during the interval between the two status is shown in Fig. 3C. There were no significant differences of the PSMA alteration (dPSMA) across the different categories of SGARA, chemotherapy, or radiation in matched samples (Supplementary Table 8). Figure 3D-G illustrates the different patterns of intra-patient PSMA expression heterogeneity.
Comparison of PSMA expression profile between HSPC-PL and mCRPC-BML status. (A) Box plot showing the comparison of PSMA MHscore between PL and BML, PSMA expression level in BML was significant lower than PL. (B) Violin plot showing the comparison of PSMA NMR between PL and BML, PSMA NMR in BML was also significant lower than PL. (C) Waterfall plot showing the alteration of membranous PSMA expression from PL to BML, along with the corresponding treatment history heatmap (second generation androgen receptor antagonist (SGARA), chemotherapy and radiotherapy), no significant correlation was observed between PSMA alteration and any of the various treatment history. (D, E,F, G) Different patterns of intra-patient PSMA expression heterogeneity in matched samples. (D) Heterogeneously low expression in different regions of the same HSPC sample (①③: within same regions, even adjacent tumor tissues can exhibit heterogeneous PSMA expression level, the tumor tissue in the red dashed area shows absent PSMA expression;②completely absent PSMA expression tumor region), with high and uniform PSMA expression in matched mCRPC. (E) High PSMA expression in HSPC, low expression in matched mCRPC. (F) Heterogeneously low expression in both HSPC and matched mCRPC. (G) High expression in primary HSPC, heterogeneously low expression in both matched synchronously mHSPC and metachronously mCRPC. All the black scale bars in the IHC image represent 100 μm. Brown scale bars in the IHC image represent 500 μm.
Discussion
PSMA-targeted theranostic strategies, including PSMA-PET imaging and 177Lu-PSMA-617 radioligand therapy, have significantly revolutionized the clinical management of PCa. However, a subset of patients derived limited benefit from these approaches, in part due to heterogeneous or absent PSMA expression1,5,17,18emphasizing the urgent need for alternative theranostic targets. This study systematically evaluated the spatiotemporal inter- and intra-patient heterogeneity of PSMA expression in HSPC and mCRPC, revealing the PSMA-negative expression rate, and exploring potential alternative TAA expression in PSMA-negative cases. To our knowledge, this may be the first report to comprehensively head-to-head assess the expression of a panel of clinically actionable TAAs in PSMA-negative PCa within the same cohort, using real-world clinical samples of PCa, it may provide some insights for the clinical management of PCa in future.
Although the heterogeneity of PSMA expression in PCa has been documented, the detailed patterns of the heterogeneity remain elusive5,9,18,19. This study attempts to comprehensively characterize PSMA heterogeneity at three levels: inter-patient, intra-patient, and intra-tumoral. Consistent with previous literature, inter-patient heterogeneity of PSMA expression is evident in both HSPC-PL and mCRPC-BML in our study. Inter-patient variability in PSMA expression was observed in both PL and BML, with H-score ranging from 1.42 to 197.16. Notably, we observed significantly lower PSMA expression levels in BML compared to PL, which aligns with prior findings indicating that a considerable proportion of mCRPC patients exhibit PSMA expression loss19. Our study further revealed spatiotemporally intra-patient heterogeneity patterns of PSMA expression in 27 patients with matched PCa samples, with only 2 cases consistently showing negative PSMA expression, while 6 cases exhibiting HSPC-/mCRPC + and 7 cases exhibiting HSPC+/mCRPC- PSMA expression patterns in HSPC and mCRPC status, indicating that PSMA may display certain degrees of spatiotemporally dynamic expression. To further explore intra-tumoral diversity, PSMA expression was quantified through SDI analysis. We found that over 60% of PCa cases exhibited at least moderately intra-tumoral heterogeneity, different levels of PSMA expression even observed across adjacent tumoral sites within the same tissue sample in IHC images, reflecting the complexity of tumor subgroups within the tumor microenvironment.
Tumor components with low or absent PSMA expression may not be effectively targeted, resulting in residual disease. To address the clinical challenge posed by the loss of PSMA expression in these cases, we further investigated the expression of alternative clinically actionable TAAs as potential theranostic targets. B7H3 and STEAP1 were both first described more than two decades ago and have been identified as highly expressed cell surface antigens in PCa15,20. Our findings support the high expression levels of these two TAAs in PSMA-negative mCRPC, along with relatively homogeneous expression and predominant membrane localization (Fig. 2D), suggesting their potential as effectively complementary targets to PSMA. Future multi-target strategies combining targeted radionuclide therapy with ADCs, chimeric antigen receptor T-cell therapy (CART) or bi-specific antibody drugs may significantly improve comprehensive tumor targeting and killing in lethal mCRPC.
Although PSMA is primarily a membrane antigen, many cases in our study exhibited both membranous and cytoplasmic staining, with notably higher cytoplasmic levels in mCRPC compared to primary HSPC. This finding is consistent with recent studies in PCa, which also demonstrated membranous-cytoplasmic co-staining in IHC15,21. Paschalis et al. highlighted the importance of distinguishing these staining patterns, suggesting that membranous PSMA may represent more clinically actionable target, with cytoplasmic expression affecting membranous staining assessment5. Conversely, Pereira et al. believed that endocytosis and recycling of membranous receptors may explain this co-expression phenomenon6. These dynamic processes can influence TAA density on the cell surface and contribute to cytoplasmic staining. Garassino MC et al. found that in lung cancer, the NMR index of TROP2, incorporating both membranous and cytoplasmic expression, was better at predicting prognosis and therapeutic responses to TROP2-ADCs than membranous expression alone7. Therefore, we suggest that cytoplasmic PSMA staining should not be regarded merely as a limitation but integrated into the systematic assessment of PSMA expression. Another noteworthy observation from the literature is that Watanabe et al. reported PSMA-expressing tumor endothelial cells in approximately 12% of prostate cancer patients, with PSMA expression in endothelial cells being predominantly cytoplasmic. Their findings suggest a potential role for PSMA in promoting angiogenesis in vascular endothelial cells22. However, we would like to clarify that the present study focused exclusively on the expression of targetable markers in tumor cells within prostate cancer tissues. Tumor cells and endothelial cells are morphologically distinct and readily distinguishable. Moreover, the density of endothelial cells within the analyzed tumor regions was extremely low, therefore the endothelial cells were not evaluated in this study. Nonetheless, this phenomenon is intriguing and warrants further investigation in future studies.
Despite the comprehensive profiling of PSMA expression heterogeneity and the exploration of a panel of clinically actionable well-established TAAs in PSMA-negative PCa using large-population real-world clinical samples, this study has certain limitations. Firstly, the single-center, retrospective nature of this study may introduce selection bias. Secondly, although neuroendocrine differentiation has been increasingly reported in advanced prostate cancer, its prevalence in Asian populations appears to be relatively low, and relevant clinical evidence remains limited23,24. In this context, systematic evaluation of neuroendocrine markers was not performed in our study due to the limited availability of serial sections from the clinical samples. Finally, this study primarily focused on the systemic profiling of PSMA expression, it did not correlate target expression with clinical prognosis, nor did it delve into the underlying molecular mechanisms driving the observed phenomena. Future research should address these limitations to further explore the translational potential of our findings.
In conclusion, our study provides a comprehensive analysis of PSMA expression in PCa, highlighting significant heterogeneity in both HSPC and mCRPC, underscoring the importance of considering the dynamic nature of PSMA expression. We also identified alternative theranostic TAAs in PSMA-negative PCa, offering valuable insights into potentially alternative diagnostic and treatment options for future clinical applications in PCa.
Methods
Patients and tissue samples
The experiments involved in this study were approved by the Ethics Committee of Peking University First Hospital (PKUFH) with exemption from informed consent (2023-289-001). All methods were performed in accordance with the protocol approved by the Ethics Committee.
Formalin-fixed paraffin-embedded (FFPE) tissue samples were retrospectively collected from prostate biopsies and bone metastasis surgeries of patients with PCa at PKUFH between 2013 and 2023.
A total of 127 patients had sufficient HSPC-PL samples, including 27 with matched BML samples, the remaining 100 samples were randomly selected from the central database using stratified sampling, with equal weighting assigned to each ISUP group to minimize selection bias, as ISUP group is the most influential clinicopathological factor for PCa. Seventy-six metastatic PCa samples were totally included, with 27 matched BML samples (7 mHSPC and 20 mCRPC cases) and 49 non-matched BML samples (49 mCRPC cases). All samples had sufficient FFPE tissue available for analysis.
Immunohistochemistry
IHC was performed on both cohorts to evaluate PSMA expression. Antigen retrieval was carried out in EDTA buffer (pH 9.0) using microwave heating. Endogenous peroxidase was blocked with 3% H2O2, followed by serum blocking with 3% BSA for 30 min at room temperature. Sections were incubated overnight with primary antibody at 4 °C, then with secondary antibody for 50 min at room temperature. DAB was applied for color development, followed by counterstaining with hematoxylin, differentiation, dehydration, and cover slipping.
Digital images of the slides were captured using Pannoramic DESK, P-MIDI, and P250 scanners (3DHISTECH, Hungary), with the Pannoramic Scanner software, and PSMA expression was quantified using HALO 3.2 software to calculate both the membranous H-score (MHscore) and cytoplasmic H-score (CHscore): H-score = (% weak staining × 1) + (% moderate staining × 2) + (% strong staining × 3). Three random fields per sample were evaluated, and the average score was calculated25,26. The normalized membrane ratio (NMR) was defined as: MHscore/(MHscore + CHscore). PSMA-negative PCa was defined as PSMA MHscore ≤ 205,9. Consecutive sections were derived from the PSMA-negative samples to further assessing the expression of additional TAAs: HER2, NECTIN4, TROP2, TF, STEAP1 and B7H3. Details on the antibodies usage can be found in Supplementary Table 9.
Data collection and statistical analysis
Clinical and pathological data, including age, tPSA, ISUP group and bone metastasis sites were collected. For matched samples, treatment history (ADT, second generation androgen receptor antagonist(SGARA), chemotherapy, radiotherapy) during the sampling interval was also recorded. In the mCRPC cohort, IHC results for AR and neuroendocrine differentiation-related markers, including Syn, CgA, CD56, and NSE, as well as PSA and PSAP, were re-collected and reviewed from the clinical pathology registry system of PKUFH. NEPC was defined based on the following criteria: ①at least two NE markers positive, ②negative staining for PSA/PSAP, and ③morphological features consistent with small-cell carcinoma. The interpretation of AR immunohistochemical (IHC) staining was primarily based on the proportion of AR-positive tumor cells, supplemented by staining intensity: Tumors with < 25% AR-positive cells were classified as AR-negative, those with 25–50% as low expression, 50–75% as intermediate expression, and > 75% as high expression. Linear regression was used to assess the relationship between MHscore and age, tPSA. PSMA MHscore. Other TAA MHscore in PSMA-negative PCa were compared using the Wilcoxon signed-rank test. Intra-sample heterogeneity was quantified by Shannon’s diversity index (SDI). All figures were generated using R 4.3.2 and Adobe Illustrator 27.0. A significance level of 0.05 was used.
Data availability
All data generated or analysed during this study are included in this article and its supplementary information files.
References
Roberts, M. J. et al. Using PSMA imaging for prognostication in localized and advanced prostate cancer. Nat Rev. Urol Jan. 20 (1), 23–47. https://doi.org/10.1038/s41585-022-00670-6 (2023).
Fallah, J. et al. FDA approval summary: lutetium Lu 177 Vipivotide tetraxetan for patients with metastatic Castration-Resistant prostate Cancer. Clin Cancer Res May. 1 (9), 1651–1657. https://doi.org/10.1158/1078-0432.CCR-22-2875 (2023).
Rahbar, K. et al. Response and tolerability of a single dose of 177Lu-PSMA-617 in patients with metastatic Castration-Resistant prostate cancer: A multicenter retrospective analysis. J Nucl. Med Sep. 57 (9), 1334–1338. https://doi.org/10.2967/jnumed.116.173757 (2016).
Seifert, R. et al. Second version of the prostate Cancer molecular imaging standardized evaluation framework including response evaluation for clinical trials (PROMISE V2). Eur Urol May. 83 (5), 405–412. https://doi.org/10.1016/j.eururo.2023.02.002 (2023).
Paschalis, A. et al. Prostate-specific membrane antigen heterogeneity and DNA repair defects in prostate Cancer. Eur Urol Oct. 76 (4), 469–478. https://doi.org/10.1016/j.eururo.2019.06.030 (2019).
Pereira, P. M. R., Mandleywala, K., Ragupathi, A. & Lewis, J. S. Acute Statin Treatment Improves Antibody Accumulation in EGFR- and PSMA-Expressing Tumors. Clin Cancer Res. Dec 1. ;26(23):6215–6229. (2020). https://doi.org/10.1158/1078-0432.CCR-20-1960
Garassino, M. C. S. J. et al. Normalized membrane ratio of TROP2 by quantitative continuous scoring is predictive of clinical outcomes in TROPION-Lung 01. J. Thorac. Oncol. 19 (10), S2–S3. https://doi.org/10.1016/j.jtho.2024.09.015 (2024).
Buteau, J. P. et al. PSMA and FDG-PET as predictive and prognostic biomarkers in patients given [(177)Lu]Lu-PSMA-617 versus Cabazitaxel for metastatic castration-resistant prostate cancer (TheraP): a biomarker analysis from a randomised, open-label, phase 2 trial. Lancet Oncol Nov. 23 (11), 1389–1397. https://doi.org/10.1016/S1470-2045(22)00605-2 (2022).
Sayar, E. et al. Reversible epigenetic alterations mediate PSMA expression heterogeneity in advanced metastatic prostate cancer. JCI Insight Apr. 10 (7). https://doi.org/10.1172/jci.insight.162907 (2023).
Fu, Z., Li, S., Han, S., Shi, C. & Zhang, Y. Antibody drug conjugate: the biological missile for targeted cancer therapy. Signal Transduct. Target. Ther Mar. 22 (1), 93. https://doi.org/10.1038/s41392-022-00947-7 (2022).
Colombo, R., Tarantino, P., Rich, J. R., LoRusso, P. M. & de Vries, E. G. E. The journey of Antibody-Drug conjugates: lessons learned from 40 years of development. Cancer Discov Nov. 1 (11), 2089–2108. https://doi.org/10.1158/2159-8290.CD-24-0708 (2024).
Tarantino, P. et al. Antibody-drug conjugates: smart chemotherapy delivery across tumor histologies. CA Cancer J. Clin Mar. 72 (2), 165–182. https://doi.org/10.3322/caac.21705 (2022).
Bosi, C. et al. Pan-cancer analysis of antibody-drug conjugate targets and putative predictors of treatment response. Eur J. Cancer Dec. 195, 113379. https://doi.org/10.1016/j.ejca.2023.113379 (2023).
Lopez de Sa, A. et al. Considerations for the design of antibody drug conjugates (ADCs) for clinical development: lessons learned. J Hematol. Oncol Dec. 12 (1), 118. https://doi.org/10.1186/s13045-023-01519-0 (2023).
Bhatia, V. et al. Targeting advanced prostate cancer with STEAP1 chimeric antigen receptor T cell and tumor-localized IL-12 immunotherapy. Nat Commun Apr. 11 (1), 2041. https://doi.org/10.1038/s41467-023-37874-2 (2023).
Guo, C. et al. B7-H3 as a therapeutic target in advanced prostate Cancer. Eur Urol Mar. 83 (3), 224–238. https://doi.org/10.1016/j.eururo.2022.09.004 (2023).
Wang, H. et al. Heterogeneity of prostate-specific membrane antigen (PSMA) and PSMA-ligand uptake detection combining autoradiography and postoperative pathology in primary prostate cancer. EJNMMI Res Nov. 16 (1), 99. https://doi.org/10.1186/s13550-023-01044-8 (2023).
Bakht, M. K. & Beltran, H. Biological determinants of PSMA expression, regulation and heterogeneity in prostate cancer. Nat Rev. Urol Jan. 22 (1), 26–45. https://doi.org/10.1038/s41585-024-00900-z (2025).
Bakht, M. K. et al. Landscape of prostate-specific membrane antigen heterogeneity and regulation in AR-positive and AR-negative metastatic prostate cancer. Nat Cancer May. 4 (5), 699–715. https://doi.org/10.1038/s43018-023-00539-6 (2023).
Chapoval, A. I. et al. B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol Mar. 2 (3), 269–274. https://doi.org/10.1038/85339 (2001).
Ferraro, D. A. et al. Immunohistochemical PSMA expression patterns of primary prostate cancer tissue are associated with the detection rate of biochemical recurrence with (68)Ga-PSMA-11-PET. Theranostics 10 (14), 6082–6094. https://doi.org/10.7150/thno.44584 (2020).
Watanabe, R. et al. PSMA-positive membranes secreted from prostate cancer cells have potency to transform vascular endothelial cells into an angiogenic state. Prostate Dec. 81 (16), 1390–1401. https://doi.org/10.1002/pros.24237 (2021).
Guo, A., Wen, S., Ma, Y., Wei, L. & Liu, A. Clinicopathological analysis on small cell carcinoma of the prostate in Chinese patients. J. Cancer. 5 (9), 797–803. https://doi.org/10.7150/jca.9388 (2014).
Iwamoto, H. et al. Treatment outcomes in neuroendocrine prostate Cancer. Anticancer Res Apr. 42 (4), 2167–2176. https://doi.org/10.21873/anticanres.15699 (2022).
Ichiki, T. et al. PRAME expression in fibrosarcomatous dermatofibrosarcoma protuberans. Sci Rep. Oct 3. ;14(1):22973. (2024). https://doi.org/10.1038/s41598-024-74556-5
Wang, S. et al. Identification and validation of an Apoptosis-Related gene prognostic signature for oral squamous cell carcinoma. Front. Oncol. 12, 889049. https://doi.org/10.3389/fonc.2022.889049 (2022).
Acknowledgements
This study was supported by: (1) National Natural Science Foundation of China, General Program, Beijing, China (Grant No. 82370773). (2) National High Level Hospital Clinical Research Funding (Interdepartmental Clinical Research Project of Peking University First Hospital), Beijing, China (2023IR33). (3) National High Level Hospital Clinical Research Funding (Interdepartmental Clinical Research Project of Peking University First Hospital), Beijing, China (2024IR02). 4.Beijing Physician Scientist Training Project, Beijing, China (BJPSTP-2024-20).
Author information
Authors and Affiliations
Contributions
Y.F., Q.Z. and Y.M. contributed to the conception and design of the study. Y.M., Y.T., P.C. and S.X.were responsible for the acquisition of data., Y.M., S.Q., Y.T. and K.Y. performed the analysis and interpretation of the data. Y.M., S.Q., and T.Y. drafted the manuscript. F.Y., Q.Z., Y.M., and W.Y. critically revised the manuscript for important intellectual content. Y.M., W.Y., and T.Y. performed the statistical analysis. F.Y., P.C. and Q.S. obtained the funding for the study. Z.H., F.Y., and Q.Z. provided administrative, technical, or material support. Z.H., F.Y., and Z.Q. supervised the project. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The experiments involved in this study were approved by the Ethics Committee of Peking University First Hospital with exemption from informed consent (2023-289-001).
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mulati, Y., Shen, Q., Tian, Y. et al. Characterizing PSMA heterogeneity in prostate cancer and identifying clinically actionable tumor associated antigens in PSMA negative cases. Sci Rep 15, 23902 (2025). https://doi.org/10.1038/s41598-025-06393-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-06393-z