Abstract
Advanced esophageal squamous cell carcinoma (ESCC) patients exhibit a ~ 50% objective response rate (ORR) and median progression-free survival (mPFS) of just 5–7 months when undergoing first-line immune-chemotherapeutic treatment, underscoring pronounced unmet clinical need. We assessed the efficacy and safety of Anlotinib plus Camrelizumab and chemotherapy for advanced, unresectable, or metastatic ESCC. This is an open-label, investigator-initiated, phase 2, non-randomized clinical trial enrolled patients from August 3, 2020, to August 10, 2022. Patients with treatment-naive unresectable stage III or IV ESCC received treatment which was patient-selected, including chemotherapy + camrelizumab + Anlotinib (TCAC group) or chemotherapy + Camrelizumab (TCC group) induction therapy for 4–6 cycles, followed by maintenance therapy. The primary endpoint was ORR, while secondary endpoints included mPFS, median overall survival (mOS), disease control rate (DCR), and treatment-related adverse events (TRAEs). 30 patients were included in each group. Over a median 14.5-month follow-up period, the ORR was 90.0%, 43.3%(P < 0 0.001) and the mPFS was 16.03, 7.30 months (HR 0.35, 95%CI, 0.19–0.65; P < 0 0.001) in TCAC and TCC groups, respectively. Grade 3 TRAEs were experienced by 12 patients (40.0%) in TCAC group, including decreased neutrophil counts (5 [16.7%]), decreased white blood cell counts (4 [13.3%]), reduced platelet counts (3 [10%]), and hypertension (2 [6.7%]). No patients experienced grade 4–5 TRAEs. The combination of Anlotinib plus Camrelizumab and chemotherapy had promising efficacy among patients with advanced ESCC in this study, which may be a promising first-line treatment regimen.
Trial registration: Registered with ClinicalTrials.gov, NCT04471480. 15/07/2020.
Similar content being viewed by others
Introduction
Esophageal cancer (EC) accounted for 604,100 cancer diagnoses and 544,076 deaths globally in 20201. Esophageal squamous cell carcinoma (ESCC) is the most common EC histological subtype, particularly in Southeast and East Asia2with survival rates reportedly worse than those for esophageal adenocarcinoma (EAC)3. The treatment of ESCC and EAC tumors is distinct due to significant differences in genomic characteristics, biological behaviors, and disease pathogenesis that distinguish these subtypes of EC.
Approximately 50% of patients with EC are identified at an advanced stage4. Historically, managing these patients with advanced or metastatic ESCC involved administering two cytotoxic chemotherapeutic agents. However, overall survival (OS) improvements, first-line regimens combined with anti-EGFR or other targeted therapies failed to provide survival benefits to these patients6. Recent phase III randomized controlled trials (RCTs) have demonstrated that immune checkpoint inhibitors (ICIs), particularly monoclonal anti-programmed cell death-1 (PD-1), can significantly improve ESCC patient outcomes while maintaining acceptable safety7,8,9,10,11,12. Therefore, following specific clinical guidelines, the present initial treatment approach for metastatic ESCC patients involves concurrently using ICIs and chemotherapeutic agents13,14. However, half to a quarter of patients fail to benefit from such first-line treatment, and promising second-line treatments are often unavailable. These patients may also experience progressive dysphagia resulting from tumor progression, resulting in worse nutritional status and an inability to tolerate second-line treatment.
Numerous investigations have been conducted to enhance the efficacy of combined immune checkpoint inhibitors (ICIs) and chemotherapeutic treatments for various cancers. Anti-angiogenic therapy has demonstrated encouraging synergistic effects with ICIs while ensuring tolerable safety. Adding bevacizumab (VEGFA blocking antibody) to first-line ICI-based regimens revolutionized advanced lung cancer patient treatment15 and similar efficacy has been reported in advanced EC. For example, a phase II clinical trial combining Camrelizumab (an ICI against PD-1) with Apatinib (a highly selective TKI targeting VEGFR2) and chemotherapy as first-line treatment for advanced ESCC observed improved objective response rate (ORR) and disease control rate (DCR) values of 80% and 96.7%, respectively16. Although studies indicate the potential for the treatment of ESCC patients with chemotherapy, ICIs, and anti-angiogenic drugs, it is essential to note that 90.0% of the patients encountered treatment-related adverse events (TRAEs) of grade 3–4, which raises significant safety concerns.
Anlotinib is a new type of oral medication that inhibits multiple kinases and is classified as a small-molecule TKI. According to a randomized, double-blind, multi-center research, anlotinib has been shown to achieve an ORR of 7.3% and increase progression-free survival (PFS) in patients with metastatic or recurrent ESCC17. First-line anlotinib plus TQB2450 (a novel humanized mAb against PD-L1) treatment without chemotherapy in advanced ESCC reportedly achieved promising ORRs and DCRs of 60.9% and 91.3%, respectively18. No studies to date have evaluated first-line advanced ESCC patients with treatment outcomes for anti-angiogenic drugs combined with ICIs and platinum-containing dual-drug chemotherapeutic regimens. In advanced ESCC patients, the current phase II non-randomized clinical trial (NCT04471480) was conducted to compare the safety and efficacy of chemotherapy plus Camrelizumab combined with anlotinib to chemotherapy plus Camrelizumab alone. This is the first prospective analysis of this first-line treatment regimen for advanced ESCC patients. It ultimately demonstrated that adding this anti-angiogenic small molecule to combined immunotherapy/chemotherapy regimens improved treatment outcomes, including PFS and ORRs, while protecting against adverse drug reactions.
Methods
Study design and participants
This investigator-initiated, single-center, prospective, open-label, phase II trial enrolled 18 to 80-year-old patients with treatment-naïve, histologically confirmed, locally advanced, unresectable, or metastatic ESCC measurable with the RECIST (v1.1) criteria. Unresectable Stage III includes cases with anatomical unresectability (inability to achieve complete resection) or severe comorbidities precluding tolerance for major surgery. Patients who met the eligibility criteria had a performance status of 0–1 on the Eastern Cooperative Oncology Group, a minimum expected life expectancy of 3 months, and satisfactory organ function assessed by laboratory tests and blood counts. Individuals who met the following criteria for exclusion were as follows: a clinical diagnosis of EAC, a documented history of autoimmunity, prior treatment with ICI, uncontrolled hypertension or clinically severe cardiovascular disease, active infections necessitating treatment, deep ulcer type or tumor invasion affecting large blood vessels, the trachea, or bronchus. Full eligibility criteria are listed in the study protocol (supplementary data). The Declaration of Helsinki and Good Clinical Practice guidelines were observed for this trial, which was approved by the Ethics Committee of Daping Hospital. The reference number was 2020(97), and all patients provided written informed consent.
Procedures
This is an investigator-initiated, non-randomized study. The researchers’ role is to present patients with a comprehensive overview of the treatment plan in the study. Subsequently, patients autonomously determine which treatment group to participate. As this study was initiated in 2019 when dual-drug chemotherapy was the standard treatment, three treatment groups were set, including chemotherapy + Camrelizumab + Anlotinib, chemotherapy + Camrelizumab, and chemotherapy only, and the chemotherapy group served as the control. During the research period, based on new research evidence10 and updated guidelines13immunotherapy combined with chemotherapy became the new standard treatment due to better efficacy. In order to improve patient outcomes, we modified the research protocol and concluded the recruitment of patients in the chemotherapy-alone group in February 2022. This paper will present the outcomes of the TCAC and TCC groups, where the TCC group serves as the control group. Preclinical studies have shown that low-dose (but not higher-dose) anti-VEGFR2 antibody treatment reprograms the tumor microenvironment from immune suppression to immune enhancement19. Therefore, the dose of Anlotinib used in this study was 8 mg rather than 12 mg. On day 1, TCAC group patients were treated with Camrelizumab (200 mg, i.v), 135–185 mg/m2 paclitaxel (i.v) or 240–260 mg/m2 albumin-bound paclitaxel (i.v), and 75 mg/m2 cisplatin (i.v) or carboplatin AUC 5 (i.v), with oral Anlotinib (8 mg, p.o) treatment on days 1–14 (drugs information are listed in the eTable 1). All treatments were repeated every 21 days for up to 4–6 cycles, followed by maintenance Camrelizumab and Anlotinib treatment every 3 weeks until progression. Patients in the TCC group received the identical treatment plan, except for Anlotinib, which was excluded. Each therapy was administered at intervals of 21 days for a maximum of 4–6 cycles. Subsequently, maintenance Camrelizumab treatment was given every 3 weeks until disease progression. Discontinuation of maintenance treatment in the TCAC and TCC groups occurred when there was illness progression, patient choice, or death. If the patient receives surgical treatment, the surgical date is the censore time of the patient’s PFS and OS according to the protocol.
Neither Camrelizumab nor Anlotinib doses could be reduced, but the physician had the discretion to temporarily or permanently stop Camrelizumab treatment if there were suspected immune-related adverse events. Similarly, Anlotinib may be stopped if there are signs of drug-induced uncontrolled hypertension or bleeding. Physicians were allowed to modify doses for chemotherapy drugs based on the patient’s condition, and the first cycle can start by reducing the dosage level of paclitaxel by one level, which is 135 mg/m2 paclitaxel or 240 mg/m2 albumin-bound paclitaxel .
Baseline computed tomography (CT) or magnetic resonance imaging scans were performed within 28 days before study initiation. CT scans were conducted every 2 cycles (cycles 1–6) and every 9–12 weeks after that until disease progression to monitor tumor responses as per RECIST 1.1 criteria.
Weekly laboratory tests (complete blood counts, blood chemistry) were performed, while routine tests of urine, fecal samples, coagulation function, thyroid function, and cardiac injury were conducted every 3 weeks. Electrocardiograms were performed if needed.
In order to compensate for the limitations of the research design, we used Dako 22C3 antibody to detect PD-L1 expression in the initial biopsy samples of patients after the completion of the study. This is in compliance with the original ethical review, and the informed consent form includes a clause for “future biomarker research”. The post-hoc testing of this study did not exceed the scope of consent and did not require additional ethical approval.
AEs were recorded 3 months after the end of treatment. Telephone-based follow-up was performed at 4-week intervals after therapy cessation until the time of death. The National Cancer Institute Common Terminology Criteria for Adverse Events (v5.0) was utilized to assess the severity of adverse events. All authors were granted access to the study data and thoroughly examined and endorsed the final manuscript.
Outcomes
Investigator-assess ORR which need to be confirmed by 3 investigators in order to count as a response was the primary study endpoint and was defined as the percentage of patients achieving complete response (CR) or partial response (PR) per RECIST v1.1 criteria. PFS, OS, DCR, duration of response (DOR), and safety are secondary endpoints. PFS is the interval from the initiation of study treatment to progression or all-cause mortality; DCR is the proportion of patients who achieve CR, PR, or stable disease; and OS is the interval from the initiation of study treatment to all-cause mortality. DOR is the time interval from response to disease progression or all-cause mortality. If the patient receives surgical treatment, the surgical date is the patient’s PFS and OS censor time.
Statistical analyses
Based on the NCT0360375616 and KEYNOTE59012 trials, assuming a true ORR of 90% in TCAC group, and the ORR of immunotherapy combined with chemotherapy is 45%, enrolling 21 patients in each group yielded at least 90% power to detect an improvement in ORR (two-sided α level = 0.05) from 45 to 90%. With a dropout rate of 30%, the intended enrollment for this study is 60 participants. The efficacy and safety analyses were conducted using the complete analysis set (FAS), which included all enrolled patients. The Newcombe-Wilson approach was employed to compute 95% confidence intervals (CIs) for the ORR, DOR, and DpR. The Kaplan-Meier method was used to estimate median PFS, OS, and DOR, with log-rank tests being used to calculate corresponding hazard ratios (HRs) and 95% CIs. Continuous variables were compared by Student’s t test. Categorical variables were compared by chi-square test or Fisher’s exact test.Given the non-randomized design of this study, we employed a logistic regression model to assess the association between treatment regimens and ORR, while applying a Cox proportional hazards regression model to analyze the relationship between treatment regimens and PFS, in order to control for potential confounding variables and minimize bias in the results. SPSS v23.0, GraphPad Prism v8.0.2 and R4.2.2 were used for analyses and figure construction, with p < 0.05 as the significance threshold.
Result
Study population and treatment
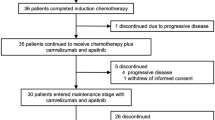
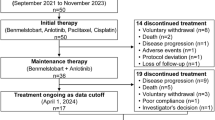
From August 3, 2020, to August 10, 2022, 60 patients were enrolled in the study, with 30 patients in the TCAC group and 30 in the TCC group. All 60 patients described previously had undergone at least one assessment of effectiveness and were included in the Full Analysis Set (FAS) and the safety sets (Fig. 1).
Patient flowchart. Patients were assigned to receive Camrelizumab plus Paclitaxel/Paclitaxel for Injection (Albumin Bound) plus Cisplatin/Carboplatin plus Anlotinib (TCAC group), or Camrelizumab plus Paclitaxel/Paclitaxel for Injection (Albumin Bound) plus Cisplatin/Carboplatin (TCC group), or paclitaxe/Paclitaxel for Injection (Albumin Bound) plus Cisplatin/Carboplatin (TC group); treatments were administered every 3 weeks for four or six cycles, after which the patients received maintenance therapy with Camrelizumab plus Anlotinib in TCAC group, Camrelizumab in TCC group. The enrollment of the TC group was terminated in February 2022, so we only report the results of the TCAC and TCC groups here. There were 10 patients in the TCAC group and 2 patients in the TCC group who underwent radical surgery due to successful conversion therapy and were censored on the date of the surgical date. In addition, 2 patients in the TCC group were lost to follow-up. We conducted ORR, PFS, and OS analyses of the FAS population. This figure depicts the efficacy and safety populations analyzed in this study, with data current through the cutoff date of April 12, 2025.
The baseline characteristics of the FAS are listed in Table 1. A standardized scoring scale (eTable 2) was used to determine the dysphagia level. All patients are treatment naive and have not received Concurrent radiotherapy. A total of 58 patients (96.7%) had discontinued the study treatment, while 2 patients were still receiving treatment in the TCAC group. In the TCAC (n = 10) and TCC (n = 2) groups, patients undergoing radical surgery after successful conversion therapy were censored at surgery. Among the 10 surgical patients in the TCAC group, 5 patients achieved pathologic complete response (pCR), which means the pCR rate was 50% (eTable 3). Initial surgical candidacy and final outcomes are detailed in eTable 4, with safety data for surgical patients provided in eTable 5. As of the April 12, 2025 data cutoff, the median follow-up duration was 14.5 months (range: 1.9–45.9 months).
Response to treatment
In the FAS population, the ORR was 90.0% (27 of 30;95% CI, 73.5–97.9%),43.3% (13 of 30;95% CI, 25.5–62.6%) in the TCAC, and TCC group, respectively(P < 0.001) (Fig. 2; Table 2). Logistic regression analysis revealed a significant protective effect of TCAC treatment regime on objective response rate, with robust associations observed in both univariate(P = 0.001) and multivariate analyses(P = 0.002) (eTable 6). A notable observation is that 10.0% of the patients in the TCAC group exhibited complete responses, while just 3.3% of the patients in the TCC group showed the same.
In terms of DpR, 15(50%) and 6(20%) patients achieved a reduction of over 50% in target lesions in TCAC and TCC groups, respectively (Table 2).
Progression-free survival
As of April 12, 2025, 16 (53.3%) and 27 (90.0%) had disease progression or deaths in the FAS of the TCAC and TCC groups, respectively (Fig. 1). The estimated median PFS was 16.03 months (95% CI, 9.49–22.58; 16 progression or deaths), 7.30 months (95% CI, 4.08–10.52; 27 progression or deaths), in TCAC and TCC groups, respectively (HR, 0.35; 95% CI, 0.19–0.65; P < 0.001) (Fig. 3A). The Cox proportional hazards regression model demonstrated that the TCAC treatment regimen exhibited a significant protective effect in univariable analysis (HR = 0.33, 95% CI: 0.18–0.63; P < 0.001) and remained statistically significant in the multivariable regression analysis incorporating all variables from eTable 7 (adjusted HR [aHR] = 0.39, 95% CI: 0.18–0.84; P = 0.016). Figure 4A demonstrates the progression-free survival in various subgroups of the FAS. It reveals that nearly all subgroups saw consistent advantages. Additionally, Fig. 4B presents a swimmer plot depicting the follow-up period among patients in the TCAC and TCC groups. In the FAS population, the mDOR was 14.30 months (95% CI, 10.03–18.57) in the TCAC group and 6.30 months (95% CI, 4.00-8.60) in the TCC group (HR, 0.37 [95% CI, 0.15–0.91]; P = 0 0.009) (Fig. 3B).The subsequent treatment information of progressed patients can be found in eTable 8.
Overall survival
A total of 37 of the 60 patients in FAS (61.7%) had died, including 15(50.0%) and 22(73.3%) deaths occurring among TCAC and TCC groups. The estimated median OS was 24.50 months (95% CI, 12.43–36.57) and 17.97 months(95% CI, 11.43–24.50) in TCAC and TCC groups, respectively(HR, 0.75; 95% CI, 0.39–1.43; P = 0.37) (Fig. 3C).
Safety
TRAEs observed in both groups are summarized in Table 3. All patients experienced at least one any-grade TRAEs (100% incidence). Grade 3 TRAEs occurred in 8 (26.7%) and 12 patients (40.0%) from the TCC and TCAC groups, respectively. The TCAC group demonstrated the following grade 3 TRAEs: decreased neutrophil count (5 [16.7%]), decreased white blood cell count (4 [13.3%]), thrombocytopenia (3 [10%]), lymphocytopenia (2 [6.7%]), and hypertension (2 [6.7%]). Among grade ≥ 3 events in TCAC, two hypertension cases lasted 3 and 7 days, one rash persisted for 6 days, and one proteinuria continued for 12 days, all resolving through symptomatic management (antihypertensive adjustment, topical therapy, and hydration) without dose reduction.Hematologic toxicities and anorexia/dysgeusia were generally transient. Management approaches incorporated growth factor support, single-level chemotherapy dose reduction(TCAC:3 cases; TCC:2 cases) and supportive care including antiemetics and hydration. Treatment discontinuation due to grade 3 TRAEs occurred in 3 patients from the TCAC and 2 patients in the TCC group.The incidence of reactive cutaneous-capillary endothelial proliferation (RCCEP) was 20% and 66.7% in TCAC and TCC groups, respectively. No Anlotinib related hemorrhage occurred. No grade 4–5 TRAEs occurred.
Discussion
ESCC is an aggressive cancer that is often first diagnosed at an advanced stage, contributing to high mortality rates20. This prospective, open-label, controlled phase II clinical research evaluated the safety and effectiveness of combining immune-chemotherapy regimens with Anlotinib as the initial treatment for advanced ESCC. This study met its primary endpoint with an ORR of 90.0% and 10.0% of patients achieving the best response of CR compared to ORRs of 43.3% in the chemo-immunotherapy group and adding Anlotinib also significantly prolonged mPFS from 7.30 to 16.03 months, suggesting that Anlotinib combined with immune-chemotherapy can synergistically treat advanced ESCC. Furthermore, no new safety signals or unmanageable safety profiles lead to death in the TCAC group. These results highlight the promise of this regimen as a first-line treatment for advanced ESCC.
Since pembrolizumab was first approved as a first-line treatment for advanced ESCC12several PD-1 inhibitor/chemotherapy combinations have shown promising efficacy and manageable toxicity in phase III trials10,11,12,21,22,23,24such that ICI plus chemotherapy combinations are the new first-line standard of care for ESCC13,14. Chemo-immunotherapy treatment has been tied to modest 10.0-21.1% increases in ORRs relative to chemotherapy alone10,11,12,21,22,23,24. The ORR in the TCC group in this trial was consistently only 43.3%, indicating that most patients with advanced ESCC show primary or acquired resistance to ICI-based treatment. Despite studies focusing on tumor cell-intrinsic and -extrinsic signatures, no conclusive biomarkers identifying ESCC patients likely to respond to immunotherapy have been found, possibly due to sample size limitations and the intrinsic spatiotemporal heterogeneity of tumor immune microenvironment25,26,27. Therefore, treatment combinations based on ICI must be improved to overcome primary resistance. Only then can predictive biomarkers be successfully utilized in clinical practice.
Angiogenesis provides nutrients, oxygen, growth factors, and avenues of metastasis that support tumor growth28,29prompting efforts to inhibit VEGF and VEGFR to suppress cancer progession30,31,32. In previously treated advanced ESCC patients, the VEGFR inhibitors Apatinib and Anlotinib exhibited similar ORRs (7.3–7.5%) and DCRs, with manageable safety profiles17. While combining Apatinib and chemo-immunotherapy for first-line advanced ESCC treatment was associated with an ORR of 80% in a phase II trial, 90.0% of patients experienced grade 3–4 TRAEs16. The synergistic efficacy of immunotherapy + Anlotinib in the first-line treatment of esophageal squamous cell carcinoma has been demonstrated in clinical trials, leading to the testing of this combination for the first time18,33,34,35,36,37,38,39. Anlotinib increased the ORR to 90.0%, more than 2-fold higher than that for the control group, with a corresponding 2-fold increase in deep responders (> 50% DpR) when comparing the TCAC and TCC groups, consistent with Anlotinib-mediated reductions in tumor burden. Anlotinib may effectively treat lymph node metastasis, which is the primary ___location of ESCC metastasis and accounts for over 80% of all target lesions. More profound responses are often related to better treatment results40,41implying that response depth may be a feasible surrogate endpoint with more investigation into the ideal cutoff value. The TCAC group experienced a significant reduction in tumor burden, leading to higher rates of successful surgery. All 10 patients who underwent surgery achieved R0 resection and confirmed a 50% pathological complete response rate. This highlights the potential for conducting additional studies on this treatment regimen in the neoadjuvant or conversion setting for earlier-stage ESCC.
In contrast to other trials of targeted therapies, a significant 10.76-month improvement in mPFS was observed when Anlotinib was combined with chemo-immunotherapy (16.03 vs. 7.30 months; HR: 0.35) in the FAS population. Owing to the short (generally < 4-month) interval between inclusion and surgery for all patients who underwent esophagectomy, these patients did not contribute to the mPFS of the TCAC group. In addition, censoring more patients who underwent surgery based on successful conversion therapy may lead to underestimation of mPFS in the TCAC group. While Anlotinib and Apatinib are both VEGFR2 inhibitors, Anlotinib use in this trial was associated with better PFS than prior trials using Apatinib, potentially owing to its ability to inhibit other receptor tyrosine kinases, including VEGFR1-4, PDGFR α/β, fibroblast growth factor receptor (FGFR) c-kit, etc42,43,44,45.Although the overall survival difference did not achieve statistical significance (24.50 vs. 17.97 months), the clinically meaningful survival advantage of over 6 months warrants attention. The observed lack of statistical significance likely stems from the study design, which prioritized sample size calculation based on the primary endpoint of ORR. This approach resulted in a small cohort and limited statistical power (16%). Using the Schoenfeld formula with an assumed HR of 0.7, a confirmatory Phase III trial would require 494 participants (247 per group) to achieve the conventional 80% statistical power.
Although the TCAC group demonstrated a higher incidence of grade ≥ 3 TRAEs compared to the TCC group (40.0% vs. 26.7%), the rates of treatment discontinuation due to adverse events were comparable between the two groups (TCAC: 10% vs. TCC: 6.7%), underscoring the critical role of close monitoring and early interventions (e.g., dose adjustments, supportive care) in maintaining adherence to higher-toxicity regimens. Notably, Zhang et al.16 reported a grade ≥ 3 TRAEs rate of 90% in a regimen combining anti-angiogenic small-molecule TKI, immune checkpoint inhibition, and taxane/platinum-based chemotherapy, which is substantially higher than the 40% observed in our TCAC group. We speculate that this discrepancy may be linked to the high inhibitory potency of Anlotinib against VEGFR2 (IC50: 0.2 nmol/L)41,46 and the use of a lower Anlotinib dose in our study, which may more conducive to immune activation and reduced toxicity19.The improved ORR and prolonged mPFS observed in the TCAC group may be attributed to the optimized dosing strategy of Anlotinib, which appears to synergistically reduce toxicity while enhancing therapeutic efficacy. Furthermore, the incidence of RCCEP47 differed markedly between the TCC and TCAC groups (66.7% vs. 20%), suggesting that Anlotinib and Apatinib, both VEGFR2-targeted agents, may share similar efficacy in mitigating Camrelizumab-associated RCCEP, potentially through overlapping mechanisms of VEGF pathway modulation. These findings highlight the importance of dose optimization and pharmacodynamically tailored combinations to improve the safety profiles of novel therapeutic regimens while preserving efficacy.
There are several advantages in ethics and scientific designs in our research.Firstly, as an IIT study, we fully respect the patient’s willingness to choose the treatment group. Secondly, we timely updated the protocol and terminated the chemotherapy group which has been proven to have poorer efficacy by the latest research, which minimizes the damage to the patient’s treatment effect. Thirdly, we chose a low-dose Anlotinib of 8 mg, which not only avoids the side effects of higher doses, but also benefits the synergistic effect of Anlotinib with ICIs. Lastly, given that patients with esophageal cancer have poorer nutritional status, physicians can lower the dosage level of chemotherapy drugs by one dose level at the first use of medication based on the patient’s condition. Therefore, the rate of grade 3 TRAEs in the TCC arm was just 26.7%, which was 47% in Checmate 648 and 63% in ESCORT-1st.
Limitation
This study is limited by its non-randomized controlled design. Although we have employed multivariable logistic regression models and multivariable Cox proportional hazards regression models to further confirm that the TCAC regimen is an independent protective factor for ORR and PFS, residual confounding inherent to non-randomized studies cannot be fully ruled out, such as unmeasured socioeconomic factors or treatment preferences.
Conclusions
The current trial concluded that, for patients with ESCC who are incurable and non-biomarker screened, adding Anlotinib to Camrelizumab plus chemotherapy was linked with a manageable safety profile and promising efficacy. Conducting more RCT-based validation for this possible first-line treatment plan is necessary.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Sung, H. et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71 (3), 209–249 (2021).
Arnold, M. et al. Global incidence of oesophageal cancer by histological subtype in 2012. Gut 64 (3), 381–387 (2015).
Morgan, E. et al. International trends in oesophageal cancer survival by histological subtype between 1995 and 2014. Gut 70 (2), 234–242 (2021).
Ku, G. Y. Systemic therapy for esophageal cancer: chemotherapy. Chin. Clin. Oncol. 6 (5), 49 (2017).
Ajani, J. A. et al. Esophageal and esophagogastric junction cancers, version 2.2019, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Canc Netw. 17 (7), 855–883 (2019).
Moehler, M. et al. Cisplatin and 5-fluorouracil with or without epidermal growth factor receptor Inhibition panitumumab for patients with non-resectable, advanced or metastatic oesophageal squamous cell cancer: a prospective, open-label, randomised phase III AIO/EORTC trial (POWER). Ann. Oncol. 31 (2), 228–235 (2020).
Kojima, T. et al. KEYNOTE-181 investigators. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal Cancer. J. Clin. Oncol. 38 (35), 4138–4148 (2020).
Kato, K. et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 20 (11), 1506–1517 (2019).
Shen, L. et al. RATIONALE-302 investigators. Tislelizumab versus chemotherapy as Second-Line treatment for advanced or metastatic esophageal squamous cell carcinoma (RATIONALE-302): A randomized phase III study. J. Clin. Oncol. 40 (26), 3065–3076 (2022).
Luo, H. et al. ESCORT-1st investigators. Effect of camrelizumab vs placebo added to chemotherapy on survival and Progression-Free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: the ESCORT-1st randomized clinical trial. JAMA 326 (10), 916–925 (2021).
Doki, Y. et al. CheckMate 648 trial investigators. Nivolumab combination therapy in advanced esophageal Squamous-Cell carcinoma. N Engl. J. Med. 386 (5), 449–462 (2022).
Sun, J. M. et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet 398 (10302), 759–771 (2021).
NCCN Guidelines for Patients® Esophageal Cancer, (2022).
Obermannová, R. et al. ESMO guidelines committee. Electronic address: [email protected]. Oesophageal cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann. Oncol. 33 (10), 992–1004 (2022).
Lam, T. C. et al. Combination atezolizumab, bevacizumab, pemetrexed and carboplatin for metastatic EGFR mutated NSCLC after TKI failure. Lung Cancer. 159, 18–26 (2021).
Zhang, B. et al. Phase II clinical trial using camrelizumab combined with apatinib and chemotherapy as the first-line treatment of advanced esophageal squamous cell carcinoma. Cancer Commun. (Lond). 40 (12), 711–720 (2020).
Huang, J. et al. Anlotinib for previously treated advanced or metastatic esophageal squamous cell carcinoma: A double-blind randomized phase 2 trial. Cancer Med. 10 (5), 1681–1689 (2021).
Zhang, Z. et al. Updated results of anlotinib combined with TQB2450 (PD-L1 blockade) as first-line treatment for advanced esophageal squamous cell carcinoma (ESCC): A single-arm, multicenter, open-label phase II clinical trial. J. Clin. Oncol. 41 16_suppl, 4041–4041. (2023).
Huang, Y. H. et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc. Natl. Acad. Sci. U. S. A. 1090 (2012).
Abnet, C. C., Arnold, M. & Wei, W. Q. Epidemiology of esophageal squamous cell carcinoma. Gastroenterology 154 (2), 360–373. https://doi.org/10.1053/j.gastro.2017.08.023 (2018).
Wang, Z. X. et al. Toripalimab plus chemotherapy in treatment-naïve, advanced esophageal squamous cell carcinoma (JUPITER-06): A multi-center phase 3 trial. Cancer Cell. 40 (3), 277–288e3 (2022).
Song, Y. et al. ASTRUM-007 investigators. First-line Serplulimab or placebo plus chemotherapy in PD-L1-positive esophageal squamous cell carcinoma: a randomized, double-blind phase 3 trial. Nat. Med. 29 (2), 473–482 (2023).
Lu, Z. et al. ORIENT-15 study group. Sintilimab versus placebo in combination with chemotherapy as first line treatment for locally advanced or metastatic oesophageal squamous cell carcinoma (ORIENT-15): multicentre, randomised, double blind, phase 3 trial. BMJ 377, e068714 (2022).
Xu, J. et al. Tislelizumab plus chemotherapy versus placebo plus chemotherapy as first-line treatment for advanced or metastatic oesophageal squamous cell carcinoma (RATIONALE-306): a global, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 24 (5), 483–495 (2023).
Jia, Q. et al. Heterogeneity of the tumor immune microenvironment and its clinical relevance. Exp. Hematol. Oncol. 11 (1), 24 (2022).
Hudson, W. H. & Wieland, A. Technology Meets tils: Deciphering T cell function in the -omics era. Cancer Cell. 41 (1), 41–57 (2023).
Xue, R. et al. Liver tumour immune microenvironment subtypes and neutrophil heterogeneity. Nature 612 (7938), 141–147 (2022).
Qin, S. et al. Recent advances on anti-angiogenesis receptor tyrosine kinase inhibitors in cancer therapy. J. Hematol. Oncol. 12 (1), 27 (2019).
Goel, H. L. & Mercurio, A. M. VEGF targets the tumour cell. Nat. Rev. Cancer. 13 (12), 871–882 (2013).
Zhao, M. et al. Molecularly imprinted nanomedicine for Anti-angiogenic Cancer therapy via blocking vascular endothelial growth factor signaling. Nano Lett. 23 (18), 8674–8682 (2023).
Lee, S. et al. Phase II study of ramucirumab in advanced biliary tract Cancer previously treated by Gemcitabine-Based chemotherapy. Clin. Cancer Res. 28 (11), 2229–2236 (2022).
Herbst, R. S. et al. Ramucirumab plus pembrolizumab in patients with previously treated advanced non-small-cell lung cancer, gastro-oesophageal cancer, or urothelial carcinomas (JVDF): a multicohort, non-randomised, open-label, phase 1a/b trial. Lancet Oncol. 20 (8), 1109–1123 (2019).
Xu, Q. et al. Efficacy and safety of sintilimab plus anlotinib for PD-L1-Positive recurrent or metastatic cervical cancer: A multicenter, Single-Arm, prospective phase II trial. J. Clin. Oncol. 40 (16), 1795–1805 (2022).
Liu, J. et al. Phase II study of TQB2450, a novel PD-L1 antibody, in combination with anlotinib in patients with locally advanced or metastatic soft tissue sarcoma. Clin. Cancer Res. 28 (16), 3473–3479 (2022).
Su, Y. et al. Anlotinib induces a T Cell-Inflamed tumor microenvironment by facilitating vessel normalization and enhances the efficacy of PD-1 checkpoint Blockade in neuroblastoma. Clin. Cancer Res. 28 (4), 793–809 (2022).
Tian, L. et al. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature 544 (7649), 250–254 (2017).
Liu, S. et al. Anlotinib alters tumor immune microenvironment by downregulating PD-L1 expression on vascular endothelial cells. Cell. Death Dis. 11 (5), 309 (2020).
Fukumura, D. et al. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat. Rev. Clin. Oncol. 15 (5), 325–340 (2018).
Yuan, M. et al. Anlotinib enhances the antitumor activity of High-Dose irradiation combined with Anti-PD-L1 by potentiating the tumor immune microenvironment in murine lung Cancer. Oxid. Med. Cell. Longev. 2022, 5479491 (2022).
Morgensztern, D. et al. Association between depth of response and survival in patients with advanced-stage non-small cell lung cancer treated with first-line chemotherapy. Cancer 125 (14), 2394–2399 (2019).
Saad, E. D. & Buyse, M. Exploratory analysis of the association of depth of response and survival in patients with metastatic non-small-cell lung cancer treated with a targeted therapy or immunotherapy. Ann. Oncol. 28 (11), 2629–2630 (2017).
Shen, G. et al. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J. Hematol. Oncol. 11 (1), 120 (2018).
Xie, C. et al. Preclinical characterization of anlotinib, a highly potent and selective vascular endothelial growth factor receptor-2 inhibitor. Cancer Sci. 109 (4), 1207–1219 (2018).
Lin, B. et al. Anlotinib inhibits angiogenesis via suppressing the activation of VEGFR2, PDGFRβ and FGFR1. Gene 654, 77–86 (2018).
Ooki, A. et al. Potent molecular-targeted therapies for advanced esophageal squamous cell carcinoma. Ther. Adv. Med. Oncol. 15, 17588359221138377 (2023).
Tian, S. et al. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci. 102 (7), 1374–1380 (2011).
Xu, B. & Sun, H. C. Camrelizumab: an investigational agent for hepatocellular carcinoma. Expert Opin. Investig. Drugs. 31 (4), 337–346 (2022).
Acknowledgements
We would like to thank the patients for their participation and commitment to clinical research. We also thank Beijing Bethune Charitable Foundation for providing anlotinib to the participants for free.
Funding
This work was supported by National Natural Science Foundation of China (NSFC, No. 82002443), Fund of Science-Health Joint Medical Scientific Research Project of Chongqing (CQMHC, No. 2021MSXM009).
Author information
Authors and Affiliations
Contributions
Mingfang Xu: Conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, software, supervision, validation, visualization, writing-original draft, writing- review & editing. Yu Pu: Data curation, formal analysis, investigation. Yuzhu Jiang: Data curation, formal analysis, investigation. Yingda Liu: Data curation, formal analysis, investigation, software validation, visualization. Yan Feng: Data curation and investigation. Xiaodong Zhao: Conceptualization, methodology, supervision, writing-review and editing. Mengxia Li: Conceptualization, writing-review and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xu, M., Pu, Y., Jiang, Y. et al. Anlotinib plus camrelizumab and chemotherapy as first-line treatment in patients with advanced esophageal squamous cell carcinoma. Sci Rep 15, 22275 (2025). https://doi.org/10.1038/s41598-025-06625-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-06625-2