Abstract
Dabigatran acylglucuronide, an active metabolite of dabigatran, exhibits higher plasma concentrations and distinct anticoagulant effects compared to dabigatran, suggesting that it may play a more significant role in overall anticoagulant activity than previously assumed. Idarucizumab, a monoclonal antibody fragment, binds to both free and thrombin-bound dabigatran, neutralizing its anticoagulant effects. However, its efficacy in reversing anticoagulation induced by dabigatran acylglucuronide remains unclear. This study aimed to evaluate whether idarucizumab differentially reverses the anticoagulant effects of dabigatran and dabigatran acylglucuronide. In vitro experiments were conducted using blood from healthy, drug-free donors. Plasma samples were spiked with either dabigatran or dabigatran acylglucuronide, followed by idarucizumab. Standard coagulation assays, including prothrombin time (PT), activated partial thromboplastin time (aPTT), thrombin time (TT), as well as thrombin generation assays (TGA), were performed. At a concentration of 1 µM, idarucizumab demonstrated significantly greater reversal of dabigatran acylglucuronide-induced anticoagulation than that of dabigatran in PT, aPTT, and TT assays. Consistently, TGA showed stronger neutralization of dabigatran acylglucuronide, with IC50 values for Cmax, endogenous thrombin potential, and lag time at least 2.0-fold higher than those of dabigatran. These findings indicate that idarucizumab exerts a more potent reversal effect on dabigatran acylglucuronide compared to dabigatran.
Similar content being viewed by others
Introduction
Dabigatran (DAB) is a direct thrombin inhibitor extensively employed in clinical settings for the treatment and prevention of thrombus formation and pulmonary embolism, and to reduce the risk of systemic embolism and ischemic stroke1,2. Unlike heparin, which interacts exclusively with free thrombin, DAB can bind to and inhibit both free and clot-bound thrombin, thus offering a broader spectrum of anticoagulant activity3.
Upon administration, the prodrug dabigatran etexilate (DABE) undergoes metabolic conversion by carboxylesterase 2 (CES2) in the intestine, resulting in the formation of an intermediate metabolite, dabigatran ethyl ester (M2). Subsequently, M2 is metabolized into the active metabolite, DAB, by carboxylesterase 1 (CES1) in the liver4,5. DAB is further metabolized in the liver by uridine 5-diphospho (UDP)-glucuronosyltransferase (UGT) enzymes into dabigatran acylglucuronide (DABG), an active metabolite. Among the UGT enzymes involved in the glucuronidation of DAB (e.g., UGT1A9, UGT2B7, and UGT2B15), UGT2B15 plays the predominant role in humans6.
Although previous pharmacological evaluations suggested that DABG has anticoagulant properties comparable to those of DAB, its role was considered minor due to its lower concentration as only approximately 20% of DAB is converted to DABG7,8,9,10. However, our recent findings contradict prior assumptions regarding the marginal role of DABG in anticoagulation, revealing that DABG is present at higher plasma concentrations than DAB11,12. Specifically, systemic exposure to DABG was 2.4-fold higher than that to DAB following a 150 mg dose of DABE administration11 suggesting that DABG has a potentially more significant role in anticoagulation than previously recognized9. This discrepancy in DABG concentrations likely results from the traditional indirect method of assessing total DAB concentrations, which sums the concentrations of DAB and DABG after glucuronic acid cleavage13,14,15,16. This method can significantly degrade DAB through strong alkaline hydrolysis, leading to artificially low DABG concentrations17. Additionally, we found that the anticoagulant effect of DABG was weaker than that of DAB, as a comparative analysis showed that DAB was 2.10-fold more potent than DABG in inhibiting thrombin generation18.
Meanwhile, Idarucizumab is a humanized DAB-specific monoclonal antibody generated by immunizing mice with DAB-derived haptens coupled to carrier protein, and is used as an antidote to reverse the anticoagulant effects of DAB19. The binding affinity of idarucizumab for DAB is approximately 350-fold greater than that of thrombin, with dissociation constant [KD] values of 2 pmol/L and 0.7 nmol/L, respectively20. Although DAB and DABG coexist in plasma following DABE administration, it remains unclear how idarucizumab differentially interacts with each compound11,12. Given the distinct anticoagulant activities of DAB and DABG, a differential reversal effect of idarucizumab on each may have potential clinical relevance18. Therefore, this study aimed to determine whether idarucizumab has differential reversal effects on anticoagulation induced by DAB versus DABG.
Materials and methods
Reagents
Idarucizumab (Commercialized product: Praxbind®) was purchased from Boehringer Ingelheim (Ingelheim an Rhein, Germany). DAB and DABG were procured from TRC Canada (Toronto, ON, Canada). Recombinant tissue factor (Hemoliance® RecombiPlasTin) was from the Instrumentation Laboratory (Milan, Italy)21. Synthetic phospholipids (PL) were obtained from Avanti Polar Lipids Inc. (Alabaster, AL, USA), and phospholipid vesicles were composed of phosphatidylserine (PS), phosphatidylethanolamine (PE), and phosphatidylcholine (PC) (1:1:3, mol: mol: mol). The thrombin-specific fluorogenic substrate, Z-Gly-Gly-Arg-aminomethyl coumarin (ZGGR-AMC), was purchased from Bachem Co. (Basel, Switzerland). Technothrombin® TGA kit was purchased from Technoclone (Vienna, Austria). All other reagents used in this study were of high purity and analytical grade, and were obtained from commercial suppliers.
Sample collection
All sample collections were performed in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Anam Hospital, Korea University College of Medicine, Seoul, Korea (approval number: 2021AN0477). Written informed consent was obtained from all participants prior to blood collection, in compliance with institutional and ethical guidelines.
Blood samples were drawn from healthy and drug-free donors by peripheral venipuncture into conventional vacuum containers containing 0.109 mol/L (3.2%) buffered trisodium citrate. (Blood: 3.2%, 9:1 v/v, Becton Dickinson, NJ, USA). Plasma samples were separated through double centrifugation at 1,500 × g for 15 min at 4 ℃.
Preparation and incubation of plasma samples
DAB and DABG were spiked into citrated plasma at their respective test concentrations (0.5, 1, or 2 µM) and preincubated for 30 min. Following this, idarucizumab was added at a fixed concentration (either 0.5, 1, or 2 µM, depending on the experimental condition) and further incubated for 30 min under the same conditions. The samples were then immediately subjected to thrombin generation assay (TGA) and standard coagulation assays including PT, aPTT, TT measurement. Each concentration of idarucizumab was tested independently in all conditions, including individual and combined treatment with DAB and DABG.
Standard coagulation assay (PT, aPTT, TT)
The following four coagulation tests were performed using STA® Compact Max (Stago, Asnières, France) and same batch of reagents were used.
In brief, prothrombin time (PT), was measured by mixing 100 µL STA® Neoplastine® CI reagent with a plasma sample (50 µL) in a cuvette. To measure the activated partial thromboplastin time (aPTT), allowed to react for a while in the presence of STA®-PTT (50 µL) which was added 50 µL of plasma sample as with PT in the cuvette, thereafter following by the addition of CaCl2 0.025 M (50 µL) for recalcification. For the thrombin time (TT) measurement, a plasma sample (100 µL) was mixed with STA®-thrombin in a cuvette. Calibrators and internal controls were used according to the manufacturers’ specifications. All measurements were performed in duplicates for each sample.
Thrombin generation assay (TGA)
Thrombin generation was assessed using the Technothrombin® TGA kit by Technoclone22,23. To implement thrombin generation, the development of a relative fluorescence unit was monitored with an automated, multi-mode microplate reader (Flexstation 3; Molecular Devices, San Jose, CA, USA) set at a wavelength of 360 nm (excitation), 460 nm (emission), and mode to kinetics, using adapted software (Technothrombin TGA, Technoclone, Vienna, Austria). Sample loading was performed as previously described18. In brief, 30 µL of platelet-poor plasma samples in which DAB (and/or DABG) with idarucizumab was spiked in advance were loaded into a 96-microplate well, followed by 25 µL of 400 µmol/L fluorogenic substrate (Z-Gly-Gly-Arg-AMC; Bachem, Bubendorf, Switzerland) that was supplemented. The assay was triggered by dispensing 10 µL of recombinant human tissue factor (final concentration 5 pmol/L) lipidated with synthetic phospholipids (final concentration 4 µmol/L) and adding 10 µL of CaCl2 to reach a concentration of 15 mmol/L for recalcification. All samples were analyzed in duplicate in every experiment.
Thrombin generation was measured and assessed in terms of peak thrombin concentration (Cmax), endogenous thrombin potential (ETP, represented as area under the curve, AUC), lag time.
Statistical analysis
All data are presented as mean ± standard deviation (SD). IC50 values were estimated from the inhibition plot using nonlinear regression analysis. Group differences were analyzed using one-way analysis of variance (ANOVA), followed by Bonferroni’s test or Student’s t-test, as applicable. Statistical analyses were performed using SAS® 9.4 (SAS Institute Inc., Cary, NC, USA) and Microsoft Excel 2021 (Microsoft Corp., Redmond, WA, USA). Statistical significance was set at P < 0.05.
Results
Differential anticoagulant effects of DAB and DABG
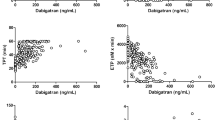
First, we compared the anticoagulant effects of DAB and DABG using a TGA. Both DAB and DABG exhibited dose-dependent anticoagulant effects on thrombin generation, with DAB exhibiting a stronger anticoagulant effect than DABG (Fig. 1). When comparing the inhibitory potencies of DAB and DABG on thrombin generation, the calculated IC50 values for DAB and DABG were 371.1 ± 27.1 nM and 669.2 ± 44.4 nM for Cmax, and 324.1 ± 26.4 nM and 505.2 ± 30.5 nM for ETP, respectively. In addition, DAB exhibited a longer lag time than DABG (Fig. 1).
Reversal effect of idarucizumab on DAB- and DABG-induced thrombin generation inhibition
Based on the inhibition of thrombin generation induced by DAB and DABG, we evaluated the reversal effects of idarucizumab. Idarucizumab dose-dependently reversed the DAB- and DABG-induced inhibition of thrombin generation (Fig. 2). However, idarucizumab exhibited a more potent reversal effect on DABG-induced inhibition than DAB. 1 µM idarucizumab fully reversed DABG-induced thrombin generation inhibition at 1 µM, while a higher concentration of 2 µM idarucizumab was required to fully reverse the effects of DAB at 1 µM, indicating a stronger reversal effect on DABG-induced anticoagulant activity. Moreover, when evaluating the inhibitory potency of idarucizumab against 1 µM of DAB and DABG on thrombin generation, the drawn IC50 values for Cmax, ETP, and Lag time differed at least 2-fold (Fig. 3; Table 1).
We also evaluated the reversal effect of idarucizumab on the anticoagulant effects of DAB and DABG as measured by PT, aPTT, TT. Idarucizumab reversed the anticoagulant effects of DAB and DABG on PT, aPTT, and TT in a dose-dependent manner. Consistent with the thrombin generation results, idarucizumab exhibited a more potent reversal effect on DABG than on DAB (Fig. 4). Comparing the inhibitory potency of idarucizumab against 1 µM of DAB and DABG on PT, aPTT, and TT, the calculated IC50 values of each parameter represented a clear difference between them, especially the 2-fold difference on aPTT. The IC50 values of idarucizumab are listed in Table 1.
Reversal of idarucizumab on thrombin generation in DAB and DABG co-incubated samples
Following the administration of DABE, both DAB and DABG are detected in the human blood. In this study, we evaluated the ability of idarucizumab to reverse the inhibition of thrombin generation by DAB and DABG. These compounds were mixed and co-incubated, resulting in concurrent and dose-dependent suppression of thrombin generation. Additionally, the anticoagulant effects of DAB at a constant dose were intensified by DABG treatment and reversed by the introduction of idarucizumab. Similarly, the anticoagulant effect of DABG at a fixed dose was amplified by DAB treatment and similarly reversed by idarucizumab. Furthermore, the reversal effect of idarucizumab was more pronounced for DABG anticoagulant effects compared to DAB at equivalent concentrations. Specifically, idarucizumab at 2 µM nearly fully reinstated normal thrombin generation levels in the presence of 2 µM DABG and 0.5 µM DAB, whereas it only restored approximately 20% in samples with 0.5 µM DABG and 2 µM DAB (Fig. 5).
Discussion
In patients taking DABE, there may be a need to reverse the anticoagulant effects of DAB in various situations, such as invasive procedures, emergency surgery, or when the patient experiences uncontrolled or life-threatening bleeding24. The DAB-binding region of idarucizumab is structurally similar to the DAB-binding region of thrombin, and its binding to DAB is mediated by hydrophobic interactions, H-bonds, and a salt bridge, resulting in a stronger affinity for DAB than the affinity of DAB for thrombin19. The strong affinity of idarucizumab to DAB is due to the high affinity corresponding with a rapid-on and very slow-off rates, resulting in an almost irreversible binding of idarucizumab to DAB20. Additionally, idarucizumab has no off-target effects and, therefore, has a low potential to interfere with the coagulation system, and has no procoagulant effect because of its selectivity for DAB, even at high doses25,26,27,28. Given the structural similarity between DABG and DAB, we believe that idarucizumab may reverse the antithrombotic effect induced by DABG, similar to that induced by DAB. However, whether idarucizumab can reverse the antithrombotic effect of DABG in addition to DAB, and if so, to what extent compared to DAB, was unknown.
We first assessed the antithrombotic effects of DAB and DABG, demonstrating that DAB exhibited a more potent antithrombotic effect than DABG (Fig. 1). Specifically, the IC50 values for Cmax and ETP were 1.80-fold and 1.56-fold lower, respectively, for DAB than for DABG in the TGA. These results are consistent with previous findings18. Furthermore, the parameters measured by the TGA provided direct evidence of the absence of procoagulant activity of idarucizumab29. When we compared the reversal effects of idarucizumab on both DAB- and DABG-induced antithrombotic activity, both showed dose-dependent responses (Figs. 2, 3, 4 and 5). Notably, idarucizumab exhibited differential potency in reversing the antithrombotic effects induced by DAB and DABG. For the antithrombotic effects induced by DAB and DABG at a concentration of 2 µM, idarucizumab completely reversed the effect of DABG, whereas only partial reversal (approximately 50%) was observed for DAB (Figs. 2 and 3). These results suggest that idarucizumab exerts a more potent neutralizing effect on DABG than on DAB19,25. We propose that this difference in reversal efficacy may stem from the disparity in thrombin-binding affinity between DAB and DABG, which contributes to their distinct antithrombotic activities and, consequently, to the differential neutralization effects observed with idarucizumab.
Although the absolute [KD] values of thrombin obtained from molecular modeling (e.g., 76.8 nM for DAB and 107.8 nM for DABG) differ from the experimentally reported value for DAB (~ 0.7 nM), the modeling was conducted under identical computational conditions for both ligands18,20 allowing for a meaningful comparison of their relative binding strengths. These in silico results are not intended to provide precise quantification of binding affinities, but rather to qualitatively characterize the relative impact of glucuronidation on thrombin binding. To our knowledge, no prior studies have experimentally compared the thrombin-binding affinity of DABG to that of DAB. Thus, further validation using direct biophysical binding assays will be necessary to confirm this difference. Nevertheless, the observed trend of weaker thrombin affinity for DABG supports its lower anticoagulant activity and offers a plausible mechanistic explanation for the more efficient reversal of DABG by idarucizumab. In other words, based on our findings, it can be assumed that idarucizumab may have a relatively higher or equivalent affinity for DABG compared to that for DAB. Therefore, the antithrombotic effect of DABG is thought to be abolished more easily by idarucizumab than that of DAB.
Co-incubation of both DAB and DABG with idarucizumab was also conducted to better mimic physiological conditions in which both compounds coexist in plasma (Fig. 5). The reversal effect of idarucizumab was more potent against the DABG-induced antithrombotic effect than the DAB-induced effect in this scenario. When DAB (0.5 µM) and DABG (1 µM) were co-incubated with idarucizumab (1 µM), thrombin generation was partially restored: Cmax inhibition decreased from 95.3 to 24.1%, and ETP inhibition from 93.8 to 47.8%. In contrast, under the reversed condition—1 µM DAB and 0.5 µM DABG—the same concentration of idarucizumab yielded a weaker reversal effect, with residual inhibition levels of 51.2% for Cmax and 63.6% for ETP (from initial decreases of 96.8% and 96.7%, respectively). This marked difference between the two conditions clearly demonstrated that idarucizumab exerts a more potent neutralizing effect on DABG than on DAB, supporting the conclusion that the idarucizumab interacts with each compound in a distinct manner.
These findings support the possible clinical relevance of monitoring the blood levels of DAB and DABG individually following administration of the clinical dose of DABE (e.g., 150 mg twice daily), as DAB and DABG exhibit distinct antithrombotic activities, and their relative concentrations may influence the efficacy of idarucizumab during anticoagulation reversal. This may be particularly important when deviations from the expected concentrations or ratios occur, such as in cases of single nucleotide polymorphism or comorbidities that affect the pharmacokinetics of DAB and DABG in individual patients. As DAB is converted to DABG by UGT2B15 in the body, interference with its metabolism can inhibit DAB conversion, leading to increased DAB levels and decreased DABG levels. Therefore, it is important to acknowledge that patients taking UGT2B15 inhibitors or those with UGT2B15 polymorphism-related genetic variations may exhibit a stronger antithrombotic effect from DAB than from DABG, along with a reduced reversal effect of idarucizumab10,30,31,32. However, this assumption has not yet been explored, highlighting the need for further research. Additionally, in patients with impaired renal function, plasma concentrations of DAB have been reported to increase by up to 2.41-fold compared to those with normal renal function33. Considering that the typical maximum plasma concentration (Cmax) of DAB following administration of DABE 150 mg twice daily is approximately 175–240 ng/mL (0.37–0.51 µM), the plasma level in patients with renal impairment may exceed 1 µM, thereby increasing the risk of bleeding due to drug accumulation. Such clinical scenarios are likely attributable to prolonged systemic retention and redistribution of DAB, particularly in patients with impaired clearance capacity, as DAB that has distributed into extravascular compartments may later re-enter the circulation, potentially limiting the efficacy of idarucizumab34. Indeed, clinical case reports have documented insufficient reversal of DAB with the recommended 5 g dose of idarucizumab in patients with either severe renal impairment or multiple trauma. In one case, DAB plasma levels rebounded to 96% of initial concentrations within 8 h following idarucizumab administration, necessitating hemodialysis to support further clearance of the drug in the context of acute kidney injury35. In another report, a trauma patient exhibited secondary bleeding despite initial reversal, which was only resolved after an additional idarucizumab dose, suggesting that the standard fixed dose may be insufficient in cases of high thrombin inhibitor burden36. Prolonged systemic exposure to DAB facilitates its gradual conversion to DABG via UGT2B15, resulting in the concurrent presence of both DAB and DABG in plasma. In such cases, the DABG-to-DAB ratio may increase, resulting in a shift of the circulating pool toward a less potent but more abundant metabolite. Under these conditions, the relatively higher neutralization efficiency of idarucizumab against DABG may enable more rapid restoration of coagulation function. These findings highlight the importance of considering the relative plasma concentrations of DAB and DABG when determining reversal strategies in clinical settings. Such considerations underscore the need for individualized anticoagulation reversal approaches based on renal function and the circulating composition of DAB species.
In conclusion, DABG exhibits a weaker anticoagulant effect than DAB. Furthermore, idarucizumab demonstrated distinct reversal effects, more effectively neutralizing the anticoagulant activity of DABG than that of DAB.
Data availability
The data that support the findings of this study are available from the corresponding author on reasonable request.
References
Blommel, M. L. & Blommel, A. L. Dabigatran etexilate: A novel oral direct thrombin inhibitor. Am. J. Health Syst. Pharm. 68, 1506–1519. https://doi.org/10.2146/ajhp100348 (2011).
Cid-Conde, L., López-Castro, J. & Liu, T. (IntechOpen) (2013).
Wienen, W., Stassen, J. M., Priepke, H., Ries, U. J. & Hauel, N. In-vitro profile and ex-vivo anticoagulant activity of the direct thrombin inhibitor Dabigatran and its orally active prodrug, Dabigatran etexilate. Thromb. Haemost. 98, 155–162 (2007).
Shi, J. et al. Dabigatran etexilate activation is affected by the CES1 genetic polymorphism G143E (rs71647871) and gender. Biochem. Pharmacol. 119, 76–84. https://doi.org/10.1016/j.bcp.2016.09.003 (2016).
Laizure, S. C., Parker, R. B., Herring, V. L. & Hu, Z. Y. Identification of carboxylesterase-dependent Dabigatran etexilate hydrolysis. Drug Metab. Dispos. 42, 201–206. https://doi.org/10.1124/dmd.113.054353 (2014).
Blech, S., Ebner, T., Ludwig-Schwellinger, E., Stangier, J. & Roth, W. The metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humans. Drug Metab. Dispos. 36, 386–399. https://doi.org/10.1124/dmd.107.019083 (2008).
Li, X. et al. Bioequivalence and pharmacodynamics of a generic Dabigatran etexilate capsule in healthy Chinese subjects under fasting and fed conditions. Pharmacol. Res. Perspect. 8, e00593. https://doi.org/10.1002/prp2.593 (2020).
Moj, D. et al. A comprehensive Whole-Body physiologically based Pharmacokinetic model of Dabigatran etexilate, Dabigatran and Dabigatran glucuronide in healthy adults and renally impaired patients. Clin. Pharmacokinet. 58, 1577–1593. https://doi.org/10.1007/s40262-019-00776-y (2019).
Stangier, J. Clinical pharmacokinetics and pharmacodynamics of the oral direct thrombin inhibitor Dabigatran etexilate. Clin. Pharmacokinet. 47, 285–295. https://doi.org/10.2165/00003088-200847050-00001 (2008).
Ebner, T., Wagner, K. & Wienen, W. Dabigatran acylglucuronide, the major human metabolite of dabigatran: in vitro formation, stability, and Pharmacological activity. Drug Metab. Dispos. 38, 1567–1575. https://doi.org/10.1124/dmd.110.033696 (2010).
Park, I. H. et al. Development and validation of LC-MS/MS method for simultaneous determination of Dabigatran etexilate and its active metabolites in human plasma, and its application in a Pharmacokinetic study. J. Pharm. Biomed. Anal. 203, 114220. https://doi.org/10.1016/j.jpba.2021.114220 (2021).
Nouman, E. G., Al-Ghobashy, M. A. & Lotfy, H. M. Development and validation of LC-MSMS assay for the determination of the prodrug Dabigatran etexilate and its active metabolites in human plasma. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 989, 37–45. https://doi.org/10.1016/j.jchromb.2015.02.042 (2015).
Stangier, J., Stähle, H., Rathgen, K., Roth, W. & Shakeri-Nejad, K. Pharmacokinetics and pharmacodynamics of Dabigatran etexilate, an oral direct thrombin inhibitor, are not affected by moderate hepatic impairment. J. Clin. Pharmacol. 48, 1411–1419. https://doi.org/10.1177/0091270008324179 (2008).
Stangier, J. & Clemens, A. Pharmacology, pharmacokinetics, and pharmacodynamics of Dabigatran etexilate, an oral direct thrombin inhibitor. Clin. Appl. Thromb. Hemost. 15 (Suppl 1), 9s–16s. https://doi.org/10.1177/1076029609343004 (2009).
Stangier, J., Rathgen, K., Stähle, H. & Mazur, D. Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral Dabigatran etexilate: an open-label, parallel-group, single-centre study. Clin. Pharmacokinet. 49, 259–268. https://doi.org/10.2165/11318170-000000000-00000 (2010).
Stangier, J., Stähle, H., Rathgen, K. & Fuhr, R. Pharmacokinetics and pharmacodynamics of the direct oral thrombin inhibitor Dabigatran in healthy elderly subjects. Clin. Pharmacokinet. 47, 47–59. https://doi.org/10.2165/00003088-200847010-00005 (2008).
Balaji, M., Srinivasarao, V., Rao, K. & Ch Appa, R. K. A new and stability indicating liquid chromatographic method for the determination of Dabigatran in bulk drug and pharmaceutical dosage form. Anal. Chem. Indian J. 14, 264–273 (2014).
Kim, J. M. et al. Dabigatran acylglucuronide, the major metabolite of dabigatran, shows a weaker anticoagulant effect than dabigatran. Pharmaceutics https://doi.org/10.3390/pharmaceutics14020257 (2022).
Schiele, F. et al. A specific antidote for dabigatran: functional and structural characterization. Blood 121, 3554–3562. https://doi.org/10.1182/blood-2012-11-468207 (2013).
Eikelboom, J. W., Quinlan, D. J., van Ryn, J. & Weitz, J. I. Idarucizumab: the antidote for reversal of Dabigatran. Circulation 132, 2412–2422. https://doi.org/10.1161/circulationaha.115.019628 (2015).
Gerotziafas, G. T. et al. Towards a standardization of thrombin generation assessment: the influence of tissue factor, platelets and phospholipids concentration on the normal values of Thrombogram-Thrombinoscope assay. Thromb. J. 3, 16. https://doi.org/10.1186/1477-9560-3-16 (2005).
Ay, L. et al. Thrombin generation in morbid obesity: significant reduction after weight loss. J. Thromb. Haemost. 8, 759–765. https://doi.org/10.1111/j.1538-7836.2010.03766.x (2010).
Holm, E., Zetterberg, E., Lövdahl, S. & Berntorp, E. Patients referred for bleeding symptoms of unknown cause: does evaluation of thrombin generation contribute to diagnosis?? Mediterr. J. Hematol. Infect. Dis. 8, e2016014. https://doi.org/10.4084/mjhid.2016.014 (2016).
Cortese, F. et al. Idarucizumab: what should we know?? Curr. Drug Targets. 19, 81–88. https://doi.org/10.2174/1389450118666170925155943 (2018).
Glund, S. et al. Safety, tolerability, and efficacy of Idarucizumab for the reversal of the anticoagulant effect of Dabigatran in healthy male volunteers: a randomised, placebo-controlled, double-blind phase 1 trial. Lancet 386, 680–690. https://doi.org/10.1016/s0140-6736(15)60732-2 (2015).
Glund, S. et al. A randomised study in healthy volunteers to investigate the safety, tolerability and pharmacokinetics of idarucizumab, a specific antidote to Dabigatran. Thromb. Haemost. 113, 943–951. https://doi.org/10.1160/th14-12-1080 (2015).
Reilly, P. A., van Ryn, J., Grottke, O., Glund, S. & Stangier, J. Idarucizumab, a specific reversal agent for dabigatran: mode of action, pharmacokinetics and pharmacodynamics, and safety and efficacy in phase 1 subjects. Am. J. Emerg. Med. 34, 26–32. https://doi.org/10.1016/j.ajem.2016.09.050 (2016).
Schmohl, M. et al. Idarucizumab does not have procoagulant effects: assessment of thrombosis biomarkers in healthy volunteers. Thromb. Haemost. 117, 269–276. https://doi.org/10.1160/th16-05-0385 (2017).
Herrmann, R. et al. Thrombin generation using the calibrated automated thrombinoscope to assess reversibility of Dabigatran and Rivaroxaban. Thromb. Haemost. 111, 989–995. https://doi.org/10.1160/th13-07-0607 (2014).
Shnayder, N. A. et al. Using pharmacogenetics of direct oral anticoagulants to predict changes in their pharmacokinetics and the risk of adverse drug reactions. Biomedicines https://doi.org/10.3390/biomedicines9050451 (2021).
Court, M. H. et al. UDP-glucuronosyltransferase (UGT) 2B15 pharmacogenetics: UGT2B15 D85Y genotype and gender are major determinants of Oxazepam glucuronidation by human liver. J. Pharmacol. Exp. Ther. 310, 656–665. https://doi.org/10.1124/jpet.104.067660 (2004).
Chung, J. Y. et al. Effect of the UGT2B15 genotype on the pharmacokinetics, pharmacodynamics, and drug interactions of intravenous lorazepam in healthy volunteers. Clin. Pharmacol. Ther. 77, 486–494. https://doi.org/10.1016/j.clpt.2005.02.006 (2005).
Padrini, R. Clinical pharmacokinetics and pharmacodynamics of direct oral anticoagulants in patients with renal failure. Eur. J. Drug Metab. Pharmacokinet. 44, 1–12. https://doi.org/10.1007/s13318-018-0501-y (2019).
Melicine, S. et al. Dabigatran-reversal failure using standard dose of idarucizumab: a systematic review and meta-analysis of cases. Res. Pract. Thromb. Haemost. 7, 100201. https://doi.org/10.1016/j.rpth.2023.100201 (2023).
Simon, A. et al. The recommended dose of Idarucizumab May not always be sufficient for sustained reversal of Dabigatran. J. Thromb. Haemost. 15, 1317–1321. https://doi.org/10.1111/jth.13706 (2017).
Ströhle, M., Rugg, C., Schmid, S., Fries, D. & Oswald, E. Difficult reversal of Dabigatran with Idarucizumab in a multiple-trauma patient: A question of dose? Trauma. Case Rep. 32, 100422. https://doi.org/10.1016/j.tcr.2021.100422 (2021).
Funding
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number : RS-2024-00408877) and Korea University grant.
Author information
Authors and Affiliations
Contributions
J.-M.K.: Conceptualization, Writing – original draft, Writing – review & editing, Formal analysis, Methodology, Data curation, Investigation, Validation, Visualization; Y.Y.B.: Writing – review & editing, Data curation, Investigation; K.-A.K.: Writing – review & editing, Methodology, Data curation, Software, Validation; J.-W.P.: Supervision, Writing – original draft, Writing – review & editing, Methodology, Data Curation, Validation; J.-Y.P.: Conceptualization, Project administration, Supervision, Writing – original draft, Writing – review & editing, Methodology, Software, Visualization.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kim, JM., Bang, Y.Y., Kim, KA. et al. Idarucizumab more effectively reverses the anticoagulant effects of dabigatran acylglucuronide than dabigatran. Sci Rep 15, 21967 (2025). https://doi.org/10.1038/s41598-025-08417-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-08417-0