Abstract
This article aims to describe the current diagnostic and therapeutic practices for managing chronic rhinosinusitis with nasal polyps (CRSwNP) among Austrian ENT specialists. A cross-sectional, nationwide survey was conducted between November and December 2022 in Austria. A total of 50 ENT specialists, evenly split between hospital- and office-based physicians, participated. The questionnaire covered demographics, diagnostic and therapeutic approaches. CT imaging, nasal endoscopy, and blood eosinophil count were the most utilized diagnostic tools. Most participants applied the SNOT-22 for patient-reported outcomes. Local corticosteroids were the most frequently prescribed treatment. Systemic corticosteroid overuse and limited biologic adoption were noted. Hospital-based physicians managed significantly more patients with biologics. The initiation of biologic therapy was considered most appropriate following one FESS by the vast majority of respondents. Adherence to guideline-based evaluation criteria for biologic treatment response was suboptimal, highlighting gaps in clinical practice. Our survey highlights strengths in guideline adherence and areas for improvement, particularly in the diagnostic approach, systemic corticosteroid usage, and biologic treatment. Addressing educational gaps and refining clinical practices could enhance patient outcomes, reduce invasive procedures, and optimize resource utilization in CRSwNP management.
Similar content being viewed by others
Introduction
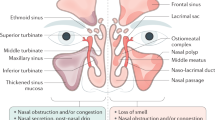
Chronic rhinosinusitis with nasal polyps (CRSwNP) is a chronic inflammation of the sinonasal mucosa characterized by typical rhinologic symptoms including nasal blockage, rhinorrhea, facial pain and impaired sense of smell1. The prevalence in Western countries has been estimated at 2–4%2,3. CRSwNP commonly displays a type 2 inflammatory profile in western countries, marked by elevated levels of IL-4, IL-5, and IL-13, along with eosinophilic infiltration4,5. Asthma bronchiale and non-steroidal anti-inflammatory drug-exacerbated respiratory disease (N-ERD), often coexist as comorbidities due to shared pathomechanisms. The presence of these comorbidities is linked to a higher disease burden and a greater risk of CRSwNP recurrence6,7. Diagnosis of CRSwNP is typically based on both clinical presentation and objective findings, including nasal endoscopy and computed tomography (CT) imaging, which reveal the characteristic nasal polyp formations. Given the chronic nature of the condition and the frequent need for individualized treatment, management often requires a combination of medical and surgical approaches. Traditional treatments for CRSwNP include local corticosteroids (LCS), systemic corticosteroids (SCS), and functional endoscopic sinus surgery (FESS)1. While LCS as first-line treatment are used to manage inflammation locally1,8, SCS may be employed in short-term bursts to provide more immediate symptom relief, though with potential side effects1,9. FESS is indicated when appropriate medical therapies fail to control symptoms sufficiently. However, a substantial proportion of patients experience disease recurrence10,11. Recent therapeutic advancements introduced biologic treatments targeting key cytokines involved in the inflammatory process of CRSwNP. Currently approved biologics for the treatment of CRSwNP include dupilumab, mepolizumab, and omalizumab12. These agents have shown promising results for efficacy and safety in clinical trials13 as well as in real life studies14,15,16, leading to their incorporation in treatment guidelines with established criteria for indication and evaluation1. However, several factors still need consideration such as the high cost and the (potential) lifelong treatment duration. Therefore, it is important to select the right candidate for biological therapy, as a considerable proportion of patients do not respond well to the treatment17. Moreover, certain questions remain open, like which biologic is most suited for which patient, which biomarkers are suitable as predictors for treatment response or are there any long-term side effects18.
Recent nationwide surveys on CRSwNP management have underscored how management practices vary across countries, reflecting differences in healthcare infrastructure, accessibility to biologics, practice patterns and adherence to guidelines19,20,21,22,23. In Austria, there is limited research on the current state of CRSwNP management, making it essential to examine how ENT specialists approach diagnosis and treatment of CRSwNP. Understanding these practices can provide valuable insights into areas where healthcare support systems, educational campaigns or national guidelines may need to evolve, particularly concerning the recently introduced biologic treatment. Therefore, this article aimed to describe the current state of CRSwNP management among ENT specialists in Austria, with a focus on diagnostic and therapeutic approaches.
Materials and methods
Study design and participants
A cross-sectional, nationwide survey was conducted to assess current diagnostic and therapeutic practices for CRSwNP among Austrian ENT specialists. The questionnaire was administered through online interviews or telephone calls between November 14 and December 2, 2022. A total of 50 ENT specialists, evenly split between hospitals (n = 25) and private practices (n = 25) across Austria, participated in this survey. The inclusion criteria required participants to be active ENT physicians treating CRSwNP patients. Selection followed a purposive sampling strategy to ensure a balanced representation of practice types and regions. Participation was voluntary and continued until the target sample size was reached.
Study questionnaire
The survey was divided into four sections: (1) Demographics and practice characteristics: years of experience, type of practice (hospital or private office), number of treated CRSwNP patients in the last 6 months, and working ___location; (2) Diagnostic management of CRSwNP: Participants were asked about the usual diagnostic pathway, which diagnostic tools they use, and which patient-reported outcome measures (PROMs) they apply. (3) Treatment practices for CRSwNP: this section included questions on the most common treatments for CRSwNP, including LCS, SCS, FESS, and biologic therapy; (4) Comorbidities and interdisciplinary care: prevalence of relevant comorbidities and interdisciplinary collaborations were assessed. Question types varied between single-choice, multiple-choice, proportional-scales, and open-text responses. The questionnaire was developed by the author team based on current clinical guidelines and expert knowledge, followed by a pilot test involving six ENT specialists to ensure content validity. Based on their feedback, minor modifications were made. Due to the descriptive and exploratory nature of the survey, and because most items were structured and factual in nature, formal psychometric reliability testing was not conducted.
Data analysis
SPSS © statistical software, version 29.0 (IBM ©, Armonk, NY, USA) was used. Quantitative data were reported using descriptive statistics. For the exploratory subgroup analyses (hospital-based vs. office-based), Whitney-U-Test was utilized to compare results of proportional-scaled questions (Rosenthal’s r was applied as effect size). χ2-test was used to compare categorical data (effect size was expressed with Cramer’s V). In contingency tables greater than 2 × 2, Bonferroni-adjusted Z-tests were used as post hoc test in case of significant χ²-test. Statistical significance level α was set at p < 0.05, two sided.
Sample size calculation and power analysis
According to publicly available records, the total population of ENT specialists in Austria in 2022 was 795. Based on a desired confidence level of 80% and a margin of error of 10%, a minimum sample size of 39 participants would be sufficient for generalizable survey estimates (Sample size calculator, SurveyMonkey©). Our final sample consisted of 50 participants, exceeding this threshold and thus ensuring a robust representation of the national ENT specialist population. Given the exploratory nature of our subgroup analyses, we conducted post-hoc power analyses, given α-level, sample size and effect size (G*Power, Version 3.1.).
Ethics
The survey was carried out in accordance with relevant guidelines and regulations. Ethical approval for the survey was waived by the Institutional Review Board of the Medical University of Graz, as the data were fully anonymized. Informed consent was obtained from all participants.
Results
Survey participants
A total of 50 ENT specialists from Austria participated in the survey, evenly divided between hospital-based (n = 25, 50%) and office-based settings (n = 25, 50%). The participants represented a broad range of professional experience, spanning from less than 5 years to over 30 years. The majority (62%, n = 31) of the ENT physicians were based in urban areas with a population exceeding 50,000 inhabitants. All participants had treated at least 10 patients with CRSwNP in the past 6 months, with 74% (n = 37) managing more than 30 patients during this period. Detailed data are given in Table 1.
Diagnostic pathway in CRSwNP
ENT specialists reported that 96% of CRSwNP patients were referred by another physician, most frequently pulmonologists and primary care physicians, while the remaining 4% were seen without a referral. Half of the CRSwNP patients (50%) were pre-treated by primary care physicians before consulting an ENT specialist. By far the most frequently applied diagnostic tools were CT imaging (74%, n = 37), nasal endoscopy (72%, n = 36) and the assessment of blood eosinophil count (BEC) (72%, n = 36). Comprehensive results of diagnostic tool utilization are presented in Fig. 1. The most commonly used PROM was SNOT-22 (92%, n = 46), followed by overall symptom severity score (74%, n = 37), nasal congestion score (54%, n = 27), asthma-specific test like asthma control test or asthma quality of life questionnaire (34%, n = 17) and impaired sense of smell severity score (24%, n = 12).
Treatment of CRSwNP
According to participants’ responses, 93% of CRSwNP patients received LCS, while 34% received SCS, 51% had FESS treatment and 11% were treated with biologics. Among the 51% with FESS treatment, 23% underwent more than one procedure. All participants (100%, n = 50) regarded more than one revision FESS within 10 years as excessive. The majority of ENT physicians (84%, n = 42) prescribe SCS as a treatment option for CRSwNP, with 67% indicating a treatment duration of less than two weeks, 31% reporting a duration of 2–4 weeks, and 3% extending treatment to 4–8 weeks. A dosage of 2.5–7.5 mg prednisolone-equivalent per day was reported in 60% of cases. On average, 49% of CRSwNP patients received < 1 SCS course per year, while 28% received 1–2 courses and 22% received 3–4 courses annually. All participants (100%, n = 42) agreed that SCS prescriptions should be limited to the shortest duration needed. Adverse effects related to SCS therapy were reported by only 4% of participating physicians (high blood pressure and worsening of diabetes mellitus). Detailed data on SCS treatment patterns can be found in Table 2.
Participants were asked to state known biologics approved for CRSwNP treatment as open-text response. All participants (100%) stated at least one biologic agent, while 3% (n = 6) stated more than one. The best-known biologic medication was Dupixent® (Dupilumab), named by 60% (n = 30), followed by Nucala® (Mepolizumab, 28%, n = 14) and Xolair® (Omalizumab 18%). Chosen reasons to prescribe biologics were: reduction of need for FESS (96%, n = 48), improvement of sense of smell (60%, n = 30), high surgical or anesthesiologic risk (56%, n = 28), reduction of SCS therapy (56%, n = 28) insufficient symptom control (20%, n = 10) and accordance with guidelines (12%, n = 6). The start of biologic therapy was considered most appropriate following one FESS by 98% (n = 49) of participants, prior to any FESS by 2% (n = 1) and by nobody (0%) following 2 or > 2 FESS. The most commonly preferred dosing interval of biologics was every 4 weeks (74%, n = 37), followed by 2-weeks (24%), while 2% (n = 1) let their patients decide for themselves. Response to biologics was most commonly assessed by improvement of sense of smell, (70%, n = 35), reduction of nasal polyp score (NPS) (58%, n = 29), reduction of nasal congestion score (30%, n = 15) and improvement of quality-of-life score (SNOT-22) (28%, n = 14). Comprehensive results on biologic treatment are depicted in Table 3.
Comparison between hospital-based and office-based settings
While many practices were consistent across hospital and office-based ENT specialists, few notable differences emerged: Referrals by pulmonologists were more prevalent in hospital settings (80%) compared to office-based settings (68%), while referrals by general practitioners were more frequent in office-based settings (96%) compared to hospital settings (4%) Hospital-based physicians managed significantly more CRSwNP patients who were treated with biologics, compared to office-based specialists (13% vs. 8%, p = 0.021, r = 0.33, power = 0.80). Moreover, they were significantly more likely to prescribe SCS for longer durations (p = 0.006, V = 0.47, power = 0.86). Detailed results on setting comparisons are presented in Tables 1, 2 and 3.
Comorbidities and interdisciplinary aspects
The most commonly reported comorbidity in CRSwNP was allergic rhinitis (35%), followed by asthma (28%) and N-ERD (16%). Collaboration with pulmonologists was the most frequent form of interdisciplinary care with 86% (n = 43), followed by allergologists (8%, n = 16) and dermatologists (12%, n = 6).
Discussion
Our survey provides key insights on the diagnostic and therapeutic practices for the management of CRSwNP patients among Austrian otorhinolaryngologists, shedding light on adherence to guidelines, the utilization of diagnostic tools, treatment approaches and the incorporation of advanced biologic therapies.
Austria’s healthcare system allows patients to access contracted health care providers easily at all levels. General practitioners are often the first point of contact; however, they don’t act formally as gatekeepers, so patients can see most specialists, including ENT, directly without a referral24. Hospital-based specialists reported a higher proportion of referrals by pulmonologists compared to office-based specialists whereas referrals from general practitioners were far more common in office-based settings than in hospitals. This pattern likely reflects the structured referral pathways within Austria’s healthcare system, where hospitals often handle more complex cases initiated by pulmonologists, and office-based care typically begins with referrals from general practitioners. Additionally, half of the CRSwNP patients were pre-treated by primary care physicians before consulting an ENT specialist, indicating that initial management often starts outside specialized care. These findings underscore the importance of interdisciplinary collaboration and the need for clearer referral and increased awareness among primary care physicians to ensure timely and proper management of CRSwNP, thereby preventing the progression of the disease.
EPOS defines CRSwNP clinically by the presence of typical sinonasal symptoms alongside objective evidence of mucosal inflammation. As CRSwNP symptoms very often overlap with those of other rhinologic diseases like (non)allergic rhinitis, it can be challenging to distinguish between these conditions based on symptoms alone1. Adding endoscopy/imaging significantly improves diagnostic accuracy and specificity25. EPOS advises to use nasal endoscopy as a standard part of the diagnostic process and recommends CT imaging if symptoms continue and endoscopy remains abnormal after proper medical treatment1. In our survey, the key diagnostic tools CT imaging and nasal endoscopy were indeed mentioned most frequently. However, given their importance as standard diagnostic methods, the observed rates should ideally be even higher. In comparison, a survey from Spain reported that 98% of surveyed ENT specialists routinely use nasal endoscopy, and 74% rely on CT scans to support diagnosis21. An international survey from the Young Otolaryngologists of the International Federation of Oto-rhino-laryngological Societies (Yo-IFOS) revealed that 62% of the participants order a CT scan immediately upon endoscopic CRSwNP diagnosis19.
The next most commonly used diagnostic tool in our survey was the assessment of BEC, a biomarker for type-2 diseases. Another frequently-cited type-2 marker, total IgE, was marked far less by the participants. More and more ENT specialists recognize the recently introduced endotype-based classification system, which categorizes CRSwNP into distinct endotypes (type 2 and non-type 2) based on the molecular pathways driving mucosal inflammation. CRSwNP in the western world typically presents a type-2 inflammatory process within the nasal mucosa, which is characterized by the infiltration of eosinophils and locally elevated IgE4,5. Eosinophil count, either in blood serum or nasal mucosa tissue, and blood total IgE are increasingly used as biomarker for type 2 disease. A tissue eosinophil count of ≥ 10/hpf, BEC of ≥ 150 cells/mL and total IgE count of ≥ 100 kU/L currently serve as cut-off values for the “evidence of type-2” EPOS/EUFOREA criterion in the context of indicating biologic treatment in CRSwNP18. Recent research reported that BEC on its own can fulfill the evidence of type-2 criterion in 96.3%, while total IgE is required in only 2 − 5% to meet the criterion26. Moreover, high BEC may predict a good response to SCS treatment or biologicals and can thus play a valuable role in guiding therapy planning27,28. Furthermore, eosinophilic CRSwNP has been associated with a higher recurrence rate, hence, high BEC can be used to aid in patient counseling29,30. In addition, elevated BEC and total IgE levels are both strong predictors of coexisting asthma6. Increased total IgE levels can also help identify patients who may benefit from anti-IgE therapy31. Although the 2020 EPOS guidelines could not reach a clear consensus on recommending the routine measurement of blood EOS or total IgE in CRSwNP diagnosis1, the importance of these biomarkers has increased substantially over the past years. For patients receiving dupilumab, the most recent EPOS/EUFOREA update advises measuring BEC at one and three months after initiation of therapy, with more frequent assessments for those with high baseline BEC (> 500/mL). This recommendation arises due to the potential for dupilumab to cause transient hypereosinophilia within the first months, necessitating close monitoring to avoid potential organ damage18.
Less than half of the survey participants evaluated for allergic disease in CRSwNP with skin prick test or specific IgE assessment32. In comparison, the Yo-IFOS survey reported that 66% of respondents evaluate for potential allergic triggers—via skin or blood tests—when managing CRSwNP19. Although the relationship between allergy and CRSwNP is not fully elucidated, these diagnostic tools can be utilized to determine whether an allergic component may be contributing to CRSwNP33. The prevalence of inhalant allergy in CRSwNP was 31% in a large epidemiologic UK study34. Given that inhalant allergies are treatable traits, performing a skin prick test as part of routine clinical practice is advisable when the patient’s history suggests the presence of an allergic condition35. Smell testing is performed solely by a fifth of the participated ENT physicians in CRSwNP diagnosis. Olfactory dysfunction in CRSwNP is very common, affecting between 60% − 80% of patients36,37. However, many patients are either unaware of their olfactory impairment or unable to accurately evaluate its severity, making subjective testing insufficient38. To address this, smell tests like the Sniffin’ Sticks are additionally advised to objectively evaluate the extent of olfactory impairment39. Acoustic rhinometry, peak nasal inspiratory flow and rhinomanometry were mentioned the least in the survey. While these nasal patency tests objectively measure the nasal air flow, they offer little additional value for CRSwNP diagnosis1.
Several PROMs specifically designed for CRS management have been created, with the SNOT-22 currently being the most commonly utilized and recommended PROM for quality-of-life (QoL) assessment. Moreover, the SNOT-22 is also the recommended QoL instrument for the indication and evaluation in biological treatment of CRSwNP. Nearly all participants reported using routinely the SNOT-22 as a key tool in CRSwNP management, reflecting a good adherence to international guidelines (1). About half of ENT specialists in Spain reported routine use of the SNOT-2221. In contrast, only one-third of the participating ENT physicians reported using asthma-specific PROMs in the management of CRSwNP, a figure that may appear low given the high prevalence of comorbid asthma and its role as a key EPOS/EUFOREA response criterion for biological treatments (18). However, this limited use could be explained by the strong interdisciplinary collaboration observed. These findings align with trends seen in Spain and Germany, where interdisciplinary management is more commonly used to assess these domains21,23. The close partnership may reduce the reliance on PROMs within ENT practices, as pulmonologists likely take a leading role in assessing and monitoring asthma-related outcomes.
The survey findings also provide detailed insights into the therapeutic strategies used by Austrian ENT specialists for managing CRSwNP. LCS remain the cornerstone of treatment. The observed high rate in our survey aligns with their established role as a first-line, guideline-recommended intervention1. Similar results were reported from a German survey23. However, while LCS are effective for mild-to-moderate cases, more severe or refractory cases often require additional therapies. Our survey revealed that SCS were prescribed for about every third CRSwNP patient. A quite similar tendency was observed in Germany, where 52% of ENT physicians administer SCS23. A much higher SCS use of 80% was reported in a Spain survey21. SCS are known for their rapid anti-inflammatory effects, reducing polyp size and relieving nasal congestion. Despite these benefits, the transient nature of their effects and the associated risks of systemic side effects necessitate their careful and limited use regarding frequency and duration. According to 2020 EPOS guidelines, no more than two courses of SCS should be given per year to avoid cumulative side effects1,40. However, in clinical practice, SCS overuse is common41. The observed rate of SCS use in our survey indicates the need of awareness-efforts. The 2020 EPOS guidelines also recommend restricting SCS use to short-term courses1,40. In this context, the survey results show that most ENT specialists adhere to this recommendation. Hospital-based physicians were significantly more likely to prescribe longer SCS courses. This may reflect the complexity and severity of cases typically seen in hospital settings, where patients often present with more advanced or refractory disease. FESS represents an important therapeutic option for CRSwNP patients when medical treatment fails to provide adequate relief. The main aims of FESS in CRSwNP are the removal of diseased tissue, including nasal polyps, and to functionally open the sinus drainage pathways. According to EPOS, primary ESS is generally indicated for severe CRSwNP cases with symptoms that persist or recur despite comprehensive medical treatment, including LCS and usually one or more courses of SCS within the preceding two years1,40. The participating ENT physicians in Austria reported that approx. half of patients underwent FESS treatment. Similarly, in Spain, 49% of ENT specialists identified FESS as the primary option for managing uncontrolled CRSwNP21. Our observed survey findings of revision surgery rates match with a recent meta-analysis calculating an overall FESS revision rate of 18.6% in CRSwNP patients42. Repeated surgeries often increase the risk of complications, reduce the likelihood of success, and result in permanent scarring10,11. In this context, all survey participants agreed that more than one revision FESS within 10 years is excessive.
In the last years, newly introduced biologics have revolutionized the treatment landscape for recalcitrant CRSwNP, offering highly effective and personalized therapeutic options. Our survey revealed that hospital-based ENT specialists managed significantly more CRSwNP patients with biologic treatment than office-based physicians. Hospitals often serve as referral centers for more complex or severe cases and may also provide more experience with newer, cutting-edge therapies, such as biologics, due to the ongoing exposure to the latest clinical advancements. We have asked ENT physicians to state known biologics approved for CRSwNP treatment. Currently approved biologics in Austria are dupilumab, mepolizumab and omalizumab12. All participants stated at least one biologic agent, while only 3% were able to name more than one. It still remains elusive which biologic is best suited for which patient and reliable biomarkers are lacking. German ENT specialists reported that dupilumab was the most common prescribed biologic, followed by mepolizumab and omalizumab23. Similar results were observed in a national US survey20. Nevertheless, ENT physicians should be familiar with all approved medications. Some patients may not respond well to the prescribed biologic or experience medication side effects. In such cases, switching to an alternative biologic can be considered. Recent studies reported that 4−16% of patients required switching to an alternative biologic43,44. Austrian ENT physicians should therefore be more thoroughly informed about the various biologics and the option of switching biologic agents. In Austria, Dupilumab and Mepolizumab are approved as add-on therapies to LCS for adults with severe, recurrent CRSwNP who have undergone surgical treatment and either failed to respond, are intolerant, or have contraindications to SCS45. In contrast, Omalizumab is approved without requiring prior treatment with or contraindications to SCS17. The current EPOS/EUFOREA criteria suggest performing a complete FESS prior to initiating biologic therapy. However, it remains unclear what extent of surgery is necessary, whether revision surgery vs. biologics represents the superior approach or whether it is preferable to start biologic treatment with “clean sinuses” after surgery18. In our survey, the vast majority of ENT physicians considered initiating biologic therapy most appropriate after performing one FESS. Similar, in the German survey, 72% of the ENT specialists regarded one FESS as the required amount before initiating biologic therapy23. This high consensus underscores the perceived effectiveness of biologic treatments. Nevertheless, prospective studies are urgently required to address these unanswered questions with robust, evidence-based data. The approved dosing intervals for biologics vary, with Mepolizumab administered every 4 weeks, Dupilumab every 2 weeks, and Omalizumab offering flexibility between 2- and 4-week intervals depending on IgE levels12. In our survey, most ENT-physicians preferred the 4-weeks dosing interval. While it is understandable that a notable concern for patients is the discomfort associated with repeated injections, particularly as the therapy may need to be continued for life, a recent study demonstrated that tapering of dupilumab treatment up to a 6-week interval was possible in the majority of cases, when an initial good response is seen46. Tapering data for mepolizumab and omalizumab in CRSwNP treatment are currently not available. Further studies are urgently warranted to further examine the process of tapering in biologic treatment. The EPOS/EUFOREA expert panel provided current criteria for evaluation of response to biologics in 2023, which are reduced NPS of ≥ 1; SNOT-22 score of ≥ 40 or reduction of ≥ 12; improved sense of smell; no need of SCS/revision surgery; and reduced impact of comorbidities18. To qualify for reimbursement in Austria, the national Federation of Social Insurances requires solely a reduction in NPS of ≥ 2 for dupilumab, and ≥ 1 for mepolizumab and omalizumab within 6 months of treatment initiation45. The suboptimal use of response criteria in our survey might be attributed to several factors including insufficient awareness among physicians, gaps in professional trainings, limited consultation time, inconsistencies in local practice protocols, and reimbursement challenges.
The current survey has identified strengths in clinical practice as well as areas for improvement. To address these gaps, few targeted actions are warranted. Updated national guidelines and educational workshops might enhance the usage of key diagnostic tools, biomarkers and PROMs. A nationwide training program on biologic therapies may enhance ENT specialists’ familiarity with approved agents, eligibility criteria, and response assessment. Structured referral pathways and shared care models might improve interdisciplinary collaboration between ENT specialists, pulmonologists, and allergists, for a better disease management.
There are some limitations that need to be acknowledged. First, the survey relied on self-reported data, which may introduce recall bias and does not permit verification of responses. Second, although the sample size exceeded the calculated minimum for adequate representation, it may still limit the generalizability of the findings to all ENT specialists in Austria. Third, accidental or intentional bias in responses cannot be entirely ruled out. Despite these limitations, the study has several strengths. It is the first of its kind to provide a nationwide overview of CRSwNP management practices in Austria, offering a comprehensive analysis of both hospital- and office-based settings. Furthermore, the survey’s insights into the adoption of biologics offer a valuable foundation for future policy and educational initiatives aimed at optimizing patient outcomes. Given the dynamic nature of medical advancements, it would be beneficial to repeat this survey in a few years to track changes in practice patterns and evaluate the long-term impact of educational and policy interventions. Such a longitudinal approach could provide meaningful insights into emerging trends and further refine the strategies for CRSwNP management in Austria. Comparing future findings with the current baseline data will allow for a better understanding of progress and areas that still require attention.
Conclusions
This report provides important insights into current practices for managing CRSwNP in Austria, identifying strengths in adherence to guidelines as well as areas for improvement. Key challenges include improving diagnostic approach, optimizing treatment strategies, increasing awareness of biologic therapies, and standardizing evaluation methods.
Data availability
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
References
Fokkens, W. J. et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology 58(Suppl S29), 1–464. https://doi.org/10.4193/Rhin20.600 (2020).
Chaaban, M. R., Walsh, E. M. & Woodworth, B. A. Epidemiology and differential diagnosis of nasal polyps. Am. J. Rhinol. Allergy 27(6), 473–478. https://doi.org/10.2500/ajra.2013.27.3981 (2013).
Campion, N. J. et al. Prevalence and symptom burden of nasal polyps in a large Austrian population. J. Allergy Clin. Immunol. Pract. 9(11), 4117–4129e2. https://doi.org/10.1016/j.jaip.2021.06.037 (2021).
Tomassen, P. et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J. Allergy Clin. Immunol. 137(5), 1449–1456e4. https://doi.org/10.1016/j.jaci.2015.12.1324 (2016).
Stevens, W. W. et al. Associations between inflammatory endotypes and clinical presentations in chronic rhinosinusitis. J. Allergy Clin. Immunol. Pract. 7, 2812–2820E3 (2019).
Laidlaw, T. M., Mullol, J., Woessner, K. M., Amin, N. & Mannent, L. P. Chronic rhinosinusitis with nasal polyps and asthma. J. Allergy Clin. Immunol. Pract. 9, 1133–1141 (2021).
Walters, B. K. et al. Aspirin-exacerbated respiratory disease and the unified airway: A contemporary review. Otolaryngol. Clin. North. Am. 56(1), 107–124 (2023).
Chong, L. Y. et al. Intranasal steroids versus placebo or no intervention for chronic rhinosinusitis. Cochrane Database Syst. Rev. 4(4), CD011996 (2016).
Hox, V. et al. Benefits and harm of systemicsteroids for short- and long-term use in rhinitis and rhinosinusitis: An EAACI position paper. Clin. Transl. Allergy 10, 1 (2020).
DeConde, A. S. et al. Prevalence of polyp recurrence after endoscopic sinus surgery for chronic rhinosinusitis with nasal polyposis. Laryngoscope 127(3), 550–555 (2017).
Levi, L. et al. Patterns of recurrence in patients with CRSwNP who underwent complete FESS. Eur. Arch. Otorhinolaryngol. 281, 5847–5856 (2024).
Wang, H. et al. Efficacy of different biologics for treating chronic rhinosinusitis with nasal polyps: A network meta-analysis. Eur. Arch. Otorhinolaryngol. 26 https://doi.org/10.1007/s00405-024-08903-7 (2024).
Domínguez-Sosa, M. S. et al. Real-life effectiveness of mepolizumab in refractory chronic rhinosinusitis with nasal polyps. Biomedicines 11(2), 485 (2023).
Lombardo, N. et al. Real-life effects of Omalizumab on chronic rhinosinusitis with nasal polyposis. J. Pers. Med. 14(1), 3 (2023).
Seys, S. F. et al. CHRINOSOR consortium. Real-world effectiveness of dupilumab in a European cohort of chronic rhinosinusitis with nasal polyps (CHRINOSOR). J. Allergy Clin. Immunol. (2024).
Chong, L. Y. et al. Biologics for chronic rhinosinusitis. Cochrane Database Syst. Rev. 3(3), CD013513 (2021).
Fokkens, W. J. et al. EPOS/EUFOREA update on indication and evaluation of biologics in chronic rhinosinusitis with nasal polyps 2023. Rhinology 61(3), 194–202 (2023).
Maza-Solano, J. et al. Chronic rhinosinusitis with nasal polyps management in the biologic therapy era: An international YO-IFOS survey. Eur. Arch. Otorhinolaryngol. 280(5), 2309–2316 (2023).
Ayoub, N. F., Sbeih, F. & Bleier, B. S. Contemporary practice patterns for chronic rhinosinusitis with nasal polyps. Int. Forum Allergy Rhinol. 14(11), 1830–1833 (2024).
Alobid, I. et al. Management of patients with chronic rhinosinusitis with nasal polyps in spain: Learnings from a nationwide survey of otorhinolaryngologists. Eur. Arch. Otorhinolaryngol. 281(1), 227–235 (2024).
Kim, H. A. J. et al. Assessing the use of Patient-Reported outcome measures in the routine clinical care of chronic rhinosinusitis patients: A Canadian perspective. J. Otolaryngol. Head Neck Surg. 53, 19160216241288806 (2024).
Deuss, E. et al. Ergebnisse einer umfrage Zur aktuellen behandlung der Chronischen rhinosinusitis Mit Nasalen polypen [Results of a survey on the current management of chronic rhinosinusitis with nasal polyps in Germany]. Laryngorhinootologie 103(9), 646–654 (2024). (German).
Bachner, F. et al. Austria: Health system review. Health Syst. Trans. 20(3), 1–256 (2018).
Amine, M., Lininger, L., Fargo, K. N. & Welch, K. C. Outcomes of endoscopy and computed tomography in patients with chronic rhinosinusitis. Int. Forum Allergy Rhinol. 3(1), 73–79 (2013).
n der Lans, R. et al. Eosinophils are the dominant type2 marker for the current indication of biological treatment in severe uncontrolled chronic rhinosinusitis with nasal polyps. Rhinology 62(3), 383–384 (2024).
Deng, J. et al. Blood eosinophils to direct oral corticosteroid treatment for patients with nasal polyps—An open label, non-inferiority, randomized control trial. Rhinology 61(4), 328–337 (2023).
Habenbacher, M. et al. Investigation of blood count-based inflammatory biomarkers as predictors of response to dupilumab treatment in patients with chronic rhinosinusitis with nasal polyps. Pharmaceutics 16(11), 1370 (2024).
Zhong, B. et al. The role of preoperative blood eosinophil counts in distinguishing chronic rhinosinusitis with nasal polyps phenotypes. Int. Forum Allergy Rhinol. 11(1), 16–23 (2021).
Brescia, G. et al. The prognostic role of serum eosinophil and basophil levels in sinonasal polyposis. Int. Forum Allergy Rhinol. 7(3), 261–267 (2017).
Humbert, M. et al. IgE-mediated multimorbidities in allergic asthma and the potential for Omalizumab therapy. J. Allergy Clin. Immunol. Pract. 7(5), 1418–1429 (2019).
Alimuddin, S., Rengganis, I., Rumende, C. M. & Setiati, S. Comparison of specific immunoglobulin E with the skin Prick test in the diagnosis of house dust mites and cockroach sensitization in patients with asthma and/or allergic rhinitis. Acta Med. Indones 50(2), 125–131 (2018).
Gevaert, P., Wong, K., Millette, L. A. & Carr, T. F. The role of IgE in upper and lower airway disease: More than just allergy! Clin. Rev. Allergy Immunol. 62(1), 200–215 (2022).
Philpott, C. M. et al. Prevalence of asthma, aspirin sensitivity and allergy in chronic rhinosinusitis: Data from the UK National chronic rhinosinusitis epidemiology study. Respir Res. 19(1), 129 (2018).
Yii, A. C. A. et al. Precision medicine in united airways disease: A treatable traits approach. Allergy 73(10), 1964–1978 (2018).
Soler, Z. M., Kohli, P., Storck, K. A. & Schlosser, R. J. Olfactory impairment in chronic rhinosinusitis using threshold, discrimination, and identification scores. Chem. Senses 41(9), 713–719 (2016).
Kohli, P. et al. The prevalence of olfactory dysfunction in chronic rhinosinusitis. Laryngoscope 127(2), 309–320 (2017).
Lötsch, J. & Hummel, T. Clinical usefulness of self-rated olfactory performance-—A data science-based assessment of 6000 patients. Chem. Senses 44(6), 357–364 (2019).
Whitcroft, K. L. et al. Position paper on olfactory dysfunction: 2023. Rhinology 61(33), 1–108 (2023).
Hellings, P. W. et al. EUFOREA/EPOS2020 statement on the clinical considerations for chronic rhinosinusitis with nasal polyps care. Allergy 79(5), 1123–1133 (2024).
Khan, A. et al. The global allergy and asthma European network (GALEN rhinosinusitis cohort: A large European cross-sectional study of chronic rhinosinusitis patients with and without nasal polyps. Rhinology 57(1), 32–42 (2019).
Loftus, C. A. et al. Revision surgery rates in chronic rhinosinusitis with nasal polyps: Meta-analysis of risk factors. Int. Forum Allergy Rhinol. 10(2), 199–207 (2020).
van der Otten, J. et al. Evaluation of switching or simultaneous use of biologic treatment in patients with severe chronic rhinosinusitis with nasal polyps and severe asthma. Considerations in clinical decision making. Expert Rev. Clin. Immunol. 19(8), 1041–1049 (2023).
Dorling, M. et al. Switching biologics in chronic rhinosinusitis with nasal polyps: A multicenter Canadian experience. Int. Forum Allergy Rhinol. (2024). https://doi.org/10.1002/alr.23466
n der Lans, R. J. L. et al. Two-year results of tapered dupilumab for CRSwNP demonstrates enduring efficacy established in the first 6 months. Allergy 78(10), 2684–2697 (2023).
Funding
The primary market research carried out by IQVIA was financed by GSK Austria. No other funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
All authors have provided substantial contributions to the conception or design of the work acquisition, analysis or the interpretation of data for the work. All worked on the draft or revised it critically for important intellectual content. The final version was approved for publishing by all authors. The authors agree on accountability for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
Janina Kay is a GSK employee, Philipp Guenzl and Laura Walrave are GSK employees and hold financial equities in GSK. The other authors declare that they have no conflict of interest. The primary market research carried out by IQVIA was financed by GSK Austria.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Walla, K., Habenbacher, M., Kay, J. et al. Current diagnostic and therapeutic management of chronic rhinosinusitis with nasal polyps in Austria: insights and unmet needs from a nationwide survey. Sci Rep 15, 23051 (2025). https://doi.org/10.1038/s41598-025-07658-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-07658-3