Abstract
Microplastics (MPs) are a common long-lasting pollutant of aquatic ecosystems. Microalgae are primary producers of aquatic systems, and MP contamination could have a high impact on the aquatic food web. Therefore, the present study utilized polyethylene (PE) particles (0 to 150 mg/L) for investigating the half-maximal inhibitory concentrations (IC50) of Chlorella sorokiniana and also studied their impacts on growth rate, biomass, pigments and other biochemical components of the microalgae. After 96 h of incubation, PE of 100 mg/L resulted in the half-maximum inhibition (IC50). After reaching the stationary phase (14 d), harvesting was made for MP-exposed cultures to reveal a biomass production of 0.89 g/L, while it was 0.96 g/L for the control. A slight reduction in pigment and lipid contents was also observed, while the protein and carbohydrate contents were high in MP-exposed C. sorokiniana cells. Under the MP stress, reactive oxygen species (ROS) and phenolic levels were reduced, whereas flavonoid content increased. PE particles were characterized using Scanning Electron Microscopy-Energy Dispersive X-ray Spectroscopy (SEM-EDX) and Fourier Transform infrared spectroscopy (FT-IR) for their size, shape, chemical composition, and interaction with C. sorokiniana, followed by micro-Fourier Transform infrared spectroscopy (µ-FT-IR) for the mapping of MP. This research contributes to a deeper understanding of how MP contamination can disrupt aquatic food webs, guiding future ecological assessments and pollution management strategies.
Similar content being viewed by others
Introduction
Microplastic (MP) is a ubiquitous piece of plastic debris originating from a variety of sources. Once released into the environment, it has an adverse impact on human health and microbiota due to its high persistence in the environment1. Although there is room for debate, it is reported to carry chemical pollutants and can cause physical harm if accumulated by organisms. The concentration of MPs in aquatic environments is increasing at a very rapid rate because of the continuous increase in plastic production2. It has been reported that in 2016, about 335 million metric tonnes (MMT) of plastic products were produced, while in 2017, this number increased by 348 MMT, which indicates an annual bump of around 4% in plastic production globally3.
According to statistics, 4,392.5 million metric tonnes (MMT) of plastic was generated between 1950 and 2020; and if present utilization trends continue, it is anticipated to extend to 17,312 MMT by 2050, globally4. Consequently, nearly 8-MMT plastic waste directly flows into the aquatic ecosystem every year, and this amount is predicted to increase fourfold by 20502. According to the National Oceanic and Atmospheric Administration (NOAA), MPs can be classified as plastic fragments that possess a size of less than 5 mm. Because of the inappropriate release of domestic and industrial wastewater, MPs are extensively disseminated in oceans, surface water, and stormwater5. Examples of commercially available plastics include polypropylene (PP), polyvinyl chloride (PVC), low-density polyethylene (LDPE), polyethylene terephthalate (PET), and high-density polyethylene (HDPE)6. Among various plastics, polyethylene (PE), polypropylene (PP), polystyrene (PS) and polyethylene terephthalate (PET) are the most prevalent in marine environments7. In the environment, MPs have various shapes, including fragments, fibres, beads, and pellets. Due to their high surface area to volume ratio, MPs can adsorb other chemical pollutants, raising concerns about their interactions with environmental contaminants. These plastic particles also can be adsorbed to other organic solids in the water because of their hydrophobic nature, and the smaller the size, the easier they can build up to a harmful concentration1,8. Additionally, many environmental pollutants like polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyls (PCBs), 2, 2-bis (p-chlorophenyl) 1, 1, 1-trichloroethane (DDTs), and polycyclic aromatic hydrocarbons (PAHs) are very well known to be easily adsorbed onto microplastics9.
Microplastic pollutants in aquatic environments may affect organisms at different trophic levels via adsorption, food intake, and transformation through the food chain10,11. The food chain transfer between tropical levels is majorly associated with their shape, size, accumulation and residence time. The longer residence time of MP in biota will facilitate their easy movement across the trophic levels, which will possess a definite effect on the overall ecosystem11. Due to bioaccumulation, such organic pollutants pose a consequential challenge to the aquatic environment5. Aquatic bodies are a huge abode of renewable sources of natural substances12. Microalgae (photosynthetic microbes) are primary producers that consume inorganic substances to synthesize protein, lipids and carbohydrates. Being primary producers, microalgae play a major part in preserving environmental stability. Increasing exposure to MPs could influence the internal components of microalgae and the aquatic environment. Microalgae have a short growth cycle, are easy to cultivate, and are sensitive to toxic substances, which makes them suitable for performing eco-toxicity assays13. Microplastics have been reported to adversely affect microalgae populations by reducing their photosynthetic efficiency and increasing reactive oxygen species (ROS) generation14,15. The amount of MPs in aquatic settings varies greatly; in virgin waters, it is quite low, but in contaminated places, it is much higher. In a study performed on the Zuari River in Goa, India, MPs were detected at all sampling sites with concentrations ranging from 0.01 to 1.38 particles/L16. These concentrations demonstrate the pervasiveness of MPs in aquatic systems, even though they are below several experimental criteria. Lang et al.17 investigated the effect of different polystyrene (PS) concentrations (25–200 mg/L) on marine diatom P. tricornutum for 96 h. At lower concentrations (25–50 mg/L), diatom produced more trihalomethanes as a result of oxidative stress. However, higher concentrations (100–200 mg/L) resulted in significant cellular damage and decreased the formation and release of trihalomethanes. A similar study examined the combined toxicity of 50-mg/L MPs and six concentrations of sulfadiazine (5, 10, 20, 50, 100, and 200 mg/L) on Chlamydomonas reinhardtii, observing notable cellular damage and oxidative stress18. Previous findings indicated that MPs serve as a substrate for the attachment and proliferation of microbes19. The proliferation and colonization can alter microbes’ diversity, interaction between species, metabolic activity and microbial composition. From this point of view, the major concern is that MP can serve as a vector for hazardous contaminants and a host for pathogenic microbes, which could spread when coming into contact with them20,21.
Current research are still investigating the effect of MPs on creatures and ecosystem, involving their influence on ecosystem services, food webs and biodiversity. Studies have stated that MPs accumulation may lead to those of contaminants like dichloro-diphenyl-trichloroethane (DDTs), bisphenol A (BPA), polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) in aquatic life that are likely included in the food web thus indirectly affecting the human health. Moreover, MP accumulation in sediments can affect benthic species and interrupt the pathways of nutrient cycling. Therefore, minimizing MP pollution is important for protecting aquatic life as they are crucial for the processes like nutrient cycling and carbon sequestration22. For better understanding of these interactions, advanced analytical techniques such as scanning electron microscopy with energy dispersive X-ray spectroscopy (SEM-EDX) and Fourier transform infrared spectroscopy (FT-IR) are frequently used. SEM-EDX can provide the detailed visualization of the surface and elemental composition of MPs, while FT-IR detects specific polymer types and chemical bonds, enabling accurate characterization of MPs in environmental samples23. A study utilizing SEM-EDX and FT-IR to examine the interaction between MPs and Spirulina sp., revealed significant biochemical alterations in the microalgae when exposed to polyethylene terephthalate (PET) and polypropylene (PP) MPs24. Similarly, a study comparing µ-FT-IR and SEM-EDX for assessing MPs released from plastic bottles confirmed that both methods were effective25.
The exploration of the connection between microalgae and MP warrants evaluating the potential impact of plastics on aquatic ecosystems26. The impact of MPs on the ecosystem and human health is a dynamic area of research, and efforts are ongoing to minimize their production and presence in the environment through regulation of manufacturing practices. Generally, many reports are available regarding interaction, colonization, and toxic effects of MPs on aquatic life and microalgae. However, very few reports concerning the characterization and its influence on biochemical compositions of microalgae have yet been documented.
This study presents a novel approach by evaluating the effects of polyethylene (PE) microplastics (MP) on the growth and biochemical parameters (biomass, lipid, pigments, carbohydrates, and proteins) of Chlorella sorokiniana. Contrasting with previous studies that primarily focused on MP toxicity at extremely high concentrations, this research determines the half-maximal inhibitory concentration (IC50) of PE MP on C. sorokiniana, establishing a critical threshold for microalgal response. This study investigates the underexplored concentration-dependent effects of polyethylene microplastics on ROS dynamics and pathways in Chlorella sorokiniana. The comparative analysis of growth rate, biomass production, pigment content, biochemical compounds, production of phenols and flavonoids was carried out for the control (devoid of MP) and the MP-treated C. sorokiniana. The interactions between MPs and microalgae were also investigated by using advanced characterization techniques like SEM-EDX, FT-IR, and µ-FT-IR thus providing detailed insights into the morphological and chemical properties of the MP. Additionally, it systematically evaluates multiple concentrations of MP, offering valuable data on the concentration-dependent effects on algal health and biochemical composition.
Materials and methods
Chemicals and reagents
All the chemicals and reagents used in the present study were purchased from Sigma-Aldrich, India. Polyethylene granules were purchased from Chemika Biochemika, India.
Preparation of PE MP
One gram PE granules were dispersed in 10-mL xylene by stirring on a magnetic stirrer at 500 rpm and 70 °C for 1 h. The prepared dispersions were cooled down at room temperature, followed by the addition of 20-mL ethanol. The mixture was further stirred for an additional 30 min to ensure proper homogenization. Microplastics were filtered and washed with ethanol and dried at room temperature. Then, the dried MP were collected in a dried zip-lock bag. Prepared MP were dispersed in distilled water and after sonication the particle size distribution, mean particle size and polydispersity index were analysed using a particle size analyzer (Zetasizer Lab, Malvern, India). An overall scheme of the present investigation is illustrated in Fig. 1.
Microalgae cultivation and experimental design
The previously isolated microalgal strain Chlorella sorokiniana UUIND6 (GenBank accession number KY780616) was used in the current study27. The culture was grown and maintained in Bold’s Basal Media (BBM), under white light emitting diodes (LED) light at an intensity of around 300 µmol/(m2 s) with continuous illumination and incubated at 25 ± 1 °C for 10 days. The cultures were gently shaken every 6 to 8 h, and growth was monitored each day by measuring optical density (OD) at 730 nm using a UV-VIS spectrophotometer (Agilent & Cary-60 UV-Vis). After 10 days incubation, the cultures were harvested and prepared inoculum was used for further experiments.
The experiments were conducted to examine the half-maximal inhibitory concentrations (IC50) by utilizing different concentrations of PE MP (25–150 mg/L) (average size 1.42 μm) in BBM. The flask without PE MPwas taken as the control. After 96-hr incubation period, the growth of microalgae cultures was assessed by measuring their OD at 730 nm, and percent inhibition was calculated by using Eq. 128. Later on, a graph was plotted between inhibition percentage and MP concentrations. The IC50 value of MP concentration was utilized to study its effect on growth, biomass content and biochemical compounds of C. sorokiniana.
The IC50 was calculated and found to be approximately 100 mg/L, which was then selected for use in subsequent experiments to ensure consistent and physiologically relevant exposure.
Estimation of biochemical components of microalgae
Growth curve and biomass collection
The cell growth was measured by taking OD values during incubation. The culture was incubated for up to 14 d. All the cultures (control and PE-treated) were harvested after 14-d incubation by centrifugation in pre-weighted falcon tubes. The biomass thus obtained was dried at 60 °C in a hot-air oven, weighed and stored for further analysis.
Pigments estimation
Pigments content was measured following the method of Lichtenthaler29 with slight modifications. 2-mL sample was poured into a tube and centrifuged for 5 min at 5000 rpm on 10th day for microalgae cultivation. The supernatant was removed, and 2-mL methanol was added to the pellet. The suspension was kept in a water bath at 45 °C until the pellets turned colourless. At last, the suspension was centrifuged and then supernatant was collected; the absorbance of the supernatant was measured at 665.2, 652.4, 470 and 750 nm in a UV-Vis spectrophotometer. The values obtained from 665.2, 652.4, and 470 nm were corrected by subtracting the value obtained from 750 nm. Chl-a, Chl-b and Car were calculated by using following equations (Eq. 2 to 4):
Lipid extraction
The dry weight of the obtained cells was used for lipid extraction following Bligh and Dyer’s method with slight modification30. Initially, a 3-mL mixture of chloroform and methanol (v/v = 2/1) was added to the disrupted cells. The suspension was incubated in a shaker at 180 rpm for 3 h under room temperature. After that, the mixture was centrifuged for 10 min at 5000 rpm. The upper layer was separated in a fresh tube, and the collected solution was treated with 3 mL of 0.034% MgCl2 to remove polar impurities and enhance separation and again centrifuged at 5000 rpm for 10 min. Then, the upper phase was discarded, and to remove residual methanol and contaminants 3 mL of 2 N KCl was added to the lower phase and again centrifuged. Once more, the upper phase was discarded, and the lower phase was treated with 3 mL of the mixture of chloroform, methanol, and distilled water in the ratio of 3:47:48 (v/v/v), followed by centrifugation for 5 min at 5000 rpm. At last, the resulting lower phase was collected and dried at 45 °C for 6–7 h in a hot air oven to ensure complete solvent removal and obtain total lipids. The dried lipid was weighed, and then the lipid percentage was calculated by using the given equation (Eq. 5).
Total protein and carbohydrate Estimation
Elemental carbon, hydrogen and nitrogen analyzer (Thermo Fisher) was used to estimate the total nitrogen content in microalgae. The amount of crude protein was calculated by using the following equation (Eq. 6)31.
For total carbohydrate estimation, lipid-extracted microalgal biomass was hydrolyzed by 5% H2SO4 and autoclaved. Then, released sugar was assessed using phenol-sulfuric acid method; d-glucose was kept as reference (Eq. 7)32 as shown in Fig. S1.
Determination of phytochemical compounds and ROS
Determination of total phenolic content (TPC)
For microalgae extract preparation 5 g of freeze-dried algal biomass were mixed with 100 mL of 80% methanol (v/v). The mixture was sonicated for 10 min with the temperature maintained below 40 °C. Then solution was centrifuged at 8000 rpm for 10 min. Supernatant was concentrated using rotary evaporator (Dlab RE 100-Pro, China made), marked as methanol extract and stored at − 20 °C for further analysis. Total phenolic content of the control and the MP-treated microalgae were determined spectrophotometrically using Folin-Ciocalteu (FC) colorimetric method adopted from Bisht et al.33 with slight modification. In the present study, gallic acid was used to obtain standard calibration curve as shown in Fig. S2. The sample solution was made by dissolving 10 mg in 100 µL of methanol. Before use, the FC reagent was mixed with distilled water in 1: 9 ratio. In a test tube, 20 µL of microalgae extract was diluted with 450 µL of distilled water to make 500 µL total volumes. To this solution, 250 µL of FC reagent was added and incubated for 1 min. Further, the solution was supplemented with 2 mL of 7.5% sodium carbonate, and the mixture was left to stand for 90 min. The absorbance against the blank was measured at 765 nm and expressed as gallic acid equivalent (GAE) per g of dry weight (DW). TPC was determined using the following equation (Eq. 8).
Where c is the polyphenol content from the calibration curve expressed in mg GAE/mL, v is the volume of chemical used in the test (mL), and m is the sample weight (g DW).
Determination of total flavonoid content (TFC)
Total flavonoid contents of the control and the MP-treated microalgae extract were assessed spectrophotometrically using the colorimetric technique reported by Bisht et al.33 with minor modifications. A 5-mL volumetric flask was used to hold 20-µL sample solution. Furthermore, 2.5 mL of distilled water was added to the flask, followed by 0.15 mL of 5% NaNO2 and 5 min of stirring. After that, 0.15 mL of 10% AlCl3 and 1 M NaOH were added at 5 min intervals, and the volume was increased to 5 mL with distilled water. The solution was vortexed, and absorbance v/s blank was measured at 510 nm using spectrophotometer. The blank sample was made in the same way as described above. The results were determined against quercetin standard curve (Fig. S3) and represented in mg quercetin equivalent per g DW. TFC was determined using the following equation (Eq. 9).
Where c is the flavonoid concentration from the calibration curve expressed in mg quercetin/mL, v is the volume of chemical used (mL), and m is the sample weight (g DW).
Determination of reactive oxygen species (ROS)
The amounts of ROS produced in the control and the MP-treated microalgae extract were measured in terms of H2O2 scavenging. The microalgae extract (20–100 µg/mL) was added to a 40-mM H2O2 solution buffered with phosphate (pH 7.4). After 10 min, absorbance was measured at 230 nm, and the scavenging percentage was calculated using the Eq. 1034:
.
Characterization of PE MP
The prepared PE MPs were characterized to check their properties by their shape, size, and elemental analysis. Before characterization, fixation of microalgal cells for scanning electron microscopic analysis (SEM; Carl Zeiss EVO 18) was done by treating them overnight in the dark at 4 °C with 2.5% glutaraldehyde solution, followed by washing with phosphate-buffered saline (PBS) to remove excess fixative. Then, microalgal cells were dehydrated by treating with 10–100% ethanol solutions for 10 min at each step. The dehydrated samples were then gold-sputter-coated to enhance conductivity for SEM analysis35.
SEM analysis was done to perceive the changes in the surface morphology of C. sorokiniana, PE MP, and their interaction. Whereas, SEM coupled with EDX (Carl Zeiss EVO 18; Energy dispersive X-ray) was used to estimate the alteration in elemental composition and quantify alterations in elemental percentages during the interaction of C. sorokiniana with PE MP. The technique was chosen due to its ability to provide high-resolution surface imaging and elemental mapping, enabling us to understand MP interaction at the microscale. The elemental composition data obtained from the SEM-EDX analysis were cross-validated with FT-IR (Themo Scientific Summit lite) analysis to confirm changes in the organic compositions and surface chemistry of C. sorokiniana. Microalgal biomasses cultivated with and without (control) PE MPs were dehydrated and analyzed using the potassium bromide (KBr) pellet technique with FT-IR in the range of 4000 –400 cm-1. Zeta potential was measured at room temperature on a Zeta size analyzer (Malvern Instrument, ltd, UK). Zeta potential is used to assess the reduction in repulsive forces and the formation of larger flocs before and after the coagulation process between microalgae and MPs36. It plays a crucial role in algal aggregation, separation and flotation efficiency37.
Mapping of PE microplastic using µFT-IR
The supernatant was used for µ-FT-IR analysis of MPs in media containing PE MP after harvesting microalgal biomass. The supernatant was filtered by Anodisc filter paper. The resulting filters were analyzed using an FT-IR microscope (Nexus 670, Thermo Electron Corporation, USA) in transition mode and spectral range of 1300–1400 cm− 1 for mapping and followed by the procedure described by Song et al.38. The number of particles on one of the filters was estimated using the Particle Wizard option of profiling feature in the Omnic Picta software 9.12. For mapping, 200,000-300,000 spectra were collected using the ultrafast mapping mode with a linear array detector and 25-µm steps over a single section (~ 12 × 12 mm), for semi-automated and automated PE MP analysis on Anodisc filter papers (n = 5). The peaks of CO2 (2200–2400 cm− 1) were removed, and the entire collected spectra were matched with reference spectra using the spectrum.
Statistical analysis
To evaluate the impact of microplastic (MP) treatment on various physiological and biochemical parameters of microalgae, statistical analyses were conducted using the online tool OPSTAT (Operational Statistics). A one-way analysis of variance (ANOVA) was employed to determine whether the differences observed between the treatment and control groups were statistically significant. The input data for the analysis consisted of experimentally measured values obtained from biological triplicates (n = 3) for control and MP-treated groups. The data are expressed as mean values ± standard deviation (SD). Statistical significance was set at p < 0.05. The detailed ANOVA results are provided in Table S1.
Results and discussion
Particle size distribution of MP
Through the particle size analysis of MP dispersion, the mean particle size was determined as 1420 nm (1.42 μm) (Fig. 2). The polydispersity index was found to be 1, which revealed a highly polydisperse PE. PE MPs are becoming more prevalent in surface water, marine environment, and wastewater as micropollutants. According to recent studies, prolonged exposure to higher PE MPs may harm the aquatic biosystem39,40.
Cell viability and IC50 of Chlorella sorokiniana under MP exposure
C. sorokiniana was exposed to MPs with different concentrations ranging from 25 to 150 mg/L to examine its cell viability by measuring OD. After a 96-hr incubation period, a decrease in OD of microalgae cultures was seen from 50 mg/L to 150 mg/L of MP concentration. The %-inhibition was calculated by using Eq. 1 (Section “2.2”) and a graph was plotted between %-inhibition and MP concentrations as shown in Fig. S4. The IC50 value was found to be 100 mg/L, indicating that this concentration of MP decreased the growth of C. sorokiniana by 50%, when exposed for 96 h. Therefore, MPs of 100 mg/L were utilized further for treating microalgae cultures and their effect on biomass content, pigments and other biochemical components was compared with that of the control.
Effect of MP exposure on growth rate and biomass content of C. sorokiniana
The effect of microplastic (MP) exposure (100 mg/L) on the growth of Chlorella sorokiniana was assessed by measuring optical density at 686 nm over a 14-day cultivation period (Fig. 3A). Both control and MP-treated cultures showed an initial increase in cell density; however, a visible lag in growth was observed in MP-treated cultures starting from day 4. Notably, while the MP-treated group exhibited slower growth, particularly up to 96 h consistent with the inhibitory concentration (IC₅₀) of 100 mg/L this difference was not statistically significant when comparing the overall biomass accumulation between the groups.
According to the one-way ANOVA analysis (Table S1), the F-calculated value for biomass content was 0.637 with a p-value of 0.51, indicating no significant difference (p > 0.05) in biomass between control and MP-exposed cultures by the end of the cultivation period. These results suggest that while MP exposure temporarily suppressed growth kinetics during early stages, it did not cause a statistically significant reduction in final biomass yield under the tested conditions.
The slight reduction in growth rate can be due to the adsorption of MPs onto algal cells can obstruct light from reaching their photosynthetic apparatus, potentially diminishing photosynthetic efficiency41,42. Moreover, MPs can carry toxic additives (like copper, bromium & titanium) into the medium, which damage the cell membranes of microalgal cells, hence reducing their growth rate43. The observed results were in accordance with previous studies that had also noticed a reduction in microalgal growth rate upon treatment with MPs42,43,44,45.
Biomass did not show statistically significant changes (p > 0.05), suggesting minimal or variable responses under the MP stress. Biomass content was also observed in the MP treatment, i.e., 0.89 g/L, as compared to that in control groups, i.e., 0.96 g/L (Fig. 3B). In another study, Cunha et al.46 also stated that Phaeodactylum tricornutum cultures exposed to MPs (polymethyl methacrylate and PS) showed a low biomass yield as compared to control ones. Nguyen et al.47 also found that high concentrations of MPs can negatively impact the growth and biomass content of microalgae. Hence, the results indicate that the presence of MPs might cause light scattering, preventing microalgal cells from absorbing enough light, thus adversely influencing their photosynthetic efficacy and growth46.
Effect of MP exposure on pigments and biochemical compounds of C. sorokiniana
In plants and algae, chlorophyll is considered a crucial pigment participating in photosynthesis, which absorbs light energy. Therefore, photosynthetic action of microalgal cells is proportional to the chlorophyll content48. Moreover, carotenoids are bioactive compounds, which possess a crucial role in bioavailability mechanisms, regulation of gene transcription and the activity of pro-vitamin A49. C. sorokiniana exhibits various adaptive responses when exposed to microplastics, highlighting the resilience of microalgae in polluted environments. It shows significant adaptations in its photosynthetic machinery when exposed to microplastics. These adaptations include modifications in chlorophyll florescence, adjustments in photosynthetic efficiency and changes in energy transfer within photosystems50. Hence, examining the effect of MPs on microalgal pigments is of great importance. As revealed by experimental data, the content of the pigments (i.e., Chl a, Chl b and car) of the MP-treatment was lower than that of control groups. The contents of Chl a, Chl b and car of the MP-treatment were 3.99, 2.01 and 1.47 µg/mL, respectively, while those of the control were 5.06, 2.60 and 1.64 µg/mL, respectively (Fig. 4A). Hence, it has been suggested that MP may negatively impact the primarily photosynthetic pigment while not strongly affecting Chl b and carotenoids. Reduced contents of chlorophyll in MP-treated groups might be due to hindrance in photosynthetic activity. MPs might block pores for cellular respiration in the cells, resulting in cellular injury and then impeding chlorophyll synthesis4. These findings were consistent with Xu et al.51 studies that reported a reduction in chlorophyll content of Skeletonema costatum by 20% when exposed to 50 mg/L of PVC MPs. Similarly, Jiang et al.52 also noticed that exposure to PET MPs reduced the accumulation of Chl a, Chl b and car within Scenedesmus sp. as compared to a control group. However, the factors like exposure time, MP types and microalgae species greatly influence the effect of MP exposure on photosynthetic activities of microalgae51,53. Another study examining the complex interaction between microalgae and MPs revealed that exposure to MPs led to a reduction in Chl-a content. This exposure disrupts electron transfer within the photosynthetic system, damaging electron transport and oxidative stress. Major biological functions like energy production, carbon fixation, lipid metabolism, and nucleic acid metabolism may also be impacted by these effects54.
Moreover, biochemical analysis revealed that, as compared to the control, the lipid content was decreased, while the protein and carbohydrate contents were increased in MP-treated groups. In particular, the lipid, protein and carbohydrate contents in control were 35.7, 32.2 and 22.3%, whereas in MP-treated groups, they were 26.7, 37.6, 24.4%, respectively (Fig. 4B). As shown in the ANOVA table, parameters such as chlorophyll “a” (p = 0.04), lipid content (p = 0.004), and carbohydrate content (p = 0.000) exhibited statistically significant differences upon MP exposure, indicating that microplastics had a notable impact on these traits. In contrast, biomass, chlorophyll “b,” carotenoids, and protein content did not show statistically significant changes (p > 0.05), suggesting minimal or variable responses under the same conditions. In contrast, Jiang et al.52 observed a decrease in intracellular carbohydrates and proteins in Scenedesmus sp. exposed to PET MPs. Similar inhibitory effects of MPs (i.e., PVC, PP, HDPE) were observed by Ansari et al.45 on the protein yield of Acutodesmus obliquus. However, culture conditions largely influence the accumulation of different metabolites in microalgal biomass55. For example, Ansari et al.45 observed that PP and HDPE MPs increased the carbohydrate yield in A. obliquus at a concentration of 50–150 mg/L, while a decreasing trend was observed beyond 150 mg/L. Similarly, Li et al.56 stated that PS with a concentration of 50 mg/L downregulated the carbohydrate metabolism in Skeletonema costatum. The heterocoagulation of microalgae and MPs can obstruct the matter and energy transfer, thus delaying carbon fixation at the time of photosynthesis and carbohydrate production57. In addition, amino acids are essential raw components for the synthesis of proteins, and MPs can cause a shift of energy in microalgae from photosynthetic carbon integration to the amino acid catabolism pathway. Thus, the improvement in amino acid catabolic pathways might consequently influence the buildup of carbohydrates and proteins51,58. Moreover, a decrease in lipid content might be due to MP stress disrupting physiological homeostasis in microalgae and the generation of ROS and intracellular oxygen-free radicals results in cellular oxidative injury. Production of ROS can lead to lipid peroxidation by damaging the polyunsaturated fatty acids (PUFAs) of microalgae. Malondialdehydes (MDA) are the ultimate product of membrane peroxidation, which can describe the oxidation rate and lipid damage in microalgae exposed to environmental stress59.
Effect of MP exposure on zeta potential and size of C. sorokiniana
Exposure to microplastics induce notable changes in C. sorokiniana including alterations in cell size and zeta potential which play a crucial role to its adaptation in microplastic pollution. The size of C. sorokiniana in the control was estimated as 2.7 μm and decreased to 2.2 μm after exposed to PE MP. In the control condition, the estimated zeta potential was − 11.3 mV and − 31.8 mV in the MP-treatment, indicating the zeta potential of the MP-treatment would be more stable. Changes in zeta potential suggest modifications in membrane properties potentially influencing nutrient uptake and stress response mechanisms. According to our findings, it was hypothesized that C. sorokiniana and PE MP interaction would promote cell dormancy. Recently, it was reported that the zeta potential of PE MP was 29.9 mV, which decreased to -25.8 mV over time60. In a previous report, it was shown that the initial zeta potentials of Chlorella and Scenedesmus were − 26.1 and − 11.3 mV, respectively. However, after they were exposed to + PS beads, the zeta potentials increased to 85.8 mV and 56.3 mV, respectively. However, with PS beads, the zeta potential decreased to 9.0 mV and 14.6 mV. These results proposed that positively charged PS beads have more binding affinity to Chlorella and Scenedesmus than negatively charged ones61.
Effect of MP exposure on phenolics, flavonoids and ROS of C. sorokiniana
The study examined the effect of PE MP exposure on phenolics, flavonoids and ROS in C. sorokiniana. The experimental data revealed that the total phenolics, flavonoids and ROS levels in the control were 15.3 mg GAE/g sample, 9.3 mg QUERCETIN/g sample, and 41.8%, respectively. In contrast, MP-treated samples exhibited a significantly reduced ROS (31.0%) and phenolic content (11.4 mg GAE/g). However, the flavonoid content increased to 12.2 mg QUERCETIN/g sample (Table 1). Hence, the results clearly stated that PE MP exposure would alter the oxidative balance and secondary metabolite production in C. sorokiniana.
In contrast to other MP forms, such as PET, which haven known to cause a larger accumulation of ROS in microalgae, this decrease in ROS content after MP exposure suggests a reduced oxidative stress response4,52. Microplastics typically cause oxidative stress which results in excessive electron buildup and the production of ROS by interfering with the electron transport chain44. Overproduction of ROS can cause oxidative damage to cellular organelles such as nuclei, mitochondria, and chloroplasts, which can subsequently limit photosynthesis, cause genetic instability, and decrease biomass14,62,63.
Since ROS levels were lower in PE-exposed cultures, the demand for phenolic-based antioxidant defenses was also reduced. In contrast, the increase in flavonoid content suggests an alternative non-enzymatic antioxidant defense mechanism. Flavonoids are well-known for their radical-scavenging abilities, chelation of metal ions, and DNA protection against oxidative damages64. The elevated flovonoid levels in C. sorokiniana exposed to PE MPs may indicate a compensatory response to a stress. The reduction in phenolic content in MP-treated C. sorokiniana aligns with previous reports suggesting that phenolic compounds are synthesised as part of microalgal responses to an oxidative stress65. A recent study explored the response of PS-MPs and perfluorooctanoic acid (PFOA) on C. sorokiniana. It was identified that PS-MPs primarily inhibited photosynthesis through shading effects, while PFOA induced an oxidative stress via ROS generation50. While this study focused on biochemical and oxidative stress responses, other approaches, such as molecular analysis, could provide deeper insights into regulatory pathways influenced by MP exposure. Further advanced techniques such as proteomics and metabolomics could help to identify key genes, proteins and metabolic pathways involved in the responses to an MP-stress.
SEM-EDX analysis
SEM is considered an authentic analysis for studying the surface morphology of a wide range of micro- and nano-particulate materials66. It also provides the chemical compositions of solids when equipped with the EDX detector67. Figure 5A, B and C shows SEM images and EDX spectra of MP, microalgae, and microalgae + MP. SEM image (B) revealed that C. sorokiniana has spherical structures, whereas SEM image (C) clearly shows morphological changes in C. sorokiniana cells after interaction with PE-MP. This shows that the surface of PE-MP may be positively charged, because they tend to bind the surface of the cell membrane of C. sorokiniana and form an aggregation, which plays a good role in the interaction with C. sorokiniana and supports its survival. There were no significant changes observed in the growth of C. sorokiniana exposed to PE-MP as compared to the control. However, during the experiment, it was noticed that when the biomass was harvested on the final day of the incubation period and subjected to centrifugation, small particles of PE-MP with slight shear force appeared in the supernatant after centrifugation. The interaction also hypothesized how long C. sorokiniana interacted with PE-MP before separating. It can be suggested that exposure to low concentrations of PE-MP did not result in significant interaction but did affect the growing environment. Conversely, higher concentrations of PE-MPs interacted more with C. sorokiniana, leading to aggregation. In Fig. 5, shading of C. sorokiniana is seen while the species interact with PE-MP. In a previous study, it was documented that microalgal biomass showed homo- and hetero-aggregation of PE-MPs with its surface68. They also demonstrated that PE-MPs could create physical blockage or shading effects that would ultimately hinder light intensity and availability of nutrients to the microalgae. Subsequently, this could create a photooxidative damage of PS-II69. Besides, previous reports found that PE-MPs could serve as a substrate for algal growth and enhance microalgal growth70,71.
EDX analysis revealed elemental composition of PE-MP, C. sorokiniana, and PE-MP with C. sorokiniana. The results of EDX showed a reduction in the percentage of carbon in the PE-MP + C. sorokiniana sample compared to the pure C. sorokiniana sample. This shows that PE-MP created stress in the environment and influenced C. sorokiniana. In previous studies, SEM-EDX analysis was done to reveal the presence of all superior (C, O, N, and Ca) constituents in microalgae (Stigonematales sp.)72 and Spirulina sp73. The information on the elemental composition is useful in identifying carbon-dominant plastics over other types of materials74.
FT-IR analysis
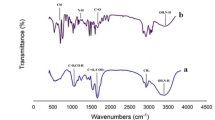
In FT-IR analysis, the sharp peaks at 2917 cm–1, 2842 cm–1, 1458 cm–1, and 708 cm–1 were observed in the PE-MP treatment (Fig. 6). The presence of peaks at 2950 cm–1 to 2800 cm–1 show the presence of an alkyl group which indicates that the PE-MPs contained the CH2 polymers that form polymer chains75,76. SEM and FT-IR analyses of C. sorokiniana biomass exposed to PE-MP elucidated the surface alteration of cells and depletion of amine groups from C. sorokiniana, which are obvious from the spectral peaks at 1238 cm–1 and 2854 cm–1. Therefore, based on the FT-IR analysis, it can be predicted that C. sorokiniana cells treated with PE-MPs have not created toxicity in terms of biomass and lipid content. The band of 1400 –1076 cm-1 was noticed in the IR spectrum of the C. sorokiniana sample, which is shown in Fig. 6. According to El-Naggar et al.77, the bands at 1300–900 cm–1 denote carbohydrates/polysaccharides biomolecules such as alkanes, carboxylic acid, CH3 linked to O or N, CH and CO stretching mode or CH2 asymmetric stretching vibration mode. Sadiq et al.78 and Clement et al.79 investigated how TiO2, a PE-MP additive, would interact with Chlorella sp. and Scenedesmus sp. They observed peak alterations in the carbonyl, amine, hydroxyl, and carboxyl groups after interaction with TiO2. A similar study was conducted by Dmytryk et al.80, who investigated the interaction of Spirullina sp. with copper, another additive in PE-MPs and observed peak shift at wavelengths 1300 to 1000 cm–1. The shift indicates the presence of -OH group, suggesting an interaction between the hydroxyl and metal ions because hydroxyl is one of the binding sites on the surface of the biomass.
Further, Fig. 7 shows the µ-FT-IR spectra-display map of PE-MP. Because the technique involves minimum sample preparation and is used to detect PE-MP straight on membrane filters81. The spectra were collected in transmission mode and used for profiling. Being non-destructive and reliable, µ-FT-IR has been the latest technique used for polymer characterization of PE-MP of > 10 μm82.
Conclusion
The present study shows the tolerance of C. sorokiniana to PE-MP stress. Synergistically, PE IC50 revealed an increase in protein, carbohydrate and total flavonoid contents, while a slight reduction was observed in the biomass and total lipid content. Notably, rather than inducing an oxidative stress, MP exposure appeared to reorient the microalgal biosynthetic activity, as indicated by a reduction in ROS and total phenol content compared to the control. SEM-EDX analysis showed morphological changes in C. sorokiniana cells after interaction with PE-MP. The elemental analysis of the cells confirmed the alterations in carbon and oxygen content, indicating environmental stress on the microalgae. Additionally, FT-IR analysis revealed the destruction and loss of functional groups in microalgal cells after exposure to PE-MP. , Thus, further research is still required to determine species-specific responses and broader ecological implications of PE-MP exposure.
Data availability
Data is provided within the manuscript or supplementary information.
References
Jaiswal, K. K. et al. Impact of aquatic microplastics and nanoplastics pollution on ecological systems and sustainable remediation strategies of biodegradation and photodegradation. Sci. Total Environ. 806, 151358 (2022).
Dey, T. K., Uddin, M. & Jamal, M. Detection and removal of microplastics in wastewater: Evolution and impact. Environ. Sci. Pollut Res. 28 (14), 16925–16947 (2021).
Europe, P. Plastics-the Facts 2015 an analysis of European plastics production, demand and waste data, Available on the website: (2017).
Khatiwada, J. R. et al. Interaction between polyethylene terephthalate (PET) microplastic and microalgae (Scenedesmus spp.): Effect on the growth, chlorophyll content, and hetero-aggregation. Environ. Adv. 13, 100399 (2023).
Cheng, Y. R. & Wang, H. Y. Highly effective removal of microplastics by microalgae Scenedesmus abundant. Chem. Eng. J. 435, 135079 (2022).
Bhuyar, P., Muniyasamy, S. & Govindan, N. Biodegradation of plastic waste by using microalgae and their toxins. J. Biotechnol. Biomater. 8, 55 (2019).
Lundebye, A. K., Lusher, A. L. & Bank, M. S. Marine microplastics and seafood: Implications for food security. Microplast. Environ. Pattern Process, 131. (2022).
Wu, P., Cai, Z., Jin, H. & Tang, Y. Adsorption mechanisms of five bisphenol analogues on PVC microplastics. Sci. Total Environ. 650, 671–678 (2019).
Wang, T. et al. Interactions between microplastics and organic pollutants: Effects on toxicity, bioaccumulation, degradation, and transport. Sci. Total Environ. 748, 142427 (2020).
Chen, G., Feng, Q. & Wang, J. Mini-review of microplastics in the atmosphere and their risks to humans. Sci. Total Environ. 703, 135504 (2020).
Du, S. et al. Environmental fate and impacts of microplastics in aquatic ecosystems: A review. RSC Adv. 11 (26), 15762–15784 (2021).
Sharifian, S. & Homaei, A. Marine-derived polysaccharides: Prospects for future pharmaceuticals and drug delivery systems. In Marine Biomaterials: Drug Delivery and Therapeutic Applications. 403–453 (Springer, 2022).
Jain, R. et al. Microplastic pollution: Understanding microbial degradation and strategies for pollutant reduction. Sci. Total Environ. 167098. (2023).
Prata, J. C., da Costa, J. P., Lopes, I., Duarte, A. C. & Rocha-Santos, T. Effects of microplastics on microalgae populations: A critical review. Sci. Total Environ. 665, 400–405 (2019).
Yang, W. et al. The combined toxicity influence of microplastics and nonylphenol on microalgae Chlorella pyrenoidosa. Ecotoxicol. Environ. Saf. 195, 110484 (2020).
Kalangutkar, N., Mhapsekar, S., Pednekar, M. M. A. & P Microplastic pollution in the surface waters of the Zuari river, goa, India. Environ. Monit. Assess. 196 (11), 1007 (2024).
Lang, X., Ni, J. & He, Z. Effects of polystyrene microplastic on the growth and volatile halocarbons release of microalgae Phaeodactylum tricornutum. Mar. Pollut. Bull. 174, 113197 (2022).
Li, Z. et al. Toxicological effects of microplastics and sulfadiazine on the microalgae Chlamydomonas reinhardtii. Front. Microbiol. 13, 865768 (2022).
Zhang, J. et al. Adsorption behavior and mechanism of 9-Nitroanthracene on typical microplastics in aqueous solutions. Chemosphere 245, 125628 (2020).
Koelmans, A. A. et al. Microplastics in freshwaters and drinking water: Critical review and assessment of data quality. Water Res. 155, 410–422 (2019).
Rai, M. et al. Microplastic pollution in terrestrial ecosystems and its interaction with other soil pollutants: A potential threat to soil ecosystem sustainability. Resources 12 (6), 67 (2023).
Ratnasari, A. et al. The crucial nexus of microplastics on ecosystem and climate change: Types, source, impacts, and transport. Water Air Soil Pollut. 235 (5), 315 (2024).
Girao, A. V. SEM/EDS and optical microscopy analysis of microplastics. In Handbook of Microplastics in the Environment (57–78). (Springer, 2022).
Khoironi, A., Anggoro, S. & Sudarno Evaluation of the interaction among microalgae Spirulina sp, plastics polyethylene terephthalate and polypropylene in freshwater environment. J. Ecol. Eng. 20 (6), 161–173 (2019).
Winkler, A. et al. Microplastic release from plastic bottles-comparison of two analytical methodologies (SEM-EDX and µ-FTIR). In Proceedings of the 2nd International Conference on Microplastic Pollution in the Mediterranean Sea 255–261. (Springer, 2020).
Mao, Y. et al. Phytoplankton response to polystyrene microplastics: Perspective from an entire growth period. Chemosphere 208, 59–68 (2018).
Kumar, V., Nanda, M., Kumar, S. & Chauhan, P. K. The effects of ultraviolet radiation on growth, biomass, lipid accumulation and biodiesel properties of microalgae. Energ. Source Part A Recov. Util. Environ. Eff. 40 (7), 787–793 (2018).
Fatima, N. et al. Toxicity of cadmium (Cd) on microalgal growth,(IC50 value) and its exertions in biofuel production. Biointerface Res. Appl. Chem. 10 (4), 5828–5833 (2020).
Lichtenthaler, H. K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Met. Enzymol. 148, 350–382 (1987).
Bligh, E. G. & Dyer, W. J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37 (8), 911–917 (1959).
Laurens, L. M. L., Olstad, J. L. & Templeton, D. W. Total Protein Content Determination of Microalgal Biomass by Elemental Nitrogen Analysis and a Dedicated Nitrogen-to-protein Conversion Factor 233–242 (Methods and Protocols, 2020).
Sturgeon, R. J. Monosaccharides. In Methods in Plant Biochemistry. Vol. 2. (ed Carbohydrates, P. M.) 1–37. (Academic, 1990).
Bisht, B. et al. Synergistic interaction between hydrocolloids and Kinnow Peel Biowaste for the development of edible fillers using response surface methodology. Environ. Sci. Pollut. Res. 31 (2), 2595–2613 (2024).
Özyürek, M., Bektaşoğlu, B., Güçlü, K., Güngör, N. & Apak, R. A novel hydrogen peroxide scavenging assay of phenolics and flavonoids using cupric reducing antioxidant capacity (CUPRAC) methodology. J. Food Compos. Anal. 23 (7), 689–698 (2010).
Arora, N. et al. A hybrid approach integrating arsenic detoxification with biodiesel production using oleaginous microalgae. Algal Res. 24, 29–39 (2017).
Nasrabadi, A., Esmaeili, M., Eydi & Bonyadi, Z. Utilizing Chlorella vulgaris algae as an eco-friendly coagulant for efficient removal of polyethylene microplastics from aquatic environments. Heliyon 9 (11) (2023).
Matho, C. et al. Bio-compatible flotation of Chlorella vulgaris: Study of zeta potential and flotation efficiency. Algal Res. 44, 101705 (2019).
Song, Y. K., Hong, S. H., Eo, S. & Shim, W. J. A comparison of spectroscopic analysis methods for microplastics: Manual, semi-automated, and automated fourier transform infrared and Raman techniques. Mar. Pollut Bull. 173, 113101 (2021).
Arias-Andres, M., Kettner, M. T., Miki, T. & Grossart, H. P. Microplastics: New substrates for heterotrophic activity contribute to altering organic matter cycles in aquatic ecosystems. Sci. Total Environ. 635, 1152–1159 (2018).
Costa Araújo, A. P., da, Gomes, A. R. & Malafaia, G. Hepatotoxicity of pristine polyethylene microplastics in Neotropical Physalaemus cuvieri tadpoles (Fitzinger, 1826). J. Hazard. Mater. 386, 121992 (2020).
Bhattacharya, P., Lin, S., Turner, J. P. & Ke, P. C. Physical adsorption of charged plastic nanoparticles affects algal photosynthesis. J. Phys. Chem. C. 114 (39), 16556–16561 (2010).
Hadiyanto, H. et al. Interactions between polyethylene and polypropylene microplastics and Spirulina sp. microalgae in aquatic systems. Heliyon, 7(8). (2021).
Zhu, X. et al. Growth Inhibition of the microalgae Skeletonema costatum under copper nanoparticles with microplastic exposure. Mar. Environ. Res. 158, 105005 (2020).
Li, S. et al. Influence of polystyrene microplastics on the growth, photosynthetic efficiency and aggregation of freshwater microalgae Chlamydomonas reinhardtii. Sci. Total Environ. 714, 136767 (2020).
Ansari, F. A. et al. Effect of microplastics on growth and biochemical composition of microalga Acutodesmus obliquus. Algal Res. 56, 102296 (2021).
Cunha, C., Lopes, J., Paulo, J., Faria, M., Kaufmann, M., Nogueira, N. & Cordeiro, N. The effect of microplastics pollution in microalgal biomass production: A biochemical study. Water Res. 186, 116370 (2020).
Nguyen, H. T., Yoon, Y., Ngo, H. H. & Jang, A. The application of microalgae in removing organic micropollutants in wastewater. Crit. Rev. Environ. Sci. Technol. 51 (12), 1187–1220 (2020).
Bhatnagar, P., Gururani, P., Rawat, J., Jaiswal, K. K., Gautam, P., Nanda, M. & Kumar, V. Influence of GABA (Gamma-aminobutyric acid) supplementation on biomass,pigments, lipid and protein content of Pseudochlorella pringsheimii under salinity stress. Curr. Res. Biotechnol. 7, 100223 (2024).
Abomohra, A. E. F., El-Sheekh, M. & Hanelt, D. Pilot cultivation of the chlorophyte microalga Scenedesmus obliquus as a promising feedstock for biofuel. Biomass Bioenerg. 64, 237–244 (2014).
Zhao, Z. et al. Response mechanisms of Chlorella sorokiniana to microplastics and PFOA stress: Photosynthesis, oxidative stress, extracellular polymeric substances and antioxidant system. Chemosphere 323, 138256 (2023).
Xu, H., Wang, Y., Qiu, K., Chen, S., Zeng, J., Liu, R., & Huang, W. Differential physiological response of marine and freshwater microalgae to polystyrene microplastics. J. Hazardous Mater., 448, 130814 (2023).
Jiang, J. et al. Effects of polyethylene terephthalate microplastics on cell growth, intracellular products and oxidative stress of Scenedesmus Sp. Chemosphere 348, 140760 (2024).
Lagarde, F. et al. Microplastic interactions with freshwater microalgae: Hetero-aggregation and changes in plastic density appear strongly dependent on polymer type. Environ. Pollut. 215, 331–339 (2016).
Li, J. et al. (ed Fan, Z.) Effect and mechanism of microplastics exposure against microalgae: Photosynthesis and oxidative stress. Sci. Total Environ 905 167017 (2023).
Luo, L., Ren, H., Pei, X., Xie, G., Xing, D., Dai, Y. & Liu, B. Simultaneous nutrition removal and high-efficiency biomass and lipid accumulation by microalgae using anaerobic digested effluent from cattle manure combined with municipal wastewater.Biotechnol. Biofuels, 12, 1–15 (2019).
Li, X., Luo, J., Zeng, H., Zhu, L. & Lu, X. Microplastics decrease the toxicity of sulfamethoxazole to marine algae (Skeletonema costatum) at the cellular and molecular levels. Sci. Total Environ. 824, 153855 (2022).
You, X., Cao, X., Zhang, X., Guo, J. & Sun, W. Unraveling individual and combined toxicity of nano/microplastics and Ciprofloxacin to Synechocystis sp. at the cellular and molecular levels. Environ. Int. 157, 106842 (2021).
Mizukami-Murata, S., Suzuki, Y., Sakurai, K. & Yamashita, H. Freshwater Alga Raphidocelis subcapitata undergoes metabolomic changes in response to electrostatic adhesion by micrometer-sized nylon 6 particles. Environ. Sci. Pollut. Res. 28, 66901–66913 (2021).
Xie, X. et al. Growth Inhibition and toxic effects of microplastics on Chlorella vulgaris. Algal Res. 78, 103378 (2024).
Wang, Z. et al. Aged microplastics decrease the bioavailability of coexisting heavy metals to microalga Chlorella vulgaris. Ecotoxicol. Environ. Saf. 217, 112199 (2021).
Bhattacharya, P., Lin, S., Turner, J. P. & Ke, P. Physical adsorption of charged plastic nanoparticles affects algal photosynthesis. J. Phys. Chem. C. 114, 16556–16561 (2010).
Ugya, A. Y., Meguellati, K., Aliyu, A. D., Abba, A. & Musa, M. A. Microplastic stress induce bioresource production and response in microalgae: A concise review. Environ. Pollutants Bioavailab. 34 (1), 51–60 (2022).
Singh, R., Paliwal, C., Nesamma, A. A., Narula, A. & Jutur, P. P. Nutrient deprivation mobilizes the production of unique tocopherols as a stress-promoting response in a new Indigenous isolate Monoraphidium Sp. Front. Mar. Sci. 7, 575817 (2020).
Gauthier, M. R., Senhorinho, G. N. A. & Scott, J. A. Microalgae under environmental stress as a source of antioxidants. Algal Res. 52, 102104 (2020).
Jerez-Martel, I. et al. Phenolic profile and antioxidant activity of crude extracts from microalgae and cyanobacteria strains. J. Food Qual. 2017 (1), 2924508 (2017).
Ranjbari, N. et al. Improved features of a highly stable protease from Penaeus vannamei by immobilization on glutaraldehyde activated graphene oxide nanosheets. Int. J. Boil Macromol. 130, 564–572 (2019).
Mariano, S., Tacconi, S., Fidaleo, M., Rossi, M. & Dini, L. Micro and nanoplastics identification: Classic methods and innovative detection techniques. Front. Toxicol. 3, 636640 (2021).
Zhao, T., Tan, L., Huang, W. & Wang, J. The interactions between micro Polyvinyl chloride (mPVC) and marine dinoflagellate Karenia mikimotoi: The Inhibition of growth, chlorophyll and photosynthetic efficiency. Environ. Pollut. 247, 883–889 (2019).
Li, Y., Liu, X., Shinde, S., Wang, J. & Zhang, P. Impacts of micro-and nanoplastics on photosynthesis activities of photoautotrophs: A mini-review. Front. Microbiol. 12, 773226 (2021).
Long, M. et al. Interactions between polystyrene microplastics and marine phytoplankton lead to species-specific hetero-aggregation. Environ. Pollut. 228, 454–463 (2017).
Miao, L. et al. Effects of biofilm colonization on the sinking of microplastics in three freshwater environments. J. Hazard. Mater. 413, 125370 (2021).
Hossain, N., Zaini, J., Mahlia, T. M. I. & Azad, A. K. Elemental, morphological and thermal analysis of mixed microalgae species from drain water. Renew. Energy. 131, 617–624 (2019).
Zakaria, Y. et al. Investigate the potential renewable energy of microalgae Spirulina Sp using proximate analyzer, SEM-EDX, and thermogravimetry. J. Mech. Eng. Sci. Technol. 6 (2), 66–73 (2022).
Gniadek, M. & Dabrowska, A. The marine nano-and microplastics characterisation by SEM-EDX: The potential of the method in comparison with various physical and chemical approaches. Mar. Pollut Bull. 148, 210–216 (2019).
Veerasingam, S. et al. Contributions of fourier transform infrared spectroscopy in microplastic pollution research: A review. Crit. Rev. Environ. Sci. Technol. 51 (22), 2681–2743 (2021).
Campanale, C., Savino, I., Massarelli, C. & Uricchio, V. F. Fourier transform infrared spectroscopy to assess the degree of alteration of artificially aged and environmentally weathered microplastics. Polymer 5 (4), 911 (2023).
El-Naggar, N. E. A., Hussein, M. H., Shaaban-Dessuuki, S. A. & Dalal, S. R. Production, extraction and characterization of Chlorella vulgaris soluble polysaccharides and their applications in AgNPs biosynthesis and biostimulation of plant growth. Sci. Rep. 10 (1), 3011 (2020).
Sadiq, I. M., Dalai, S., Chandrasekaran, N. & Mukherjee, A. Ecotoxicity study of Titania (TiO2) NPs on two microalgae Species: Scenedesmus Sp. and Chlorella Sp. Ecotoxicol. Environ. Saf. 74 (5), 1180–1187 (2011).
Clement, L., Hurel, C. & Marmier, N. Toxicity of TiO2 nanoparticles to cladocerans, algae, rotifers and plants–effects of size and crystalline structure. Chemosphere 90 (3), 1083–1090 (2013).
Dmytryk, A., Saeid, A. & Chojnacka, K. Biosorption of microelements by spirulina: Towards technology of mineral feed supplements. Sci. World J. 356328. (2014).
Harrison, J. P., Ojeda, J. J. & Romero-González, M. E. The applicability of reflectance micro-Fourier-transform infrared spectroscopy for the detection of synthetic microplastics in marine sediments. Sci. Total Environ. 416, 455–463 (2012).
Cunsolo, S., Williams, J., Hale, M., Read, D. S. & Couceiro, F. Optimizing sample Preparation for FTIR-based microplastic analysis in wastewater and sludge samples: Multiple digestions. Anal. Bioanal Chem. 413, 3789–3799 (2021).
Acknowledgements
This paper has been supported by the grant provided by DST, Govt. of India under Indo-Russian project (DST/INT/RUS/RSF/P-60/2021). The authors extend their appreciation to Ongoing Research Funding program (ORF-2025-980), King Saud University, Riyadh, Saudi Arabia for funding this research. M.V would like to thanks to NRF Korea for providing funding under Brain Pull (BP) Program (RS-2024-00406513). H. Kim and M. Verma were supported by Korea Environment Industry & Technology Institute (KEITI) through Measurement and Risk assessment Program for Management of Microplastics Project, funded by Korea Ministry of Environment (MOE, 2022003120002) and Korea Environment Industry & Technology Institute (KEITI) through Post Plastic, a specialized program of the Graduate School. This paper has also been supported by the RUDN University Strategic Academic Leadership Program. M. S. V. would like to thank Ministry of Science and Higher Education of the Russian Federation (State Assignment No. 075-00269-25-00).
Author information
Authors and Affiliations
Contributions
Jyoti Rawat: Methodology; Data curation; Writing – original draft. Pooja Bhatnagar: Writing – review & editing. Abhilasha Mishra: Conceptualization; Data curation; Writing – review & editing. Manisha Nanda: Data curation, Writing – review & editing. Prateek Gururani: Writing – review & editing. Afzal Hussain: Data curation; Writing – review & editing. Mikhail S. Vlaskin: Data curation, Writing – review & editing. Sanjay Kumar: Data curation, Writing – review & editing. Monu Verma: Writing – review & editing, Analysis. Hyunook Kim: Conceptualization, Writing – review & editing. Vinod Kumar: Conceptualization; Supervision; Writing – review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Rawat, J., Bhatnagar, P., Mishra, A. et al. Exploring biochemical responses and cellular adaptations of Chlorella sorokiniana to polyethylene microplastic exposure. Sci Rep 15, 23551 (2025). https://doi.org/10.1038/s41598-025-08674-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-08674-z