Abstract
Moyamoya disease (MMD) has been reported to be associated with a wide range of structural and functional abnormalities of bilateral hemispheres. However, whether the interhemisphere functional connectivity (FC) of MMD patients is altered and its relationship with cognitive impairments still remain unclear. A total of 20 patients with MMD and 25 healthy subjects were included, matched based on age, sex, and level of education. All participants were given cognitive assessments, and the whole-brain voxel-mirrored homotopic connectivity (VMHC) was derived through rs-fMRI analysis. The study utilized Pearson correlation analysis to examine the relationship between VMHC values and neuropsychological test results. Compared to the healthy controls, patients with MMD exhibited significantly lower VMHC in the middle frontal gyrus (MFG), orbital part of the inferior frontal gyrus (IFGorb), postcentral gyrus (PoCG) and angular gyrus (AG). Furthermore, following Pearson correction, a significant positive correlation was found between the recognition section scores of the Rey Auditory-Verbal Learning Test (AVLT) and the VMHC value for the PoCG and AG in MMD patients. This study is limited by its small sample size and cross-sectional design, which preclude the establishment of causal relationships between imaging indicators and clinical symptoms. These results suggest that VMHC analysis offers valuable insights into potential bilateral brain functional activity in MMD subjects, providing new perspectives on the neuroimaging mechanisms associated with cognitive decline, particularly memory impairment.
Similar content being viewed by others
Introduction
Moyamoya disease (MMD) is a persistent cerebrovascular condition marked by the gradual narrowing of the end segment of the internal carotid artery and the circle of Willis. This leads to the formation of a supplementary vascular network at the base of the brain1. Cognitive impairment is a prevalent concern, affecting more than 70% of individuals with MMD2,3,4 and manifesting in various domains, including intelligence, memory, attention, and computational ability3,5,6. Despite the high incidence of cognitive impairment in MMD patients, the precise mechanisms underlying these deficits are still unclear.
Homotopic functional connectivity (HoFC) refers to the functional connectivity between mirror areas of the two cerebral hemispheres, and is regarded as a manifestation of the functional integration and lateralization7. HoFC is recognized as indispensable for the maintenance of normal brain functions, encompassing cognition, sensation, mood stability, and behavior. Previous research consistently associates abnormal connectivity in the HoFC with cognitive decline8. Specifically, one study delineated a negative relationship between HoFC in the occipital fusiform gyrus and lingual gyrus and performance in tasks assessing visual long-term memory9. A subsequent investigation found that the activation of the HoFC in the ventromedial prefrontal cortex and insula was negatively correlated with performance on the color-word inhibition task, which primarily assesses executive function10. Cocaine addicts show a significant correlation between activity in the HoFC within the regions of the dorsal attention network and self-reported attention lapses11. Reduced HoFC in prefrontal and posterior cingulate regions correlated with attention and memory deficits in obstructive sleep apnea-hypopnea syndrome (OSAHS) patients12. These results highlight the connection between HoFC and cognitive function.
HoFC appears to be constrained by the structural connections within the brain, particularly the corpus callosum, which plays a crucial role in transmitting information between the two hemispheres13. This intricate network involves bilateral sensory information integration and various advanced cognition. Multiple research studies, involving both patients and individuals in good health, strongly support the argument that connectivity between cortical regions via the corticocortical corpus callosum is the fundamental structural foundation for the HoFC across hemispheres8,14. Interestingly, investigations by Su et al. have found evidence of white matter deterioration in the corpus callosum and corona radiata among patients with MMD. By comparison, the extent and severity of white matter damage in MMD surpass those observed in cerebrovascular atherosclerosis disease15. Consequently, we propose the presence of an aberration in HoFC within MMD, potentially contributing to cognitive impairment.
Resting-state functional magnetic resonance imaging (rs-fMRI) provides an opportunity to examine HoFC among patients1. Over recent years, rs-fMRI has become increasingly integral in identifying potential cognitive biomarkers associated with cognitive impairment in MMD1,16. A study reported an inverse correlation between working memory and performance speed scores and the extent of disruption in the default mode network discerned through rs-fMRI17. In particular, a significant correlation was observed between decreased functional connectivity of the left supplementary motor area (SMA) and the left orbital part of the inferior frontal gyrus (IFGorb) with cognitive function in patients with MMD18. Another investigation proposed topological metrics differentiating MMD patients with cognitive impairment, those without cognitive impairment, and healthy controls19 thereby suggesting a link between higher cognitive function and the organizational patterns of fundamental brain networks. Cumulatively, combined evidence from these studies demonstrates that cognitive impairment in MMD may originate from disrupted connections spanning various brain regions, rather than isolated regional abnormalities20. However, to date, the investigation into HoFC remains absent in studies involving MMD patients. Voxel-mirrored homotopic connectivity (VMHC) is a validated method for calculating HoFC. VMHC quantifies interhemispheric homotopic connectivity by measuring resting-state functional connectivity (RSFC) between each voxel’s time series in one brain hemisphere and that of its homotopic voxel in the contralateral hemisphere21.
In this study, VMHC was employed to investigate abnormalities in resting-state interhemispheric functional connectivity and its neural correlations with clinical cognitive impairment in patients with MMD.
Methods
This study was conducted in accordance with the principles outlined in the Declaration of Helsinki. Ethical approval was obtained from the Ethics Committee of Anhui Medical University. All patients or participants provided written informed consent prior to their participation in the study.
Participants
Twenty patients diagnosed with MMD were selected from the Department of Neurology at the First Affiliated Hospital of Anhui Medical University. Of these, 18 patients had ischemic MMD with symptoms including transient ischemic attack (n = 3), cerebral ischemia (n = 6), and dizziness (n = 9). Two patients had hemorrhagic MMD. The inclusion criteria were as follows: (1) patients diagnosed with MMD based on digital subtraction angiography (DSA) following established guidelines22; (2) age between 18 and 70 years; (3) education of at least three years, with the ability to read and calculate; (4) absence of recent (within 6 months) evidence of stroke, wherein subcortical maximum diameter > 1 cm on MRI was not observed in the cortex, basal ganglia, brainstem, or cerebellum; diffusion-weighted imaging (DWI) showed no acute intracranial lesions; (5) National Institute of Health Stroke Scale (NIHSS)23 score of 0, Modified Rankin Scale (MRS)24 ≤ 1. The exclusion criteria were: (1) severe disabilities, including vision impairment and psychiatric disorders; (2) central nervous system infections, neurodegenerative disorders, cranial trauma, or other severe neuropathies; (3) implanted devices like pacemakers, neurostimulators, metallic heart valves, or other internal ferromagnetic objects that would interfere with completing an MRI; (4) previous pharmaceutical treatments or surgical interventions that could impair cognitive function. Furthermore, a cohort of healthy individuals, matched for age, gender, and education, and assessed by a neurologist, was also enlisted. Informed consent was obtained from each patient and healthy subject.
Neuropsychological Data Acquisition
Preceding MRI scanning, we administered the Mini-Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA) to the subjects. The MMSE, widely used for assessing cognitive function, provides a brief, objective measure25,26. Multiple cognitive domains are assessed with this test, including orientation, repetition, verbal recall, attention and calculation, language, and visual construction. The MoCA is viewed as a precise instrument for identifying mild cognitive impairment (MCI)26,27. The assessment includes a 7-item questionnaire that evaluates cognitive performance in visuospatial/executive function, naming, delayed recall, attention, language, abstraction, and orientation. The Chinese version of the Rey Auditory-Verbal Learning Test (AVLT) was conducted for individuals with MMD. The AVLT evaluates immediate recall (IR), delayed recall (DR), and recognition, thereby examining both instantaneous and delayed memory function28,29.
MRI Data Acquisition
Structural and functional MRI data were obtained at the University of Science and Technology of China, Hefei, Anhui Province. Participants were instructed to recline within the scanner with closed eyes, maintaining wakefulness, and ensuring minimal head movement throughout the scanning procedure. Earplugs were employed to mitigate the impact of machine-generated noise on participants. Functional images were obtained using a 3.0 T MRI scanner (Discovery GE750w; GE Healthcare, Buckinghamshire, UK). A complete fMRI scan consisted of 217 echo-planar imaging volumes, with a total duration of 8 min and 41 s for a resting-state MRI scan. The fMRI parameters were set as follows: TR (Repetition Time) = 2400 ms, TE (Echo Time) = 30 ms, flip angle = 90°, matrix size = 64 × 64, field of view = 192 × 192 mm², slice thickness = 3 mm, and 46 slices (voxel size = 3 × 3 × 3 mm³). Additionally, T1-weighted anatomical images comprising 188 slices were acquired in the sagittal orientation with the following parameters: TR = 8.16 ms, TE = 3.18 ms, flip angle = 12°, field of view = 256 × 256 mm², slice thickness = 1 mm, voxel size = 1 × 1 × 1 mm³.
MRI Data Processing
We utilized the Data Processing Assistant for Resting-State Functional MR Imaging toolkit (DPARSF, Yan and Zang 2010, http://rfmri.org/DPARSF)30,31 which is based on the Resting State Functional MR Imaging Toolkit (REST; http://www.restfmri.net) and the statistical parametric mapping software package (SPM12; http://www.fil.ion.ucl.ac.uk/spm/software/spm12). The initial 10 volumes were excluded from the analysis. Slice timing for the remaining images was corrected, and then the images were realigned to the first volume. Head motion greater than 2 mm translation or 2 degrees rotation was not included in the following analyses. Further preprocessing included normalizing the structural T1 image to the Montreal Neurological Institute (MNI) space using the Diffeomorphic Anatomical Registration Through Exponentiated Lie algebra (DARTEL) and transferring the functional images to the MNI space based on the transformation matrix. The functional images were first smoothed using a 6 mm Gaussian kernel at full width at half maximum. Subsequently, linear detrending was performed and the data underwent band-pass filtering (0.01–0.08 Hz) to minimize low-frequency drift and high-frequency noise. Additionally, several potential sources of spurious covariance were eliminated, such as the six head-motion parameters acquired through rigid body correction and signals from white matter and cerebrospinal fluid.
Voxel-Mirrored Homotopic Connectivity
The DPARSF software was employed to investigate VMHC, calculating the Pearson correlations between the preprocessed time series of symmetrical interhemispheric voxels for each subject. The correlation values were then transformed by Fisher’s z to improve their normal distribution. We utilized the unilateral hemispheric gray matter derived from the symmetric template as a mask for conducting statistical analyses on VMHC. A two-tailed, two-sample t-test was performed using SPM12 software to discover areas showing substantial variations in VMHC among individuals with MMD and HCs. For multiple comparison adjustments, cluster-level false discovery rate (FDR) correction was applied with a corrected threshold of p < 0.005.
Statistical Analysis
Demographic and clinical data were analyzed using two-sample t-tests for age and educational years, as well as for clinical scores. Gender distribution was assessed using a chi-squared test in IBM Statistical Product and Service Solutions Version 26 (SPSS 26). To examine the association of VMHC with clinical features, we carried out correlation analyses between MMSE scores, MoCA scores, AVLT scores, and the VMHC of ROIs that showed a significant decrease in patients. We used Pearson’s correlation as the data were normally distributed, or Spearman’s correlation if the data were not normally distributed for VMHC-clinical correlation. A p-value < 0.05 (two-tailed) was considered statistically significant.
Results
Demographics and Neuropsychological Characteristics
The demographic and neuropsychological profiles of both groups are outlined in Table 1. The age of the patients with MMD ranged from 27 to 64 years. There were no differences in terms of age (t = −0.851, p = 0.400), gender (χ² = 0.538, p = 0.475), or education (t = −1.399, p = 0.172) between the MMD and HC groups. Patients with MMD had lower MMSE and MoCA scores than the HCs (p < 0.01). This indicates that MMD patients experience lower cognitive function.
Alterations in VMHC between MMD and HC groups
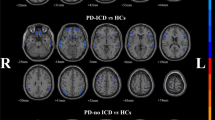
When comparing VMHC values in the MMD and HC groups, significant differences were detected in middle frontal gyrus (MFG), IFGorb, postcentral gyrus (PoCG) and angular gyrus (AG) (FDR corrected, p < 0.005, cluster size ≥ 30 voxels). Details of group differences in VMHC are shown in Table 2; Fig. 1.
Correlational Analysis
Reduced VMHC values in both the bilateral PoCG and AG were found to have a positive correlation with AVLT-recognition scores (r = 0.72, 0.67, respectively, all p < 0.05). Details of the correlation analyses are shown in Fig. 2. No significant findings emerged from the correlational analysis assessing altered VMHC in relation to other neuropsychological scales.
Brain regions with a significantly decreased VMHC in the MMD group compared to the HC group, FDR corrected (p < 0.005, cluster size ≥ 30 voxels). The color bar indicates the T value. Abbreviations: VMHC, voxel-mirrored homotopic connectivity; MMD, moyamoya disease; HC, healthy control; FDR, false discovery rate; L, left; R, right.
Discussion
In this research, we investigated the interhemispheric functional connectivity at rest in patients diagnosed with MMD. To the best of our knowledge, this is the first application of VMHC to investigate homotopic functional connectivity in patients with MMD. Our research demonstrates that MMD is characterized by abnormal interhemispheric connectivity in various brain regions, with reduced VMHC observed in the MFG, IFGorb, PoCG, and AG regions in MMD.
Generally, the decreased VMHC values herald the existence of desynchronization between cerebral hemispheres, in other words, the lateralization of brain function7. Accumulating empirical evidence indicates the potential of VMHC as an approach for facilitating a nuanced exploration of various cerebrovascular disease mechanisms32,33,34,35. Moreover, emerging findings suggest an association between VMHC and cognitive impairment, particularly in the context of memory decline. An increase in functional homotopy in the prefrontal cortex has been associated with degeneration of the corpus callosum and a decrease in working memory36. Additionally, separate investigations reveal a positive correlation between VMHC and both short-term and long-term memory test outcomes35. The relationships that have developed have piqued our interest in exploring how cognitive impairment is linked to changes in VMHC among patients with MMD.
Our study unveils a reduction in VMHC within the MFG and IFGorb region among individuals diagnosed with MMD, supported by numerous prior investigations. These studies demonstrate decreased white matter hypoperfusion37 white matter structural and network impairments38 reduced pairwise connectivity39 and altered regional homogeneity (ReHo) in the MFG among MMD patients, reinforcing our findings. Simultaneously, several studies report decreased cerebral blood flow (CBF)40 fractal dimension41 and white matter structural and network impairments38 within the IFG ___domain among MMD patients. Moreover, studies focusing on functional connectivity consistently produce significant results. Specifically, one study identifies attenuated functional connectivity between the left IFGorb and the left SMA in MMD patients, with the extent of this reduction significantly correlating with scores on the MMSE, MoCA, and Trail Making Test (TMT) Part B18. Another study documents heightened functional connectivity between the IFG and the ipsilateral PoCG, associated with limb paresthesia42. Notably, the left IFG plays a pivotal role in various cognitive domains, encompassing language, semantics, action, social cognition, and executive function43. Given its multifaceted role, compromised interhemispheric communication between bilateral IFG regions may signify disrupted modulation of coordinated repression processes, potentially contributing to the dysregulation of cognitive function observed in MMD.
A reduced VMHC was also noted in the AG and PoCG for patients with MMD. Regarding the PoCG, this region is primarily responsible for processing somatic sensory information44. Previous research has noted both structural and functional abnormalities in the PoCG of MMD patients. For instance, structural investigations by Tompkins et al.45 identified abnormal cortical thickness in the PoCG. Furthermore, Haas et al.46 reported abnormal cortical representation through regionally distributed cortical thickness measurements. On the functional side, studies utilizing EEG and fMRI have revealed alterations such as faded critical dynamics within the PoCG in adult MMD47. The convergence of these documented structural and functional impairments leads us to postulate that this diminished interhemispheric synchrony in the PoCG is a manifestation of these underlying pathologies and may contribute to the somatic sensory symptoms frequently experienced by individuals with MMD, including weakness, numbness, or paralysis48. The AG is a critical element of the default mode network (DMN), which has been implicated in various neuropsychiatric disorders such as Alzheimer’s disease, schizophrenia, major depressive disorder (MDD), generalized anxiety disorder (GAD), and others49,50,51. The observed reduction in VMHC within the AG may reflect disrupted disengagement in the DMN during states of disorder. This disruption within the DMN potentially provides a neurophysiological foundation for the elevated levels of depression and anxiety observed in MMD patients.
Interestingly, the subsequent correlational analysis revealed that significantly reduced VMHC values in the bilateral PoCG and AG were positively correlated with AVLT‑recognition scores. The AVLT is a well‑established measure of verbal learning and memory: participants are presented with two 15‑word lists across multiple trials, followed by immediate recall, delayed recall, and recognition phases, allowing precise assessment of learning rate and retention28. The AG is a brain region associated with complex language functions52 and the PoCG has intricate subcortical connections with the AG53. In a study of patients with type 2 diabetes and cognitive impairment, researchers found a positive correlation between functional connectivity of the right AG and the bilateral middle temporal gyrus (MTG) and AVLT scores54. In children during a critical period of language development, pre‑reading scores positively correlated with functional connectivity between the auditory cortex and the AG55. These findings align with our ongoing research discoveries. Given that the AVLT assesses an individual’s capacity to encode, integrate, retain, and retrieve verbal information across various stages of immediate memory54 these findings suggest that the compromised performance of these cognitive functions in MMD patients may be due to decreased VMHC in the AG and PoCG regions. However, our analysis revealed no significant associations between altered VMHC and MMSE or MoCA total scores in our first-episode MMD sample. This may be due to our relatively small sample size. Moreover, because MMSE and MoCA aggregate cover multiple cognitive domains—including orientation, memory, attention, naming, and executive function—relying solely on total scores may obscure ___domain‑specific deficits53,55. We therefore suggest that future investigations should examine VMHC relationships with individual MMSE and MoCA sub-items rather than total scores alone.
In MMD, revascularization surgery remains the mainstay of treatment and has been recognized as potentially reducing neurological insult2. Prior studies have demonstrated the ability of revascularization surgery to improve neuropsychological outcomes2 and cognitive functions, including those related to intellectual, memory, and language domains among MMD patients56,57. In rs-fMRI studies, normalization of DMN functional connectivity has been observed in patients undergoing revascularization surgery17. Additionally, a recent study identified longitudinal alterations in VMHC among stroke patients. Specifically, a notable increase in impaired VMHC within the superior precuneus at the second time point was observed. Furthermore, these VMHC changes in the superior precuneus showed a significant correlation with alterations in clinical scores34. Therefore, we speculate that revascularization surgery may also increase VMHC levels in the damaged brain regions of MMD patients, potentially contributing to the improvement of clinical symptoms. We await future longitudinal research on this matter.
In addition, several alternative explanations for our findings warrant consideration. Hemodynamic disturbances inherent to MMD, such as impaired cerebrovascular reactivity (CVR) and perfusion delays58,59 may distort Blood Oxygen Level-Dependent (BOLD) fMRI signals, thereby reducing measured VMHC independent of actual neural dysfunction60. Methodological challenges, including spatial normalization difficulties arising from structural anomalies61 and the impact of physiological noise62 may also contribute to decreased VMHC. Furthermore, disease heterogeneity—such as ischemic versus hemorrhagic presentations—adds an additional layer of complexity to interpretation63. These physiological and technical confounders necessitate a cautious interpretation of our results. Disentangling these influences is essential for a more precise characterization of interhemispheric functional integrity in MMD.
Several potential limitations warrant consideration in this study. Firstly, the inclusion of a limited number of subjects constrained the statistical power of our analysis. Additionally, the use of a cross-sectional design means that participant data were gathered at a single time point, precluding the establishment of causal relationships between imaging markers and clinical symptoms. Furthermore, some patients in our cohort had a history of ischemic attack, which may have contributed to altered functional connectivity, particularly in regions associated with stroke-related damage. Finally, no significant correlation was found between VMHC values and cognitive scores on the MMSE and MoCA. This lack of correlation may stem from the fact that MMSE and MoCA assess multiple cognitive domains, suggesting that changes in VMHC values may be just one factor contributing to overall cognitive impairments. Therefore, there is a critical need for more large-scale, longitudinal studies to further validate our findings.
Conclusion
In summary, our study delineates a distinct disruption in VMHC among individuals with MMD in contrast to HCs. Specifically, patients featured with decreased VMHC in the MFG, IFGorb, PoCG, and AG. Furthermore, deviations in VMHC metrics within the PoCG and AG regions exhibited correlations with performance on the recognition part of AVLT. These results suggest that disturbed interhemispheric functional connectivity may be a potential mechanism contributing to MMD syndrome.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Zhang, X. et al. Progression in Moyamoya disease: clinical features, neuroimaging evaluation, and treatment. Curr. Neuropharmacol. 20, 292–308 (2022).
Mitchell, D. L. et al. Post-Surgical cognitive outcomes of Moyamoya disease: A systematic review. World Neurosurg. 178, 181–190e1 (2023).
Kronenburg, A. et al. The profile of cognitive impairment and hemodynamic compromise in moyamoya: a single-center prospective cohort study. J. Neurosurg. 138, 173–184 (2023).
Wang, X. et al. Cognitive dysfunction in Moyamoya disease: latest developments and future directions. Front. Hum. Neurosci. 18, 1502318 (2024).
Shi, Z., Wen, Y. J., Huang, Z., Yu, L. B. & Zhang, D. Different aspects of cognitive function in adult patients with Moyamoya disease and its clinical subtypes. Stroke Vasc Neurol. 5, 86–96 (2020).
He, S. et al. Characteristics of cognitive impairment in adult asymptomatic Moyamoya disease. BMC Neurol. 20, 322 (2020).
Mancuso, L. et al. The homotopic connectivity of the functional brain: a meta-analytic approach. Sci. Rep. 9, 3346 (2019).
Jin, X., Liang, X. & Gong, G. Functional integration between the two brain hemispheres: evidence from the homotopic functional connectivity under resting state. Frontiers Neuroscience 14, (2020).
Gracia-Tabuenca, Z., Moreno, M. B., Barrios, F. A. & Alcauter, S. Hemispheric asymmetry and homotopy of resting state functional connectivity correlate with visuospatial abilities in school-age children. Neuroimage 174, 441–448 (2018).
Zhao, J. et al. Age-Related decreases in interhemispheric Resting-State functional connectivity and their relationship with executive function. Front. Aging Neurosci. 12, 20 (2020).
Kelly, C. et al. Reduced interhemispheric resting state functional connectivity in cocaine addiction. Biol. Psychiatry. 69, 684–692 (2011).
L, J. I. et al. Abnormal changes of brain function in patients with OSAHS: VMHC-based rs-fMRI study. Chinese J. Magn. Reson. Imaging 14, 13–18 (2023).
Szczupak, D. et al. The relevance of heterotopic callosal fibers to interhemispheric connectivity of the mammalian brain. Cereb. Cortex. 33, 4752–4760 (2023).
Wang, P., Jiang, Y. & Biswal, B. B. Aberrant interhemispheric structural and functional connectivity within whole brain in schizophrenia. Schizophr Res. 264, 336–344 (2024).
Su, J. B. et al. Microstructural damage pattern of vascular cognitive impairment: a comparison between Moyamoya disease and cerebrovascular atherosclerotic disease. Neural Regen Res. 14, 858–867 (2019).
Hu, J. et al. Preoperative brain functional connectivity improve predictive accuracy of outcomes after revascularization in Moyamoya disease. Neurosurgery 92, 344–352 (2023).
Sakamoto, Y. et al. Default mode network changes in Moyamoya disease before and after bypass surgery: preliminary report. World Neurosurg. 112, e652–e661 (2018).
Hu, J. et al. Abnormal brain functional and structural connectivity between the left supplementary motor area and inferior frontal gyrus in Moyamoya disease. BMC Neurol. 22, 179 (2022).
Lei, Y. et al. Reconfigured functional network dynamics in adult Moyamoya disease: A resting-state fMRI study. Brain Imaging Behav. 14, 715–727 (2020).
Li, X. et al. Abnormal interhemispheric functional connectivity in acute pericoronitis: A Resting-State MRI study. J. Craniofac. Surg. 35, 2099–2104 (2024).
Zuo, X. N. et al. Growing together and growing apart: regional and sex differences in the lifespan developmental trajectories of functional homotopy. J. Neurosci. 30, 15034–15043 (2010).
Research Committee on the Pathology and Treatment of Spontaneous Occlusion of the Circle of Willis & Health Labour Sciences Research Grant for Research on Measures for Infractable Diseases. Guidelines for diagnosis and treatment of Moyamoya disease (spontaneous occlusion of the circle of Willis). Neurol. Med. Chir. (Tokyo). 52, 245–266 (2012).
Chalos, V. et al. National institutes of health stroke scale. Stroke 51, 282–290 (2020).
Broderick, J. P., Adeoye, O. & Elm, J. Evolution of the modified rankin scale and its use in future stroke trials. Stroke 48, 2007–2012 (2017).
Huan, G. & Heyong, S. Longitudinal Invariance and Construct Validity of the Chinese Version of the Mini-Mental State Examination Across 10 Years in the Elderly Population. J Nurs Meas JNM-2021-0102.R1 (2023). https://doi.org/10.1891/JNM-2021-0102
Jia, X. et al. A comparison of the Mini-Mental state examination (MMSE) with the Montreal cognitive assessment (MoCA) for mild cognitive impairment screening in Chinese middle-aged and older population: a cross-sectional study. BMC Psychiatry. 21, 485 (2021).
Nasreddine, Z. S. et al. The Montreal cognitive assessment, moca: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 53, 695–699 (2005).
Rey, A. L’examen Clinique En Psychologie (Presses universitaires de France, 1964).
Dong, F. M. et al. Chinese version of the auditory verbal learning test: normative study and clinical applications in Chinese-speaking population in Shijiazhuang City. Acta Neurol. Belg. 123, 873–883 (2023).
Yan, C. G., Wang, X. D., Zuo, X. N. & Zang, Y. F. DPABI: data processing & analysis for (Resting-State) brain imaging. Neuroinformatics 14, 339–351 (2016).
Chao-Gan, Y. & Yu-Feng, Z. D. P. A. R. S. F. A MATLAB toolbox for ‘pipeline’ data analysis of Resting-State fMRI. Front. Syst. Neurosci. 4, 13 (2010).
Chen, J. et al. Altered static and dynamic voxel-mirrored homotopic connectivity in subacute stroke patients: a resting-state fMRI study. Brain Imaging Behav. 15, 389–400 (2021).
W, D. et al. altered functional connectivity in patients with subcortical vascular cognitive impairment–A Resting-State Functional Magnetic Resonance imaging Study. PloS one 10, (2015).
Li, Y., Yu, Z., Zhou, X., Wu, P. & Chen, J. Aberrant interhemispheric functional reciprocities of the default mode network and motor network in subcortical ischemic stroke patients with motor impairment: A longitudinal study. Front. Neurol. 13, 996621 (2022).
Wu, L. et al. Voxel-Mirrored homotopic connectivity associated with change of cognitive function in chronic Pontine stroke. Front. Aging Neurosci. 13, 621767 (2021).
Avelar-Pereira, B., Bäckman, L., Wåhlin, A., Nyberg, L. & Salami, A. Increased functional homotopy of the prefrontal cortex is associated with corpus callosum degeneration and working memory decline. Neurobiol. Aging. 96, 68–78 (2020).
Filimonova, E., Ovsiannikov, K., Zaitsev, B. & Rzaev, J. T1w/T2w ratio is associated with the brush sign and perfusion delay in watershed regions in patients with Moyamoya angiopathy. Clin. Neurol. Neurosurg. 231, 107821 (2023).
Sun, R. et al. White matter structural and network topological changes in Moyamoya disease with limb paresthesia: A study based on diffusion kurtosis imaging. Front. Neurosci. 16, 1029388 (2022).
Kazumata, K. et al. Investigating brain network characteristics interrupted by Covert white matter injury in patients with Moyamoya disease: insights from graph theoretical analysis. World Neurosurg. 89, 654–665e2 (2016).
He, S. et al. Impairments in brain perfusion, executive control network, topological characteristics, and neurocognition in adult patients with asymptomatic Moyamoya disease. BMC Neurosci. 22, 35 (2021).
Liu, Z. et al. Changes of cerebral cortical structure and cognitive dysfunction in ‘healthy hemisphere’ after stroke: a study about cortical complexity and sulcus patterns in bilateral ischemic adult Moyamoya disease. BMC Neurosci. 22, 66 (2021).
Sun, R. et al. Changes in sensory-related brain networks of patients with Moyamoya disease with limb paresthesia: A resting-state fMRI-based functional connectivity analysis. Neuroimage Clin. 36, 103267 (2022).
Diveica, V. et al. Graded functional organization in the left inferior frontal gyrus: evidence from task-free and task-based functional connectivity. Cereb. Cortex. 33, 11384–11399 (2023).
Kropf, E., Syan, S. K., Minuzzi, L. & Frey, B. N. From anatomy to function: the role of the somatosensory cortex in emotional regulation. Braz J. Psychiatry. 41, 261–269 (2019).
Tompkins, G., Levman, J., Ijner, P., Shiohama, T. & Takahashi, E. Cortical thickness in clinical Moyamoya disease: A magnetic resonance imaging study. Int. J. Dev. Neurosci. 81, 698–705 (2021).
Haas, P. et al. Whole-brain volumetric analysis in adult Moyamoya patients reveals significant atrophy compared to healthy controls. Brain Commun. 7, fcaf100 (2025).
Lei, Y. et al. Faded Critical Dynamics in Adult Moyamoya Disease Revealed by EEG and fMRI. Oxid. Med.Cell Longev. 6640108 (2021).
Antonov, A., Terraciano, A., Essibayi, M. A., Javed, K. & Altschul, D. J. Current Understanding of Moyamoya disease (MMD) and associated neuropsychiatric outcomes. Neuropsychiatr Dis. Treat. 19, 2673–2680 (2023).
Buckner, R. L., Andrews-Hanna, J. R. & Schacter, D. L. The brain’s default network: anatomy, function, and relevance to disease. Ann. N Y Acad. Sci. 1124, 1–38 (2008).
Mohan, A. et al. The significance of the default mode network (DMN) in neurological and neuropsychiatric disorders: A review. Yale J. Biol. Med. 89, 49–57 (2016).
Li, W. et al. Specific and common functional connectivity deficits in drug-free generalized anxiety disorder and panic disorder: A data-driven analysis. Psychiatry Res. 319, 114971 (2023).
Vatansever, D., Manktelow, A. E., Sahakian, B. J., Menon, D. K. & Stamatakis, E. A. Angular default mode network connectivity across working memory load. Hum. Brain Mapp. 38, 41–52 (2017).
Niu, M. & Palomero-Gallagher, N. Architecture and connectivity of the human angular gyrus and of its homolog region in the macaque brain. Brain Struct. Funct. 228, 47–61 (2023).
Tan, X. et al. Altered functional connectivity of the posterior cingulate cortex in type 2 diabetes with cognitive impairment. Brain Imaging Behav. 13, 1699–1707 (2019).
Benischek, A. et al. Pre-reading Language abilities and the brain’s functional reading network in young children. Neuroimage 217, 116903 (2020).
Joshi, S. B. et al. Functional and neuropsychological outcome after surgical treatment of Moyamoya disease. World Neurosurg. S1878-8750 (24), 00235–00233. https://doi.org/10.1016/j.wneu.2024.02.038 (2024).
Deckers, P. T. et al. Clinical outcome, cognition, and cerebrovascular reactivity after surgical treatment for Moyamoya vasculopathy: A Dutch prospective, Single-Center cohort study. J. Clin. Med. 11, 7427 (2022).
He, S. et al. Evaluation of cerebrovascular reactivity in Moyamoya disease using oxygen-dependent magnetic resonance imaging. iScience 27, 108923 (2024).
Zerweck, L. et al. Evaluation of the cerebrovascular reactivity in patients with Moyamoya angiopathy by use of breath-hold fMRI: investigation of voxel-wise hemodynamic delay correction in comparison to [15O]water PET. Neuroradiology 65, 539–550 (2023).
Golestani, A. M., Kwinta, J. B., Strother, S. C., Khatamian, Y. B. & Chen, J. J. The association between cerebrovascular reactivity and resting-state fMRI functional connectivity in healthy adults: the influence of basal carbon dioxide. Neuroimage 132, 301–313 (2016).
Crinion, J. et al. Spatial normalization of lesioned brains: performance evaluation and impact on fMRI analyses. Neuroimage 37, 866–875 (2007).
Tong, Y., Hocke, L. M. & Frederick, B. B. Low frequency systemic hemodynamic ‘noise’ in resting state BOLD fMRI: characteristics, causes, implications, mitigation strategies, and applications. Front. Neurosci. 13, 787 (2019).
Boren, S. B. et al. Longitudinal Resting-State functional magnetic resonance imaging study: A Seed-Based connectivity biomarker in patients with ischemic and intracerebral hemorrhage stroke. Brain Connect. 13, 498–507 (2023).
Acknowledgements
The authors would like to thank all the participants and staff involved in this study.
Funding
This work was supported by National Natural Science Foundation Incubation Program of The Second Affiliated Hospital of Anhui Medical University (grant number 2022GQFY13) and the Research Fund of Anhui Institute of Translational Medicine (grant number 2022zhyx-B11).
Author information
Authors and Affiliations
Contributions
J.N., P.W., C.L., Q.W., and K.W. conceived and designed the study. J.N., B.D., C.C., and S.C. were responsible for participant recruitment and data collection. J.N. and L.Q. conducted the data analyses, drafted the manuscript and prepared the figures. L.W. and Y.T. contributed to study design and critically revised the manuscript. J.N. and L.W. secured funding. All authors reviewed and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was approved by the Ethics Committee of Anhui Medical University (approval number 2021H048). Written informed consent was obtained from all participants.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Nie, J., Qi, L., Wu, P. et al. Disrupted resting-state interhemispheric functional connectivity in patients with first-episode moyamoya disease. Sci Rep 15, 23655 (2025). https://doi.org/10.1038/s41598-025-07462-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-07462-z