Abstract
The Nuss procedure is a widely used treatment for pectus excavatum (PE) that significantly enhances the effectiveness of correction and improves patient satisfaction. However, it is not without its limitations. One common complication that patients face is poor wound healing (PWH). Identifying risk factors for poor wound healing and accurately predicting its occurrence are critical for improving patient outcomes. Currently, there is limited research on poor wound healing in postoperative patients, and there is an urgent need for more robust clinical studies. Over the past decade, we collected data on about 40 indicators from 581 patients who underwent Nuss surgery in our hospital. Lasso regression and multivariate regression analysis (MRA) were used to identify factors significantly associated with poor wound healing. Based on these factors, a prediction model using categorical boosting (CatBoost) theory was developed to assess the risk of PWH. Approximately 9% of patients experienced poor wound healing after the Nuss procedure. Among the 35 potential risk factors, we identified several key clinical indicators closely related to poor incision healing, including muscle thickness (MT), Haller short diameter variation (Haller SDV), relative muscle thickness (RMT), orthopedic triangle (OT), absolute sternal rotation angle (ASRA), and body mass index (BMI). Furthermore, we discovered that the orthopedic triangle, a critical factor in orthopedic plate shaping, played a significant role in predicting PWH (χ² = 42.91, df = 3, p < 0.001). The optimal range for the orthopedic triangle was defined as 0.48 to 0.57. Using these identified factors, we developed the NOWI-model (Nuss surgical incision complication prediction model), which was based on CatBoost theory. The model performed well on the validation dataset, achieving an AUC of 0.96 (95% CI: 0.92,0.99) and an F1-score of 0.80 for predicting PWH. Additionally, an online calculator was created based on this model to help clinicians assess the risk of poor wound healing. We also found that the maximum chest wall elevation during correction should not exceed 4 cm. This study not only identified the high-risk factors for poor wound healing after Nuss surgery and defined the optimal range of some factors for the first time, but also provided a clinical prediction model and a tool for assessing poor wound healing risk. The NOWI model offers a reliable approach for predicting postoperative complications and improving patient care.
Similar content being viewed by others
Introduction
Pectus excavatum(PE) is the most common congenital deformity of the chest, characterized not only by inward depression of the sternum but also by its impact on heart and lung function1. Although the prevalence of PE is relatively low, approximately 0.1–0.3%2,3,4, the number of patients undergoing surgery has gradually increased with economic development and the shift in the bio-psychosocial medical model5,6. A milestone innovation in the treatment of PE is the introduction of the Nuss operation, proposed by Dr. Nuss in 1996, using an orthopedic plate to correct PE as an alternative to traditional open surgery7 and it was quickly adopted by thoracic surgeons worldwide. It is now considered as the standard surgical treatment for PE. Although the Nuss procedure is well known for its minimally invasive nature and postoperative complications similar to those of open surgery, complications such as postoperative pneumothorax, pleural effusion, incision bleeding, and poor wound healing(PWH) remain common8. PWH not only increase the likelihood of unplanned hospital admissions and surgeries but also affect the patient’s recovery process. In severe cases, this can lead to infection and premature removal of the orthotic plate, resulting in orthotic failure9. Chronic wounds that do not heal can also lead to a decline in the patient’s quality of life, as they require long-term dressing changes, sometimes even daily, causing severe physical and psychological impacts on the patient. Therefore, predicting which patients are likely to experience PWH after surgery is of significant clinical importance for developing postoperative management strategies, formulating personalized treatment plans, and optimizing surgical outcomes.
Currently, there are few reports on factors affecting PWH after Nuss surgery. A few studies have reported on factors such as the use of clindamycin before surgery9summer surgeries10,11,12 Haller index13 and SRA14,15. These factors have only been shown to have an impact on incision healing, but the extent of their impact and predictive role in PWH have not yet been reported. At the same time, traditional statistical testing methods cannot test or exclude the interactions between different influencing factors, such as the Student’s t-test or Mann-Whitney U test, or they cannot test the nonlinear effects of various factors on the outcome variable, such as logistic regression. However, these traditional statistical analysis methods often struggle to accurately capture these potentially complex relationships when faced with large multidimensional data. Currently, there is a lack of convenient and accurate methods for predicting PWH, but the emergence of machine learning provides the conditions for solving this problem. AI-based technologies can integrate patients’ clinical data in real time and repeatedly assess patients while interpreting results16. This technology is beneficial for taking timely and targeted preventive measures in high-risk groups, thereby improving patient prognosis and reducing the occurrence of PWH. Among them, the prediction model based on machine learning, particularly the CatBoost model for handling high-dimensional nonlinear data, is a gradient boosting-based machine learning algorithm that can effectively address the combination of categorical and numerical features. Moreover, CatBoost can directly process categorical features, thereby reducing the complexity of feature engineering. At the same time, it also has mechanisms to combat overfitting, such as built-in L2 regularization, and avoids the bias issues associated with gradient boosting algorithms. It fully utilizes patient data and, compared to traditional analytical methods, shows better performance in both sensitivity and specificity, demonstrating significant potential for clinical prognosis prediction17.
This study aimed to utilize the CatBoost model to process surgical and patient-related factors to construct a model that can effectively predict the risk of PWH after the Nuss operation. We hope that the predictive results of the model will assist clinicians in identifying high-risk patients and provide data support for postoperative management.
Method
Patients and study design
Study design
This study investigated patients who underwent minimally invasive Nuss surgery in the Department of Thoracic Surgery at West China Hospital, SCU, from January 1, 2010, to December 31, 2023. This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of West China Hospital of Sichuan University, which agreed to waive informed consent from patients. The ethics number is 2023 (review number 1986), and patient information is anonymized and kept confidential to avoid personal privacy and commercial interests.
Inclusion criteria
1. Patients who underwent Nuss operation in the thoracic surgery department of our hospital between 2010.01.01 and 2023.12.31, and had the orthopedic plate removed during this period.
Exclusion criteria
1. Patients undergoing other thoracic surgeries simultaneously with the Nuss operation for PE.
2. Patients with newly developed chest wall trauma or surgery and a history of spinal surgery between the Nuss operation for PE and removal of the orthopedic plate.
3. Patients who undergo surgery to insert a new orthotic plate after the Nuss operation.
4. Patients for whom follow-up results could not be obtained.
5. Patients with diseases requiring chemotherapy or immunosuppressive treatment.
6. Patients for whom preoperative and postoperative CT images could not be obtained.
Surgical method
The patient is placed in a supine position under general anesthesia, and routine disinfection and sterile tablecloths are performed. Take an orthopedic steel plate of appropriate length and pre-shape it according to the patient’s chest. Make a 2–3 cm incision about 1–2 cm outside the intersection of the horizontal plane of the anterior axillary line and the deepest point of the sternal depression on both sides, and make a thoracoscopic observation hole on the left chest wall. Use a guide to carefully establish a tunnel behind the sternum and in front of the pericardium. Insert the pre-shaped steel plate through the guide plate into the tunnel and flip it over, and lift the sternum. After observing that the correction effect is satisfactory, use steel wire or a matching fixing plate to fix the two ends of the steel plate to the ribs. Finally, after sufficient hemostasis, suture the incision layer by layer and bandage it, and transfer to the anesthesia recovery room for preemptive analgesia with intercostal nerve block.
Definition of poor incision healing
Patients who underwent surgery from the day of surgery to the removal of the orthopedic plate. Wound grade B or C healing, or grade A healing followed by non-traumatic wound dehiscence, exudate, subcutaneous effusion, and infection18.
Clinical and radiography data
We collected 37 factors that may be related to PWH after the Nuss operation through a literature search, consultation with experts, and our own experience as the target for patient data collection. The data set included basic patient information (such as age and sex), medical history information, physical examination (such as height, weight, blood pressure, smoking history, tobacco consumption, drinking history), surgery-related parameters (such as operation time, whether thoracoscopy was used, whether drainage tubes were used, number of orthopedic plates, orthopedic plate fixation method (wires directly fixed on the ribs or fixed with fixation plates), imaging indicators (such as Haller index(HI), chest wall thickness(CWT) and muscle thickness(MT) on the plane of the deepest point of chest wall depression19,20 sternal rotation angle (SRA), orthopedic plate shaping indicators and postoperative HI), and finally follow-up indicators (such as PWH or not (excluding poor healing of drainage tube and thoracoscopic ports)), number and cause of unplanned hospitalization after surgery, number and cause of unplanned surgery after Nuss operation). All data were obtained through electronic medical record management system and imaging records, and patients’ follow-up indicators were obtained through phone inquiry. Data were collected from 1041 patients. The dataset included clinical tests, examinations, surgeries, treatments, and demographic indicators of patients who underwent the Nuss procedure over the past 13 years. All patients were marked as having two clinical outcomes, PWH and no PWH, through medical history collection and follow-up. A flowchart for screening participants in this study is shown in Fig. 1.
Statistical analyses
This study used Pandas (version 1.2.4) and Scikit-learn (version 0.24.1) scientific computing packages in Python (version 3.8) for data preprocessing and statistical analysis21. All statistical analyses used two-sided tests, and P-Value < 0.05 was used as the standard for determining whether there was a difference. To ensure the comparability between features, all continuous variables were Z-score standardized before entering the model. The formula is: z = (x - µ)/σ, where µ is the mean and σ is the standard deviation.
To process high-dimensional data and screen key predictors, we used the least absolute shrinkage and selection operator (LASSO) regression method for preliminary data analysis22,23. LASSO can automatically perform feature selection by adding an L1 regularization term to the loss function. Its objective function is: min(‖y - Xβ‖^2 + λ‖β‖_1), where λ is the regularization coefficient24. The optimal λ value was determined by 10-fold cross validation, and features with non-zero coefficients were selected. The subsequent multivariate logistic regression analysis was used to evaluate the factor weights and variance inflation factor analysis to remove multicollinearity to further screen independent predictors, and the odds ratio (OR) and its 95% confidence interval (CI) of each factor were calculated.
The model performance was evaluated by the area under the receiver operating characteristic curve (AUC-ROC), and the calculation formula was: AUC = ∫[TPR(FPR^−1(x))]dx, where TPR represents the true positive rate and FPR represents the false positive rate. Finally, six key predictors were screened out: chest wall thickness (CWT), Heller SDV, orthopedic triangle (OT), sternal flip angle, BMI and relative muscle thickness. Among them, the missing rate of BMI was 10% (60/581), and the multiple imputation by chained equations (MICE) method was used to handle the missing data, and five imputations were performed based on the missing at random (MAR) assumption21,22. The imputed data set was divided into a training set (n = 405) and a validation set (n = 406) in a ratio of 7:3 using the random classification data package provided by Python.
In order to make full use of multi-dimensional features, we built a variety of machine learning models for comparison, including K-Nearest Neighbor (KNN), Naïve Bayes (NB), Convolutional Neural Networks (CNN), Light Gradient Boosting Machine (lightgbm), Categorical Boosting (Catboost), Extreme Gradient Boosting (XGboost), and Logistic Regression. We optimized the Catboost model parameters through grid search. The main adjustment parameters include learning rate, iterations, tree depth, and L2 regularization coefficient (l2_leaf_reg). The prediction performance of the model was evaluated using indicators such as the area under the ROC curve (AUC), accuracy, sensitivity, and specificity. Finally, in order to simplify the complexity of the model, we combined the Akaike information criterion (AIC) value to select the best model framework. The final Nuss surgical incision complication prediction model (NOWI-Model) was established and the calibration curve was used to evaluate the consistency between the predicted probability and the actual observed value25,26. To facilitate clinical application, we developed a web-based online calculator (https://catboost-api.onrender.com/predict), which was implemented using the Flask framework and supports real-time risk prediction. The flow chart of the study is shown in Fig. 2.
Results
General information on patients
This study retrospectively analyzed the clinical data of 1041 patients with PE who underwent Nuss surgery in the Department of Thoracic Surgery of our hospital from January 2010 to June 2024. All patients were aged between 8 and 35 years old, with an average age of 16 years old. There were 916 male patients (88.0%) and 125 female patients (12.0%), with a male-to-female ratio of 7:1. All patients underwent standard Nuss surgery, and the median OP retention time was 2.5 years (IQR: 2.0–3.0 years). The preoperative BMI of patients was 13.5–28.7 kg/m², with a median of 17.25 ± 2.5 kg/m², of which 68.3% (711/1041) of patients had a BMI < 18.5 kg/m² (Table 1).
The incidence of postoperative PWH was 9.0% (94/1041), of which 41 cases (43.6%) showed continuous exudation from the incision, 37 cases (39.4%) had local skin defects with exposed steel plates, and 16 cases (17.0%) developed incision infection with purulent secretions (Fig. 3). The time of PWH occurrence showed a gradual downward trend: 44.7% (42/94) occurred between 1 week and 4 months after surgery, but 32.9% (31/94) of PWH patients still had incision complications during the period of 1 to 2 years after surgery (Fig. 4).
There were 66 PWH patients in the training set (16.2%) and 28 patients (16.0%) in the validation set, and there was no significant difference in the incidence between the two groups (P = 0.89). Comparison of baseline characteristics showed that there was no statistical difference between the two groups in terms of demographic characteristics (age, gender), clinical indicators (BMI, Haller index), and surgical parameters (operation time, blood loss) (P > 0.05). The specific data are shown in Table 1. Among them, the continuous variables were tested using the Kolmogorov-Smirnov test, P = 0.67; the categorical variables were tested using the chi-square test, P = 0.23, both indicating that there was no statistical difference between the two data sets.
Lasso regression and multivariate regression analysis
The results of the LASSO (Least Absolute Shrinkage and Selection Operator) regression analysis after incorporating 37 potential risk factors showed that 11 variables (relative muscle thickness (RMT), chest wall muscle thickness (MT), sternum rotation angle (SRA), orthopedic triangle index (OT), weight, Haller SDV, BMI, orthopedic triangle adjacent side length, surgical season, and orthopedic triangle hypotenuse length) had non-zero coefficients, suggesting that these factors were potentially associated with PWH (Fig. 5). Subsequently, we used MLRA to estimate the weights of these factors. 11 variables were confirmed to be independently associated with PWH (P < 0.05), including relative muscle thickness (OR = 1.42, 95% CI:1.09–2.05), chest wall muscle thickness (OR = 0.34, 95% CI:0.23–0.53), sternal rotation angle (OR = 1.47, 95% CI: 1.29–1.68), orthopedic triangle (OR = 1.03, 95% CI: 0.42–1.06), weight (OR = 1.52, 95% CI: 1.09–2.11), Haller index standard deviation (Haller SDV, OR = 1.78, 95% CI: 1.10–2.90), BMI (OR = 0.69, 95% CI: 0.42–1.13), length of the adjacent side of the orthopedic triangle (LAS OT, OR = 2.77, 95%CI: 1.76–4.36), surgical season (Season, OR = 0.10, 95%CI:0.06–1.16) and length of the hypotenuse of the orthopedic triangle (LH OT, OR = 2.28, 95%CI: 1.51–3.44),CWT(OR = 0.34, 95%CI: 0.23–0.51) (Table 2).
LASSO Regression for model feature selection. Selection of best candidate (Lambda) through 10-fold cross validation. The area under receiver operating characteristic curve plotted against log(Lambda). Vertical dashed lines plotted using best criterion. Best Lambda produces 11 candidate values with non-zero coefficients.
Variance inflation factor (VIF) analysis revealed that the length of the LAS OT and the LH OT were strongly correlated with the orthopedic triangle (r > 0.85), weight was strongly correlated with BMI (r > 0.65), CWT and muscle thickness was strongly correlated with RMT (r > 0.42), and Haller index change was strongly correlated with Haller SDV (r > 0.50). After combining the factor weights of multivariate regression analysis, the index with the greatest correlation was retained27,28. Finally, six independent risk factors (relative muscle thickness, sternal rotation angle, orthopedic triangle index, Haller index standard deviation, BMI and chest wall muscle thickness) were determined for model construction.(The conventional statistical results of some indicators are shown in the Fig. 6A-D).
Results of routine analysis of some predictive factors. (A) With the increase of chest wall muscle thickness, the incidence of poor wound healing gradually increased (χ²=4.5, p < 0.001); (B) The orthopedic triangle index showed a nonlinear relationship with the occurrence of poor wound healing (χ²=2.3, p = 0.01); (C) With the increase of the degree of sternal lifting, the incidence of poor wound healing also increased (χ²=5.1, p < 0.001); (D) With the increase of relative chest wall thickness, the incidence of poor wound healing gradually decreased (χ²=4.1, p < 0.001).
Model establishment and verification
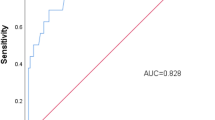
Based on the 6 key predictive variables screened, we constructed a variety of machine learning prediction models and evaluated the model performance by the area under the receiver operating characteristic curve (AUC-ROC). The Fig. 7 showed that the AUC values of each model were relatively close. Among them, the AUCs of the SVM, Light GBM, XG boost and Catboost models were 0.97, 0.92, 0.95 and 0.96, respectively. In terms of other evaluation indicators of model performance, the F1 index of each model was: SVM = 0.75, Light GBM = 0.78, XGboost = 0.73, Catboost = 0.81. Considering the AUC, F1 index and Akaike information criterion (AIC) value, the Catboost model (AIC = 112.3) was selected as the final risk prediction model framework to establish the NOWI-Model29,30,31. The NOWI-Model achieved an AUC of 0.9 (95% CI: 0.95–1.00) in the training data set (n = 405). The results of the five-fold cross-validation showed that the AUC of each round of validation was greater than 0.9, and the average AUC reached 0.96 (Fig. 8A). After substituting the independent validation set (n = 406) into the model, the model AUC = 0.96 (95%CI: 0.92–0.99), and the accuracy was 0.85 (Fig. 8B). The result classification list shows that for the prediction of PWH, the model has a recall rate of 0.88 and an F1 index of 0.81; for the prediction of no PWH, the recall rate is 0.92 and the F1 index is 0.89 (Table 3). The final probability validation graph of the model is shown in the Fig. 9.
(A) Five-fold cross-validation ROC curve of NOWI-model. The NOWI-Model’s no-fold cross-validation graph shows that the AUC for each validation is greater than 0.9, with an average AUC of 0.96, indicating that the model does not have a risk of overfitting and has strong generalization capabilities. (B) The ROC curves of the NOWI- model in the validation dataset. The performance of the candidate model on the validation set, where the closer the curve is to the top left corner, the larger the area under the curve (AUC), indicating that the model’s ability to distinguish whether poor incision healing occurs is stronger.
Clinical use
To promote the clinical application of NOWI-Model, we developed a web-based online prediction platform (https://catboost-api.onrender.com/predict)27. The platform adopts a responsive design, supports access from a variety of terminal devices, and has a simple and intuitive user interface (Fig. 9). The user interface design follows the principle of ergonomics. The main functional areas include: Data input area: Provides input boxes for 6 prediction variables. Each input box is equipped with a “?” icon on the right. When the mouse hovers over it, the detailed explanation and input requirements of the indicator are displayed. Result display area: After clicking the “Predict” button, the prediction results and risk grading are displayed in real time. Taking a typical patient as an example, when the following parameters are entered: chest wall muscle thickness: 1 cm; postoperative Haller index change: 2.5; relative muscle thickness: 0.5; sternal rotation angle: 10°; orthopedic triangular index: 0.5; BMI: 19 kg/m². The platform will return the prediction results within 0.5 s (Fig. 10). The results showed that the probability of this patient developing PWH was 85% (95% CI: 81–89%). According to the preset risk grading criteria, this patient belonged to the high-risk group.
Conclusion
Poor wound healing after pectus excavatum surgery is predictable. The occurrence of PWH is mainly related to 11 risk factors, 6 of which are highly correlated with PWH. These factors not only provide an important basis for the formulation of clinical preventive measures, but also provide a new theoretical explanation for the occurrence mechanism of PWH. The NOWI-Model we established provides a reliable and efficient tool for screening high-risk patients for PWH. Our web-based online prediction tool (https://catboost-api.onrender.com/predict) transforms this reliable tool into a convenient, fast and user-friendly way to achieve large-scale application.
Discussion
This study analyzed the clinical characteristics and risk factors of patients with PWH who underwent the Nuss procedure for PE. A new model for PWH after Nuss for PE, the NOWC-Model, was constructed and validated, providing a reliable screening tool for high-risk groups of patients with PWH after Nuss for PE. NOWC-Model includes six risk factors: RMT, ASRA, OT, Haller SDV, BMI, and CWT. According to our study, the NOWI-Model showed satisfactory accuracy and robustness in the validation dataset. PE is a rare disease, and Nuss surgery is widely used to treat PE. Compared with traditional surgery, Nuss surgery is less invasive and has a lower incidence of postoperative complications8but the problem of PWH after surgery is also very prominent9,32,33. It has been reported that the probability of PWH after Nuss operation is 2–10%32,34. The majority of cases are complicated by infection, and the probability of premature removal of the orthopedic plate due to PWH is approximately 1–6% of the population with PWH33,34 and some reports have shown that it can be as high as 70%12. The difference between PWH after the Nuss operation and other PWH is that there is an orthopedic plate under the wound, which is the key to the effect and success of the Nuss operation. Early removal of the orthopedic plate can lead to a decrease in the patient’s PE correction effect or even failure.
This study found that 9% of Nuss patients experienced post-surgery PWH, which is consistent with previous findings. To examine whether the use of clindamycin, rather than cephalosporins, before surgery increased the risk9. In this study, the patients were divided into three subgroups: those using cephalosporin antibiotics, clindamycin, and other antibiotics. The incidence of PWH in each subgroup was 8%, 10%, and 7%, respectively, and the final test results showed a p value of 0.07. These results clearly show that the occurrence of PWH was not related to the type of preoperative antibiotic used. As patients with PE generally have a lower BMI, we speculate that this may be the reason why the incidence of PWH in the Nuss operation is higher than that in other chest surgeries (1–6%)35 or abdominal surgeries (2.5%)36. Our data also emphasize the importance of improving the nutritional status of patients to a certain extent. The results of the logistic regression analysis in this study showed that BMI (OR = 0.95, p = 0.46), indicating that a high BMI value may reduce the probability of PWH. MLRA revealed that this factor was a significant risk factor for PWH, although the difference was not statistically significant. Another more detailed indicator, similar to BMI, is CWT and MT. We performed LR and MLRA for MT and CWT in patients with PE, the results showed that these two factors were closely related to PWH; however, their effects on PWH were not significantly different.
This study also found an important result that OT was closely related to poor healing in patients and was an IRF. The addition of OT values comes from our reference to orthopedic literature and clinical experience37,38. The results of the multivariate logistic regression analysis showed that the OR = 12.10, although P = 0.08 is greater than 0.05. We can see from the results of Fig. 8a that the relationship between OT and PWH is not linear. When the OT value is too large or too small, the risk of PWH increases. This result suggests that there is a limited range of the shaping shape of the orthosis for the occurrence of PWH. Although this range is not clear at present, the party class preliminarily judged that it should be between 0.48–0.57(Supplementary Tables S7-S8). The reason for this phenomenon may be that the surgical incision for funnel chest was mostly located on the mid-axillary line. The higher the OT value, the higher the chest wall lift after the Nuss operation. Simultaneously, the correction triangle is at a right angle. The larger the value of OT, the smaller the angle, which naturally means that the length of the orthopedic plate around the chest wall is longer, the corresponding ribs in contact with the orthopedic plate are greater, and the probability and degree of movement of the orthopedic plate under the influence of the rib movement are greater. A chest wall with a larger lifting amplitude and an increase in the movement of the orthopedic plate also significantly increased the stress at the fixed part. In this situation, contusion of soft tissue at the incision site may lead to long-term chronic inflammation, thereby affecting incision healing of the incision35. This is consistent with the result of the regression analysis of Haller SDV, which showed that the incidence of PWH increases with an increase in Haller SDV (OR = 1.28, p = 0.001). At the same time, this is one of the reasons why we chose the correction triangle and excluded Haller SDV as a predictive factor. At the same time, this also leads to the question of the goal of surgical treatment. Insufficient chest wall lifting affects the surgical outcome, but excessive lifting increases the incidence of complications. According to our research data, the best chest wall lift is less than 4 cm. Within this range, the incidence of PWH is significantly lower and this distance can usually achieve a good correction effect. Robert reported that, with the implementation of the Nuss procedure, the ___location of the surgical incision tended to shift to the anterior axillary line1. Simultaneously, the incidence of complications and PWH in their report also decreased. He believes that these complications mostly occur in the early stages of the learning curve and attributed this improvement to improvements in surgical techniques1. The findings of this study regarding Haller SDV and OT may be an objective reflection of the improvement in surgical techniques. The final reason for PWH is the sternal rotation angle. The sternal rotation angle had a significant impact on funnel chest treatment. The HI of many patients evaluated by CT before surgery is not high; however, owing to sternal rotation, the patient’s chest wall deformity appears very obvious39,40. In this study, the sternal rotation angle was also included in our prediction model. The results of the multivariate regression analysis were as follows: odds ratio (OR) = 1.02, p = 0.09. This result shows that an increase in the sternal rotation angle increases the likelihood of the event; however, its statistical effect is not significant, suggesting that this factor plays a prominent role in predicting PWH. However, a logistic regression analysis cannot accurately reflect this influence. The effect of the sternal rotation angle on PWH is still unclear, but our model is based on the classification principle, which is not affected by the intricate relationship between the two factors and is an end-to-end mechanism.
In addition, this study analyzed the relationship between postoperative blood test results and incision healing. We collected one blood test from patients before surgery and two at irregular intervals after surgery, as well as blood biochemical indicators including blood sugar, blood lipids, and albumin. We used the LSTM model to analyze the PWH. The results showed that previously reported indicators, such as blood sugar and albumin levels, were not effective predictors of PWH, nor the indicators screened out by our model, such as changes in white blood cell counts and white blood cell percentages, and the results of blood tests can be used to assist in judging the probability of a patient’s recovery.
Data availability
Data is available on request from the authors. The data supporting this study’s findings are available from the corresponding author Lin feng upon reasonable request.
Abbreviations
- PE:
-
Pectus excavatum
- PWH:
-
Poor wound healing
- MRA:
-
Multivariate regression analyses
- CatBoost:
-
Categorical boosting
- MT:
-
Muscle thickness
- Haller SDV:
-
Haller short diameter variation
- RMT:
-
Relative muscle thickness
- OT:
-
Orthopedic triangle
- ASRA:
-
Absolute sternal rotation angle
- BMI:
-
Body mass index
- NOWC:
-
NUSS Operation Wound Complication
- AUC:
-
Area under the curve
- SRA:
-
Sternal rotation angle
- AI:
-
Artificial Intelligence
- HI:
-
Haller index
- CWT:
-
Chest wall thickness
- LASSO:
-
Least absolute shrinkage and selection operator
- IOQ:
-
Interquartile range
- Cat-V:
-
Categorical variables
- Con-V:
-
Continuous variables
- MLRA:
-
Multivariate logistic regression analysis
- TSD:
-
Training set date
- NB:
-
Naïve Bayes
- LightGBM:
-
Light Gradient Boosting Machine
- CNN:
-
Convolutional Neural Networks
- XGBoost:
-
Extreme Gradient Boosting
- NOWC-Model:
-
NUSS operation wound complication prediction model
- LRA:
-
LASSO regression analysis
- LAS OT:
-
Length of the adjacent side of the orthopedic triangle
- LH OT:
-
Length of the hypotenuse of the orthopedic triangle
- IFRs:
-
Independent factors of risk prediction
References
Kelly, R. E. et al. Twenty-one years of experience with minimally invasive repair of pectus excavatum by the Nuss procedure in 1215 patients. Ann. Surg. 252, 1072–1081 (2010).
Westphal, F. L. et al. Prevalence of pectus carinatum and pectus excavatum in students in the City of manaus, Brazil. J. Bras. Pneumol Publicacao Soc. Bras. Pneumol E Tisilogia. 35, 221–226 (2009).
Coskun, Z. K., Turgut, H. B., Demirsoy, S. & Cansu, A. The prevalence and effects of pectus excavatum and pectus carinatum on the respiratory function in children between 7–14 years old. Indian J. Pediatr. 77, 1017–1019 (2010).
Zou, X. et al. Screening for pectus excavatum among primary students and establishment of a pectus excavatum screening program in dongguan, China. J. Thorac. Dis. 7, 868–874 (2015).
Jaroszewski, D. E. Physiologic implications of pectus excavatum. J. Thorac. Cardiovasc. Surg. 153, 218–219 (2017).
Kelly, R. E. et al. Surgical repair of pectus excavatum markedly improves body image and perceived ability for physical activity: multicenter study. Pediatrics 122, 1218–1222 (2008).
Nuss, D., Kelly, R. E., Croitoru, D. P. & Katz, M. E. A 10-year review of a minimally invasive technique for the correction of pectus excavatum. J. Pediatr. Surg. 33, 545–552 (1998).
Huerta, C. T. et al. Outcomes after pectus excavatum repair: A nationwide comparison of Nuss versus ravitch operations. J. Surg. Res. 303, 381–389 (2024).
Obermeyer, R. J. et al. Risk factors and management of Nuss bar infections in 1717 patients over 25 years. J. Pediatr. Surg. 51, 154–158 (2016).
Liang, X. et al. Subcutaneous suture can accelerate wound healing of lower midline incision: a randomized controlled trial. Am. Surg. 81, 23–30 (2015).
He, J. C. et al. Leaving the skin incision open May not be as beneficial as we have been taught. Surg. Infect. 18, 431–439 (2017).
Tanaka, K., Kuwashima, N., Ashizuka, S., Yoshizawa, J. & Ohki, T. Risk factors of infection of implanted device after the Nuss procedure. Pediatr. Surg. Int. 28, 873–876 (2012).
Zeineddine, R. M. et al. Does a high Haller index influence outcomes in pectus excavatum repair? J. Thorac. Cardiovasc. Surg. S0022-5223 (24), 00292–00297. https://doi.org/10.1016/j.jtcvs.2024.04.005 (2024).
Zeng Ruirui. Analysis of Risk Factors for Poor Wound Healing after NUSS for Pectus Excavatum and Improvement of Clinical Treatment (Zhengzhou University, 2019).
Yoshida, A. et al. Correlation of asymmetric chest wall deformity and growth in patients with pectus excavatum. J. Pediatr. Surg. 48, 771–775 (2013).
Kubat, M. An Introduction To Machine Learning (Springer Nature, 2015). https://doi.org/10.1007/978-3-319-63913-0
Ibragimov, B. & Gusev, G. Minimal Variance Sampling in Stochastic Gradient Boosting. In Advances in Neural Information Processing Systems (eds. Wallach, H. et al.) vol. 32 (Curran Associates, Inc., 2019).
Zhu, L. et al. Chinese herbal fumigant and lotion for postoperative complication in surgical wound of anal fistula: A protocol for a systematic review and meta-analysis. Med. (Baltim). 99, e22095 (2020).
Demling, R. H. Nutrition, anabolism, and the wound healing process: an overview. Eplasty 9, e9 (2009).
Guo, S. & DiPietro, L. A. Factors affecting wound healing. J. Dent. Res. 89, 219–229 (2010).
McKinney, W. Data structures for statistical computing in Python. SciPy 445, 51–56 (2010).
Zou, H. & Hastie, T. Regularization and variable selection via the elastic net. J. R Stat. Soc. Ser. B Stat. Methodol. 67, 301–320 (2005).
Lawson, M. L. et al. Impact of pectus excavatum on pulmonary function before and after repair with the Nuss procedure. J. Pediatr. Surg. 40, 174–180 (2005).
Friedman, J., Hastie, T. & Tibshirani, R. Regularization paths for generalized linear models via coordinate descent. J. Stat. Softw. 33, 1–22 (2010).
Van Calster, B. et al. A calibration hierarchy for risk models was defined: from utopia to empirical data. J. Clin. Epidemiol. 74, 167–176 (2016).
Steyerberg, E. W. & Vergouwe, Y. Towards better clinical prediction models: seven steps for development and an ABCD for validation. Eur. Heart J. 35, 1925–1931 (2014).
Tay, R. Correlation, variance inflation and multicollinearity in regression model. J. East. Asia Soc. Transp. Stud. 12, 2006–2015 (2017).
Thompson, C. G., Kim, R. S., Aloe, A. M. & Becker, B. J. Extracting the variance inflation factor and other multicollinearity diagnostics from typical regression results. Basic. Appl. Soc. Psychol. 39, 81–90 (2017).
Dorogush, A. V., Ershov, V. & Gulin, A. CatBoost: gradient boosting with categorical features support. (2018). https://doi.org/10.48550/arxiv.1810.11363
Prokhorenkova, L., Gusev, G., Vorobev, A., Dorogush, A. V. & Gulin, A. CatBoost: unbiased boosting with categorical features. (2017). https://doi.org/10.48550/arxiv.1706.09516
Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control. 19, 716–723 (1974).
Van Renterghem, K. M., von Bismarck, S., Bax, N. M., Fleer, A. & Höllwarth, M. E. Should an infected Nuss bar be removed? J. Pediatr. Surg. 40, 670–673 (2005).
Castellani, C., Schalamon, J., Saxena, A. K. & Höellwarth, M. E. Early complications of the Nuss procedure for pectus excavatum: a prospective study. Pediatr. Surg. Int. 24, 659–666 (2008).
Shin, S., Goretsky, M. J., Kelly, R. E., Gustin, T. & Nuss, D. Infectious complications after the Nuss repair in a series of 863 patients. J. Pediatr. Surg. 42, 87–92 (2007).
Pan, L. et al. Modified surgical incision suturing technique in uniportal video-assisted thoracoscopic pulmonary resection. J. Thorac. Dis. 16, 1388–1396 (2024).
Dai, X., Zhang, Y., Wang, F., Luo, Y. & Gong, Y. Effects of umbilical Preparation before Trans-umbilical Laparo-endoscopic Single-site surgery on umbilical wounds healing: a randomized controlled trial. Surg. Laparosc. Endosc Percutan Tech. 32, 632–636 (2022).
MacLeod, A. R., Serrancoli, G., Fregly, B. J., Toms, A. D. & Gill, H. S. The effect of plate design, bridging span, and fracture healing on the performance of high tibial osteotomy plates: an experimental and finite element study. Bone Jt. Res. 7, 639–649 (2018).
Verri, F. R., de Souza Batista, V. E., Santiago Jr, J. F., de Faria Almeida, D. A. & Pellizzer, E. P. Effect of crown-to-implant ratio on peri-implant stress: a finite element analysis. Mater. Sci. Eng. C. 45, 234–240 (2014).
Choi, J. H., Park, I. K., Kim, Y. T., Kim, W. S. & Kang, C. H. Classification of pectus excavatum according to objective parameters from chest computed tomography. Ann. Thorac. Surg. 102, 1886–1891 (2016).
Lee, C. et al. Measurement of sternal curvature angle on patients with pectus excavatum. Pediatr. Surg. Int. 33, 65–67 (2017).
Acknowledgements
We would like to thank the teachers and classmates of the Department of Thoracic Surgery, West China Hospital, Sichuan University for their help.
Author information
Authors and Affiliations
Contributions
SHP: Data collection, analysis and writing.LF: Project design, data analysis and review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical standards
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of West China Hospital of Sichuan University, which agreed to waive informed consent from patients. The ethics number is 2023 (review number 1986), and patient information is anonymized and kept confidential to avoid personal privacy and commercial interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sun, H., Lin, F. Analysis of risk factors for poor wound healing after Nuss procedure for pectus excavatum and establishment of a predictive model. Sci Rep 15, 22271 (2025). https://doi.org/10.1038/s41598-025-07373-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-07373-z