Abstract
Estimated pulse wave velocity (ePWV) has been proposed as a potential substitute for carotid-femoral pulse wave velocity (cfPWV), serving as an indicator for assessing aortic stiffness. Arterial stiffness has emerged as a potential marker associated with adverse outcomes in various specific diseases, yet its relationship with mortality rates in the general adult population remains unstudied. This study aims to investigate the association between arterial stiffness and both all-cause and cardiovascular mortality among US adults. Data from 48,257 participants aged 20 and older in the National Health and Nutrition Examination Survey (NHANES) from 1999 to 2018 were analyzed. Mortality details were obtained from the National Death Index (NDI). Restricted cubic spline (RCS) functions were used to visualize the association between estimated pulse wave velocity (ePWV) and mortality risk. Weighted Cox proportional hazards models were employed to assess the independent correlation between ePWV and mortality risk. Time-dependent receiver operating characteristic (ROC) curve analysis was conducted to evaluate the predictive ability of ePWV for survival. Further subgroup analyses were performed to validate the robustness of the associations. Participants were stratified into higher (> 10.92) and lower (≤ 10.92) ePWV groups. During a median follow-up of 133.69 ± 94.42 months, 8029 (16.6%) deaths, including 2641 (5.5%) cardiovascular deaths, occurred among the 48,257 participants. The weighted Cox proportional hazards model showed that after comprehensive adjustment for covariates, individuals with higher ePWV had significantly increased risks of all-cause mortality (HR 2.67, 95% confidence interval [CI] 2.50–2.84, P < 0.001) and cardiovascular mortality (HR 2.75, 95%CI 2.46–3.07, P < 0.001). RCS regression analysis revealed a nonlinear association between ePWV, a marker of arterial stiffness, and all-cause mortality with an inflection point at 8.267 (P for nonlinear = 0.0001), while a positive linear correlation was observed with cardiovascular mortality (P for nonlinear = 0.889). This association was consistent across subgroups based on age, gender, race, body mass index, education level, marital status, smoking, alcohol consumption, diabetes, and hypertension, with significant interactions observed for all-cause mortality in the hypertension subgroup (P for interaction = 0.012) and for cardiovascular mortality in smoking (P for interaction = 0.032), diabetes (P for interaction < 0.001), and hypertension subgroups (P for interaction = 0.012). The time-dependent ROC curves indicated areas under the curve (AUCs) of 0.73, 0.80, and 0.79 for 1-year, 6-year, and 10-year survival rates, respectively, for all-cause mortality, and 0.85, 0.83, and 0.83 for cardiovascular mortality. Elevated ePWV is independently associated with increased cardiovascular mortality in US adults and exhibits a significant positive correlation with all-cause mortality in US adults beyond an ePWV threshold of 8.267.
Similar content being viewed by others
Introduction
Arterial stiffness, characterized by the loss of elasticity in the arterial walls, is a hallmark of vascular aging and a significant risk factor for cardiovascular events1,2,3. As arteries stiffen, their ability to buffer the pulsatile flow of blood diminishes, leading to increased systolic blood pressure, pulse pressure, and cardiac after-load4,5. This condition has been linked to a variety of adverse health outcomes, including hypertension6, heart failure7, and stroke8. Over recent decades, the prevalence of arterial stiffness has risen due to aging populations and the increasing burden of cardiovascular risk factors such as obesity, diabetes, and hyperlipidemia9,10. Arterial stiffness is typically measured using carotid-femoral pulse wave velocity (cfPWV), the gold standard method11. However, alternative approaches, including estimated pulse wave velocity (ePWV), have been developed to assess arterial stiffness more conveniently in large-scale epidemiological studies12,13. The clinical significance of arterial stiffness lies in its strong predictive value for cardiovascular events and mortality, making it a crucial focus for both preventive and therapeutic strategies.
Estimated pulse wave velocity (ePWV) has emerged as a valuable tool for assessing arterial stiffness in clinical practice and research settings14. Unlike cfPWV, which requires specialized equipment and technical expertise, ePWV can be derived from routinely collected clinical data such as age and blood pressure. Recent studies have demonstrated that ePWV is strongly associated with traditional cfPWV and provides comparable predictive accuracy for cardiovascular events15. Moreover, ePWV has been shown to be a reliable indicator of subclinical organ damage, such as left ventricular hypertrophy16 and chronic kidney disease17. The growing body of evidence linking ePWV to adverse clinical outcomes highlights its potential utility in risk stratification and management of patients with cardiovascular disease.
Despite these advances, there remains a critical need to further explore the relationship between ePWV and mortality risk in the general population, particularly in the United States. The U.S. population is characterized by significant diversity in terms of age, ethnicity, and socioeconomic status, which may influence the association between arterial stiffness and health outcomes. Understanding the prognostic value of ePWV in predicting all-cause and cardiovascular mortality across this heterogeneous population could provide valuable insights into the broader implications of arterial stiffness as a public health concern. Therefore, we conducted this study to investigate the relationship between ePWV and the risk of all-cause and cardiovascular mortality in the general adult population, in a large, nationally representative sample of patients.
Methods
Study design and population
NHANES (National Health and Nutrition Examination Survey) is a program conducted by the Centers for Disease Control and Prevention (CDC) in the United States. NHANES is a nationally representative survey designed to assess the health and nutritional status of adults and children in the U.S. Data for NHANES are collected through interviews and physical examinations conducted by trained health professionals. Participants are selected using a complex, stratified, multi-stage probability cluster sampling design to ensure the data accurately represents the entire U.S. population. The survey received ethical approval from the National Center for Health Statistics (NCHS) Ethics Review Board and obtained informed consent from all adult participants.
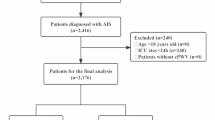
This study was classified as exempt by the Institutional Review Board (IRB) of our institution. Data for this study were obtained from ten cycles of NHANES (1999–2018), including a total of 48,257 participants (Fig. 1). We included eligible participants aged 20 years or older. Participants without complete survival or laboratory examination data, as well as pregnant individuals, were excluded from the analysis.
Ascertainment of mortality and followup
The source of mortality information was extracted from the National Death Index (NDI) database (https://www.cdc.gov/nchs/data-linkage/mortality-public.htm) of the CDC. The follow-up time for each individual was adopted from the time of participation to the date of death or until December 31, 2019 (the last update date of the NDI database). The International Statistical Classification of Diseases, 10th Revision (ICD-10) codes were used to identify cardiovascular deaths (I00-I09, I11, I13 and I20-I51)18.
Calculation of estimated pulse wave velocity
The ePWV is calculated using the following formula (8). ePWV = 9.587–0.402 × age + 4.560 × 10− 3 ×age2 − 2.621 × 10− 5 ×age2 ×mean blood pressure(MBP) + 3.176 10− 3 ×age×MBP − 1.832 × 10− 2×MBP19,20. In this formula, age is expressed in years, and mean blood pressure (MBP) is calculated as diastolic blood pressure (DBP) + 0.4 × [systolic blood pressure (SBP) - DBP]. Participants were seated quietly for 5 min before trained examiners measured blood pressure using a standardized sphygmomanometer. The blood pressure values were the average of at least three measurements. The technique for measuring blood pressure followed the latest recommendations for blood pressure measurement from the American Heart Association. For detailed information on quality assurance and quality control processes, please refer to the physician section of the MEC Operations Manual21.
Covariates
Age, gender, race, BMI, smoking status, drinking status, and hypertension were obtained from the demographic and health questionnaires of the NHANES survey. Age was treated as a continuous variable, while gender and race were categorized. Race was classified into four groups: non-Hispanic Black, non-Hispanic White, Mexican American, and other races. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared (kg/m²) and categorized as normal (< 24.9 kg/m²), overweight (24.9 ≤ BMI < 30 kg/m²), and obese (≥ 30 kg/m²).
Smoking status was divided into three categories: never smokers (defined as having smoked fewer than 100 cigarettes in their lifetime), former smokers (smoked more than 100 cigarettes but are now completely non-smokers), and current smokers (smoked more than 100 cigarettes and currently smoke every day or on some days)22. Drinking status was categorized into never drinkers (defined as having consumed alcohol fewer than 12 times in their lifetime), current drinkers (defined as having consumed alcohol ≥ 12 times in the past year or not drinking in the past year but having consumed alcohol ≥ 12 times in their lifetime), light drinkers (defined as an average of ≤ 1 drink per day for women and ≤ 2 drinks per day for men over the past 12 months), moderate drinkers (defined as 1–3 drinks per day for women and 2–4 drinks per day for men over the past 12 months), and heavy drinkers (defined as ≥ 4 drinks per day for women and ≥ 5 drinks per day for men over the past 12 months)23. Hypertension was defined as a self-reported history of hypertension, use of antihypertensive medications, an average systolic blood pressure ≥ 140 mmHg, and/or an average diastolic blood pressure ≥ 90 mmHg.
Laboratory data included white blood cell count, hemoglobin, red cell distribution width, platelet count, albumin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), total calcium, iron, sodium, potassium, globulin, blood urea nitrogen, serum cholesterol, glucose, triglycerides, uric acid, LDL-cholesterol, and HDL-cholesterol. Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) Scr equation24. The ratio of family income to poverty level was treated as a continuous variable. Educational level was categorized into four groups: college or above, some college, high school graduate or equivalent, and below high school.
Statistical analysis
We conducted our analysis in accordance with NHANES analytical and reporting guidelines25, accounting for the complex sampling design and sampling weights. Sampling weights were calculated as follows: fasting subsample 14-year mobile examination center (MEC) weight = fasting subsample 2-year MEC weight /10. Continuous variables were summarized as weighted means, while categorical variables were reported as weighted percentages. Differences between two groups for continuous variables were assessed using the Student’s t-test or Mann-Whitney U test, as appropriate. Categorical data differences were evaluated using the Chi-square test. Survival probabilities were estimated using the Kaplan-Meier method and compared with the log-rank test. Multiple imputations were performed for covariates with missing values.
The optimal ePWV cutoff point, which exhibited the most significant association with survival outcomes, was determined using maximally selected rank statistics from the ‘maxstat’ package (https://cran.r-project.org/web/packages/maxstat)26,27. This cutoff was used to classify participants into higher- and lower-ePWV groups.
Feature selection is a critical step in model development. In this study, we employed the Boruta algorithm, a supervised classification feature selection method, to accurately identify all relevant features. The Boruta algorithm was implemented using the R Boruta package with a random seed value of 2023 to ensure reproducibility of the feature selection process. This seed controls the randomization of shadow feature generation and Random Forest iterations. To assess the independent predictive value of estimated pulse wave velocity (ePWV) for mortality, we used multivariable Cox proportional hazards regression models. Three models were constructed to control for confounders: Model 1 was unadjusted, Model 2 adjusted for gender and race, and Model 3 adjusted for all-cause and cardiovascular mortality separately, based on the specific results of the Boruta algorithm. The variables included in Model 3 were: gender, race, education level, marital status, smoking status, alcohol use, hypertension, diabetes, hypercholesterolemia, arthritis, congestive heart failure, coronary heart disease, stroke, thyroid disease, cancer, weak/failing kidneys, family income-to-poverty ratio, body mass index, waist circumference, and various laboratory measures (white blood cell count, hemoglobin, red cell distribution width, platelet count, albumin, alanine aminotransferase, aspartate aminotransferase, total calcium, iron, sodium, potassium, globulin, blood urea nitrogen, serum cholesterol, glucose, triglycerides, uric acid, LDL-cholesterol, and HDL-cholesterol). Given the multiple comparisons that were made when running multiple models, it is suggested to perform the Bonferroni correction on the significance p value.
To explore the relationship between ePWV and mortality, we utilized a Cox proportional hazards regression model with restricted cubic splines (RCS). In this model, we adjusted for the same covariates as in Model 3. If a nonlinear relationship was detected, we estimated the threshold and identified the inflection point with the highest likelihood. Additionally, two-segment Cox proportional hazards models were used to examine the association between ePWV and mortality risk on either side of the inflection point.
Subgroup analyses were performed to evaluate the effect of ePWV on all-cause and cardiovascular mortality across various subgroups stratified by age (< 60 years and ≥ 60 years), gender, race (Mexican American, Other Race, Non-Hispanic White, Non-Hispanic Black), BMI (underweight, normal, overweight, obese), smoking status (yes/no), alcohol use (yes/no), educational level (below high school, high school, some college, college or above), hypertension (yes/no), diabetes (yes/no), and marital status (married/living with partner; widowed/divorced/separated/never married). A p-value < 0.05 was considered statistically significant.
Time-dependent receiver operating characteristic (ROC) curve analysis28 was conducted to assess the accuracy of ePWV in predicting survival outcomes at various time points using the ‘time-ROC’ package. Data were analyzed using R Statistical Software, version 4.3.3 (http://www.r-project.org). A two-tailed p-value < 0.05 indicated statistical significance.
Results
Characteristics of the study population
A total of 48,257 participants aged 20 years and older were recruited for this study. Using the optimal ePWV cutoff value of 10.92, which was determined based on the maximal selected rank statistics associated with the most significant correlation with survival time, participants were divided into a higher ePWV group (ePWV ≥ 10.92, n = 10,091) and a lower ePWV group (ePWV < 10.92, n = 38,166) (Fig. 2). Compared to the lower ePWV group, subjects in the higher ePWV group were older, had a higher proportion of Non-Hispanic Whites, a lower level of education, and a higher percentage of those who were widowed, divorced, separated, or never married.
In terms of comorbidities, the higher ePWV group had significantly higher proportions of hypertension, hypercholesterolemia, diabetes, arthritis, coronary heart disease, stroke, chronic bronchitis/emphysema, thyroid disease, cancer, and weak/failing kidneys. Regarding laboratory results, participants in the higher ePWV group had lower levels of WBC, RBC, hemoglobin, platelet count, albumin, ALT, AST, iron, HDL-cholesterol, and eGFR compared to the lower ePWV group. However, both all-cause mortality and cardiovascular mortality were higher in the higher ePWV group than in the lower ePWV group. Additional characteristics of the participants are presented in Table 1.
Feature selection
The results of feature selection based on the Boruta algorithm are presented in Fig. 3. After 500 iterations, one variable (asthma) was excluded for its weak association with all-cause mortality, ranked by Z-scores (see Fig. 4A and B, and Supplementary Table 1). Four variables that were not closely associated with cardiovascular mortality included liver disease, chronic bronchitis/emphysema, marital status, and asthma (see Fig. 4C and D, and Supplementary Table 2). Therefore, the variables included in our analysis were: gender, race, education level, marital status, smoking, alcohol use, hypertension, diabetes, hypercholesterolemia, arthritis, congestive heart failure, coronary heart disease, stroke, thyroid disease, cancer, weak/failing kidneys, family income-to-poverty ratio, body mass index, waist circumference, white blood cell count, hemoglobin, red cell distribution width, platelet count, albumin, alanine aminotransferase, aspartate aminotransferase, total calcium, iron, sodium, potassium, globulin, blood urea nitrogen, serum cholesterol, glucose, triglycerides, uric acid, LDL-cholesterol, and HDL-cholesterol. The Boruta algorithm results for all-cause and cardiovascular mortality are detailed in Supplementary Tables 2 and 3, respectively. Based on the Fig. 4E and F depicting the results from the Boruta algorithm for all-cause and cardiovascular mortality. The bar chart illustrates the importance of various variables in predicting mortality. The mean importance scores indicate that the most significant variable is ePWV, with a markedly higher score compared to other variables.
Panels (A) and (B) illustrate the feature selection process for all-cause mortality based on the Borutaalgorithm and the evolution of Z-scores during the screening process, respectively. Panels (C) and (D) depict thefeature selection process for cardiovascular mortality and the corresponding Z-score evolution during screening.In Panels (A) and (C), the horizontal axis represents the variable names, while the vertical axis indicates theZ-scores for each variable. In Panels (B) and (D), the horizontal axis shows the number of iterations, and the verticalaxis reflects the changes in Z-scores during the screening process. Blue boxes and lines correspond to theminimum, mean, and maximum Z-scores of shadow features. Green boxes and lines represent variablesconfirmed by the model, while red boxes and lines indicate variables that were rejected.Panels (E) and (F) display the importance of various variables in predicting all-cause and cardiovascular mortality.
Associations of the ePWV with allcause mortality
During a mean follow-up period of 133.69 ± 94.42 months, 8029 (16.64%) of the 48,257 participants died, with 2,641 (5.47%) deaths attributed to cardiovascular disease. In Model 1, the risk of all-cause mortality increased significantly with higher ePWV values (HR 1.38, 95%CI 1.37–1.40, P < 0.001) (Tables 2, 3). After multivariable adjustment, each 1-unit increase in ePWV was associated with a 10.9% increase in the risk of all-cause mortality (Model 2, HR 1.11, 95%CI 1.08–1.14, P < 0.001) and a 26.3% increase (Model 3, HR 1.26, 95%CI 1.25–1.28, P < 0.001) (Table 2).
Kaplan-Meier curves demonstrated a significantly lower survival rate in the higher ePWV group compared to the lower ePWV group (Log-rank P < 0.001) (Fig. 3A). Weighted multivariate Cox regression analysis revealed that the risk of all-cause mortality was significantly increased in the higher ePWV group, even after full adjustment for covariates (HR 2.67, 95%CI 2.50–2.84, P < 0.001) (Table 2).
Restricted cubic spline (RCS) analysis showed a nonlinear relationship between ePWV and all-cause mortality (P for nonlinearity = 0.0001) (Fig. 5A). Segmented Cox regression analysis identified an inflection point at 8.267, below which ePWV was negatively associated with all-cause mortality (Table 4). For each 1-unit increase in ePWV below this threshold, all-cause mortality decreased by 18.4% (HR 0.82, 95%CI 0.77–0.87, P < 0.001). However, when ePWV exceeded this threshold, each 1-unit increase in ePWV was associated with a 30.7% increase in all-cause mortality (HR 1.31, 95%CI 1.30–1.32, P < 0.001).
The association of ePWV with all-cause (A) and cardiovascular mortality (B) visualized by restricted cubic spline. Adjust for: gender, race, education level, marital status, smoking, alcohol use, hypertension, diabetes, hypercholesterolemia, arthritis, congestive heart failure, coronary heart disease, stroke, thyroid disease, cancer, weak/failing kidneys, family income-poverty ratio, body mass index, waist circumference, white blood cell count , hemoglobin, red cell distribution width, platelet count, albumin, alanine aminotransferase; aspartate aminotransferase, total calcium, iron, sodium, potassium, globulin, blood urea nitrogen, serum cholesterol, glucose, triglycerides, uric acid, LDL- cholesterol, HDL- cholesterol.
Subgroup analyses stratified by age, sex, race, educational level, marital status, smoking, alcohol consumption, BMI, hypertension, and diabetes were conducted to explore the relationship between ePWV and all-cause mortality. The results showed consistent associations, with significant interactions observed in the smoking and diabetes subgroups, while no interactions were found in the other subgroups (Fig. 6A).
Associations of the ePWV with cardiovascular mortality
In Model 1, an increase in ePWV was significantly associated with a higher risk of cardiovascular mortality (HR 1.41, 95%CI 1.39–1.43, P < 0.001) (Table 3). After full adjustment for covariates, each 1-unit increase in ePWV was linked to a 27.1% increase in the risk of cardiovascular mortality (Model 3, HR 1.27, 95%CI 1.24–1.30, P < 0.001) (Table 2B).
Kaplan-Meier curves demonstrated a significantly lower survival rate in the higher ePWV group compared to the lower ePWV group (Log-rank P < 0.001) (Fig. 3B). Weighted multivariate Cox regression analysis further showed that, compared to the lower ePWV group, the higher ePWV group had a significantly increased risk of cardiovascular mortality after full adjustment for covariates (HR 2.75, 95%CI 2.46–3.07, P < 0.001) (Table 3). Restricted cubic spline (RCS) analysis revealed a linear relationship between ePWV and cardiovascular mortality (P for nonlinearity = 0.8893) (Fig. 5B).
Subgroup analyses stratified by age, sex, race, educational level, marital status, smoking, alcohol consumption, BMI, hypertension, and diabetes were conducted to explore the relationship between ePWV and cardiovascular mortality. The results showed consistent associations, with significant interactions observed in the smoking, hypertension, and diabetes subgroups, while no interactions were found in the other subgroups (Fig. 6B).
The predictive ability of ePWV for allcause and cardiovascular mortality in general participates
Time-dependent ROC analysis was conducted to evaluate the prognostic value of ePWV for all-cause and cardiovascular mortality in U.S. adults. The results showed that the area under the curve (AUC) for ePWV in predicting all-cause mortality at 1 year, 6 years, and 10 years was 0.73 (95%CI 0.60–0.85), 0.80 (95%CI 0.76–0.83), and 0.79 (95%CI 0.76–0.82), respectively (Fig. 7A and B). For cardiovascular mortality, the AUC was 0.85 (95%CI 0.73–0.97), 0.83 (95%CI 0.76–0.90), and 0.83 (95%CI 0.78–0.84) (Fig. 7C and D). These findings suggest that ePWV appears to have effective predictive value for both short-term and long-term all-cause and cardiovascular mortality.
Discussion
This is a large-scale study aimed at investigating the relationship between arterial stiffness and survival outcomes in U.S. adults. In this study, which included 48,257 participants from 10 NHANES cycles (1999–2018), elevated ePWV was found to be positively associated with both all-cause and cardiovascular mortality. It was identified as an independent risk factor for poor survival, with these effects remaining significant even after adjusting for common risk factors. The relationship between ePWV and all-cause mortality was nonlinearly positive, with an inflection point at 8.267. When ePWV < 8.267, the risk of all-cause mortality decreased as ePWV increased, but once it exceeded 8.267, the association became significantly positive. Additionally, according to the time-dependent ROC, ePWV also performed well in predicting survival, particularly in predicting 6-year all-cause mortality (AUC:0.80) and 1-year cardiovascular mortality (AUC 0.85).
The estimated Pulse Wave Velocity (ePWV) is an established marker of arterial stiffness, which is closely associated with various cardiovascular and metabolic diseases29. Arterial stiffness, as measured by ePWV, provides valuable insights into the mechanical properties of the arterial wall, particularly the aorta, which is a key determinant of cardiovascular health. Higher ePWV values indicate greater arterial stiffness, which in turn reflects increased cardiovascular risk. Numerous studies have demonstrated the predictive value of arterial stiffness for adverse clinical outcomes, including coronary artery disease30, heart failure7 and stroke8. For example, pulse wave velocity estimates are robust predictors of cardiovascular events and mortality in adult patients with diabetes31 and hypertension32, independent of traditional risk factors. Similarly, current research supports these findings, indicating that even after adjusting for multiple covariates, elevated ePWV is significantly associated with increased all-cause mortality and cardiovascular mortality33,34. This strengthens the role of ePWV as a key clinical tool in patient risk stratification and management, particularly in patients with pre-existing cardiovascular disease.
The study reveals a nonlinear relationship between ePWV and all-cause mortality, with an inflection point at 8.267. Below this threshold, an increase in ePWV is associated with a reduction in all-cause mortality, while above this threshold, the association becomes significantly positive. This phenomenon can be explained by the body’s compensatory mechanisms at lower levels of arterial stiffness, where a slight increase in ePWV may reflect a physiological response to maintain adequate perfusion and oxygen delivery to vital organs. However, once the ePWV exceeds the critical threshold of 8.267, the detrimental effects of increased arterial stiffness, such as impaired vascular compliance, elevated systolic blood pressure, and reduced coronary perfusion, outweigh the compensatory benefits, leading to a higher risk of mortality. This nonlinear association has been observed in other studie35 as well, suggesting that the relationship between arterial stiffness and mortality is complex and influenced by multiple factors, including age, comorbidities, and baseline cardiovascular health .
In contrast to the nonlinear relationship with all-cause mortality, the association between ePWV and cardiovascular mortality is linear, as shown by the RCS analysis. This linearity suggests that any increase in arterial stiffness, as indicated by a higher ePWV, directly correlates with an increased risk of cardiovascular death. The linear relationship may be attributed to the fact that cardiovascular mortality is more closely linked to the pathophysiological processes driven by arterial stiffness, such as left ventricular hypertrophy, atherosclerosis, and increased afterload, which progressively impair cardiac function and lead to fatal cardiovascular events36,37. Additionally, the absence of an inflection point in this relationship indicates that the risk of cardiovascular death continues to rise with increasing ePWV, underscoring the importance of early detection and intervention to manage arterial stiffness and reduce cardiovascular risk.
The time-dependent ROC curve analysis in this study demonstrates that ePWV has strong predictive value for both short-term and long-term mortality, particularly for cardiovascular mortality at 1 year (AUC:0.85) and all-cause mortality at 6 years (AUC:0.80). The high AUC values indicate that ePWV is a reliable biomarker for identifying individuals at high risk of adverse outcomes, making it a valuable tool in clinical practice. The ability to predict long-term mortality highlights the utility of ePWV in guiding therapeutic decision-making and monitoring the effectiveness of interventions aimed at reducing arterial stiffness. Moreover, the temporal aspect of the ROC analysis emphasizes that the prognostic value of ePWV may vary over time, suggesting that serial measurements could provide additional insights into disease progression and the impact of treatment.
The subgroup analyses reveal significant interactions between ePWV and factors such as smoking, hypertension, and diabetes, indicating that the impact of arterial stiffness on mortality may differ across these populations. For instance, smokers and individuals with diabetes or hypertension may experience greater increases in mortality risk with rising ePWV due to the synergistic effects of these conditions on vascular health. Smoking, for example, accelerates arterial stiffening by promoting inflammation and endothelial dysfunction, while diabetes and hypertension exacerbate vascular damage through hyperglycemia and high blood pressure, respectively. The presence of these interactions underscores the need for personalized approaches in managing patients with high ePWV, particularly those with these high-risk factors. Understanding the mechanisms behind these interactions could lead to more targeted interventions that address the specific pathophysiological pathways contributing to increased mortality in these subgroups.
Management of increased arterial stiffness, as indicated by elevated ePWV, can be effectively addressed through a combination of pharmacological interventions and lifestyle modifications. Antihypertensive medications, particularly ACE inhibitors and ARBs, have been shown to reduce arterial stiffness38. Lifestyle changes, including regular physical activity, smoking cessation, and moderation in alcohol consumption, are also crucial in managing arterial stiffness39. Additionally, aggressive glucose control in diabetic patients can help reduce ePWV40. Weight management through a balanced diet and exercise is another strategy to decrease ePWV41. A diet rich in fruits and vegetables, such as the DASH diet, has been associated with lower arterial stiffness [42]. Regular monitoring of ePWV can aid in early detection and prompt treatment, which is beneficial in high-risk populations. These management strategies are supported by evidence from various studies and should be considered in clinical practice to improve patient outcomes.
Limitation and strength
The primary strengths of our study lie in its large individual sample size and long follow-up period, which provide robust conclusions and sufficient statistical power. Additionally, all participants were drawn from the NHANES survey, which helps prevent selection bias. However, several limitations should be noted. First, although we adjusted for several potential confounding factors in the analysis, we cannot rule out the possibility that ePWV may be influenced by other unknown factors. Second, this study was conducted among U.S. adults. Therefore, whether these findings can be generalized to other populations requires further investigation.
Conclusion
Elevated ePWV is independently associated with increased cardiovascular mortality in US adults and exhibits a significant positive correlation with all-cause mortality in US adults beyond an ePWV threshold of 8.267.The findings emphasize the importance of early detection and management of increased arterial stiffness to improve survival outcomes.
Data availability
The dataset analyzed for the current study is available in the NHANES repository [https://www.cdc.gov/nchs/nhanes/].
References
Reeve, E. H., Barnes, J. N., Moir, M. E. & Walker, A. E. Impact of arterial stiffness on cerebrovascular function: A review of evidence from humans and preclincal models. Am. J. Physiol. Heart Circ. Physiol. 326(3), H689–H704. https://doi.org/10.1152/ajpheart.00592.2023 (2024).
Moulakakis, K. G. et al. Arterial stiffness and aortic aneurysmal disease—a narrative review. Vasc. Health Risk Manag. 20, 47–57. https://doi.org/10.2147/VHRM.S410736 (2024).
Regnault, V., Lacolley, P. & Laurent, S. Arterial stiffness: From basic primers to integrative physiology. Annu. Rev. Physiol. 86, 99–121. https://doi.org/10.1146/annurev-physiol-042022-031925 (2024).
Wijdan, S. A., Ali, S., Mirza, A. & Nadeem, M. The effects of metabolic syndrome on arterial stiffness: A concise review. J. Pak Med. Assoc. 74(4), 839. https://doi.org/10.47391/JPMA.10229 (2024).
Xuereb, R. A., Magri, C. J. & Xuereb, R. G. Arterial stiffness and its impact on cardiovascular health. Curr. Cardiol. Rep. 25(10), 1337–1349. https://doi.org/10.1007/s11886-023-01951-1 (2023).
Kim, H. L. Arterial stiffness and hypertension. Clin. Hypertens. 29(1), 31. https://doi.org/10.1186/s40885-023-00258-1 (2023).
Kim, H. L. & Jo, S. H. Arterial stiffness and heart failure with preserved ejection fraction. J. Korean Med. Sci. 39(23), e195. https://doi.org/10.3346/jkms.2024.39.e195 (2024).
Chen, Y., Shen, F., Liu, J. & Yang, G. Y. Arterial stiffness and stroke: De-stiffening strategy, a therapeutic target for stroke. Stroke Vasc Neurol. 2(2), 65–72. https://doi.org/10.1136/svn-2016-000045 (2017).
Cecelja, M. & Chowienczyk, P. Role of arterial stiffness in cardiovascular disease. JRSM Cardiovasc. Dis. 1(4), cvd2012012016. https://doi.org/10.1258/cvd.2012.012016 (2012).
Sung, K. C. Arterial stiffness and incident diabetes. Pulse (Basel) 12(1), 12–18. https://doi.org/10.1159/000535775 (2023).
Albu, A., Para, I. & Bidian, C. Arterial stiffness in aortic stenosis - complex clinical and prognostic implications. Biomed. Pap Med. Fac. Univ. Palacky Olomouc Czech Repub. 166(4), 369–379. https://doi.org/10.5507/bp.2022.040 (2022).
Greve, S. V., Laurent, S. & Olsen, M. H. Estimated pulse wave velocity calculated from age and mean arterial blood pressure. Pulse (Basel). 4(4), 175–179. https://doi.org/10.1159/000453073 (2017).
Aimagambetova, B. et al. Association of estimated pulse wave velocity with cognitive function in a multiethnic diverse population: The Northern Manhattan study. Alzheimers Dement. 20(7), 4903–4913. https://doi.org/10.1002/alz.14064 (2024).
Shi, Y. et al. Estimated pulse wave velocity as a predictor of all-cause and cardiovascular mortality in patients with hypertension in china: A prospective cohort study. Front. Cardiovasc. Med. 11, 1365344. https://doi.org/10.3389/fcvm.2024.1365344 (2024).
Cheng, W., Xu, W., Luan, S., Wen, G. & Kong, F. Predictive value of estimated pulse wave velocity with all-cause and cause-specific mortality in the hypertensive population: The national health and nutrition examination surveys 1999–2014. J. Hypertens. 41(8), 1313–1322. https://doi.org/10.1097/HJH.0000000000003469 (2023).
Liu, Y., Xu, K., Wu, S., Qin, M. & Liu, X. Value of estimated pulse wave velocity to identify left ventricular hypertrophy prevalence: Insights from a general population. BMC Cardiovasc. Disord. 22(1), 157. https://doi.org/10.1186/s12872-022-02541-9 (2022).
Agarwal, R. Antihypertensive agents and arterial stiffness: relevance to reducing cardiovascular risk in the chronic kidney disease patient. Curr. Opin. Nephrol. Hypertens. 16(5), 409–415. https://doi.org/10.1097/MNH.0b013e3282063b86 (2007).
Di, D. et al. Exposure to phenols, chlorophenol pesticides, phthalate and PAHs and mortality risk: A prospective study based on 6 rounds of NHANES. Chemosphere 329, 138650. https://doi.org/10.1016/j.chemosphere.2023.138650 (2023).
Möstl, S. et al. Utility of estimated pulse wave velocity for assessing vascular stiffness: Comparison of methods. Elife 11, e73428. https://doi.org/10.7554/eLife.73428 (2022).
Huang, Y., Hu, Y. & Bao, B. Relationship of body mass index and visceral fat area combination with arterial stiffness and cardiovascular risk in cardiovascular disease-free people: NHANES (2011–2018). Endocr. Connect. 12(11), e230291. https://doi.org/10.1530/EC-23-0291 (2023).
CDC. NHANES Laboratory/Medical Technologists Procedures Manual (CDC, 2001).
Zhang, J., Chen, Y., Zou, L. & Gong, R. Prognostic nutritional index as a risk factor for diabetic kidney disease and mortality in patients with type 2 diabetes mellitus. Acta Diabetol. 60(2), 235–245. https://doi.org/10.1007/s00592-022-01985-x (2023).
Zeng, G. et al. n-3 PUFA poor seafood consumption is associated with higher risk of gout, whereas n-3 PUFA rich seafood is not: NHANES 2007–2016. Front. Nutr. 10, 1075877. https://doi.org/10.3389/fnut.2023.1075877 (2023).
Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 100 (4S), S1–S276. https://doi.org/10.1016/j.kint.2021.05.021 (2021).
Johnson, C. L. et al. National health and nutrition examination survey: Analytic guidelines, 1999–2010. Vital Health Stat. 2(161), 1–24 (2013).
Zhang, L., Chen, S., Wang, W., Wang, Y. & Liang, Y. Inflammatory and nutritional scoring system for predicting prognosis in patients with newly diagnosed multiple myeloma. J. Inflamm. Res. 16, 7–17. https://doi.org/10.2147/JIR.S390279 (2023).
Seckinger, A. et al. Clinical and prognostic role of annexin A2 in multiple myeloma. Blood 120(5), 1087–1094. https://doi.org/10.1182/blood-2012-03-415588 (2012).
Kamarudin, A. N., Cox, T. & Kolamunnage-Dona, R. Time-dependent ROC curve analysis in medical research: current methods and applications. BMC Med. Res. Methodol. 17(1), 53. https://doi.org/10.1186/s12874-017-0332-6 (2017).
Jae, S. Y. et al. Association between estimated pulse wave velocity and the risk of cardiovascular outcomes in men. Eur. J. Prev. Cardiol. 28(7), e25–e27. https://doi.org/10.1177/2047487320920767 (2021).
Boutouyrie, P., Chowienczyk, P., Humphrey, J. D. & Mitchell, G. F. Arterial stiffness and cardiovascular risk in hypertension. Circ. Res. 128(7), 864–886. https://doi.org/10.1161/CIRCRESAHA.121.318061 (2021).
Solini, A. et al. Independent association of estimated pulse-wave velocity with all-cause mortality in individuals with type 2 diabetes. QJM 117(7), 495–502. https://doi.org/10.1093/qjmed/hcae012 (2024).
Wu, L. D. et al. Estimated pulse wave velocity is associated with all-cause mortality and cardiovascular mortality among adults with diabetes. Front. Cardiovasc. Med. 10, 1157163. https://doi.org/10.3389/fcvm.2023.1157163 (2023).
Laugesen, E. et al. Estimated pulse wave velocity is associated with all-cause mortality during 8.5 years Follow-up in patients undergoing elective coronary angiography. J. Am. Heart Assoc. 11 (10), e025173. https://doi.org/10.1161/JAHA.121.025173 (2022).
Heffernan, K. S., Jae, S. Y. & Loprinzi, P. D. Association between estimated pulse wave velocity and mortality in U.S. Adults. J. Am. Coll. Cardiol. 75(15), 1862–1864. https://doi.org/10.1016/j.jacc.2020.02.035 (2020).
Huang, H. et al. Estimated pulse wave velocity is associated with all-cause and cardio-cerebrovascular disease mortality in stroke population: Results from NHANES (2003–2014). Front. Cardiovasc. Med. 10, 1140160. https://doi.org/10.3389/fcvm.2023.1140160 (2023).
Kirkman, D. L., Robinson, A. T., Rossman, M. J., Seals, D. R. & Edwards, D. G. Mitochondrial contributions to vascular endothelial dysfunction, arterial stiffness, and cardiovascular diseases. Am. J. Physiol. Heart Circ. Physiol. 320 (5), H2080–H2100. https://doi.org/10.1152/ajpheart.00917.2020 (2021).
Wilkinson, I. B., Mäki-Petäjä, K. M. & Mitchell, G. F. Uses of arterial stiffness in clinical practice. Arterioscler. Thromb. Vasc Biol. 40(5), 1063–1067. https://doi.org/10.1161/ATVBAHA.120.313130 (2020).
Cavero-Redondo, I. et al. Antihypertensive drug recommendations for reducing arterial stiffness in patients with hypertension: Machine learning-based multicohort (RIGIPREV) study. J. Med. Internet Res. 26, e54357. https://doi.org/10.2196/54357 (2024).
Zieman, S. J., Melenovsky, V. & Kass, D. A. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler. Thromb. Vasc Biol. 25(5), 932–943. https://doi.org/10.1161/01.ATV.0000160548.78317.29 (2005).
Neugebauer, R., Fireman, B., Roy, J. A. & O’Connor, P. J. Impact of specific glucose-control strategies on microvascular and macrovascular outcomes in 58,000 adults with type 2 diabetes. Diabetes Care 36(11), 3510–3516. https://doi.org/10.2337/dc12-2675 (2013).
Figueroa, A. et al. Effects of diet and/or low-intensity resistance exercise training on arterial stiffness, adiposity, and lean mass in obese postmenopausal women. Am. J. Hypertens. 26(3), 416 –423 https://doi.org/10.1093/ajh/hps050 (2013).
Funding
Project supported by Hainan Province Clinical Medical Center [2021276]. Fujian Province central guide local science and technology development fund project[2023L3009].
Author information
Authors and Affiliations
Contributions
The study was conceived by KL and ZL who were responsible for performing the data analysis and manuscript writing. KL extracted the data from the official NHANES website. YC for multiple revisions of articles, language editing, data verification, as well as grant applications.HSH contributed to the revision and review of the manuscript. KL and ZL conducted a repeat analysis of the data and verified the results.All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The NHANES protocol was approved by the Institutional Review Board of the National Center for Health Statistics (NHANES - NCHS Research Ethics Review Board Approval (cdc.gov)). All participants provided written informed consent.
Consent for publication
All authors have agreed to the submission and publication of this research.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, K., Lin, Z., Chen, Y. et al. Elevated pulse wave velocity as a marker of arterial stiffness and its association with mortality in US adults. Sci Rep 15, 23026 (2025). https://doi.org/10.1038/s41598-025-07198-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-07198-w