Abstract
Snakebite envenomation causes various toxic effects in humans, including death. Antivenoms are effectively prevent death but cannot completely block local muscle tissue damage, resulting in amputation and disability; thus, complementary therapies are needed. In this study, the ability of exopolysaccharides extracted from the microalgae Chlorella sorokiniana, Scenedesmus obliquus, Nannochloris sp. Naumann, and Scenedesmus acuminatus to inhibit the proteolytic, plasma coagulant, and phospholipase A2 (PLA2) activities of Bothrops jararaca, B. jararacussu, and B. neuwiedi venoms was assessed. Exopolysaccharides from C. sorokiniana, S. obliquus, Nannochloris sp. Naumann, and S. acuminatus inhibited the proteolytic activity of B. jararaca venom by 25%, 99%, 97%, and 13%, respectively; B. jararacussu venom by 14%, 50%, 46%, and 12%, respectively; and B. neuwiedi venom by 1%, 78%, 62%, and 1%, respectively. Exopolysaccharides from S. obliquus and Nannochloris sp. Naumann prevented the coagulation induced by snake venom and decreased the PLA2 activity by 15% and 30%, respectively. None of the four exopolysaccharides lysed red blood cells and, thus, can be considered as nonhemotoxic compounds. Therefore, microalgae exopolysaccharides may be a candidate as complementary therapies for envenomation by snakes in regions where such incidents are common.
Similar content being viewed by others
Introduction
In recent years, numerous countries with tropical and subtropical climates, particularly Africa, Asia, Latin America, India, and Oceania have experienced a surge in morbidity and mortality rates associated with venomous snake bites. These countries have a high level of biodiversity, large areas of forest, indiscriminate deforestation, and overexploitation of natural resources, bringing the population into constant contact with snakes and other reptiles1,2,3. More than 15% of all animals on Earth are considered venomous and contact with them can cause irreparable damage to human health1,4,5. Globally, the death rate of snake bite envenomation (SBE) is approximately 0.5–1%; males are more affected than females, and more children (25%) than adults experienced SBE (12%)6,8. Poorer populations are more susceptible to SBE because of inadequate bite treatment and prevention and environmental conditions, leading to pathological consequences, including local edema, tissue necrosis (myotoxicity), neuromuscular paralysis, systemic hemorrhage, coagulopathy, and death, as well as economic, social, and physicological consequences7. This led the World Health Organization (WHO) to add snakebites to the list of priority neglected tropical diseases (NTDs) in 20177,9,10.
Snake bites affect approximately 10 million people worldwide each year7,9. Asian countries have the highest rate of incidents and complications from SBE, accounting for more than 70% of all cases worldwide. India ranks first among Asian countries in terms of deaths, with over 45,000 deaths in 2020, and Africa and the Middle East rank second, with more than 35,000 deaths10. Brazil has a high rate of death, with approximately 27,000 fatalities reported annually, and pit viper species account for the highest frequency of snakebite accidents (99%)11,12. Among vipers, the genus Bothrops accounts for 87% of the registered incidents and is the largest genus with 47 species, of which B. jararaca, B. jararacussu, and B. neuwiedi are among the most important due to their association with high numbers of SBE events12.
In general, the high mortality and morbidity rates of SBE caused by pit vipers and true vipers result from the presence of three dominant protein families in the venom: including: 26.4% phospholipases A2 (PLA2s), 24% snake venom metalloproteinases (SVMPs), and 12% snake venom serine proteinases (SVSPs)13,14. The main components of snake venom (up to 95%) are proteins and peptides with a wide range of toxic activities that may cause pain, inflammation, edema, intense hemorrhage, renal and heart failure, myonecrosis, and death15. Bothrops sp. has a high content of active proteins in their venom, including SVMPs, representing as high as 74% in some species16. Other biomolecules including SVSPs, PLA2s, LAAOs, and hyaluronidases; other proteins lack catalytic activity, e.g., bradykinin-potentiating peptides (BPPs), but are associated with devastating toxic effects, including vasodilatation, decreased blood pressure, and circulation shock14,15,16,17. However, the complications, symptoms, and protein compositions may vary according to the snake species, age, diet, sex, and environmental conditions12,18,19.
Currently, SBE is effectively treated by intravenously administering immunoglobulin-based antivenoms to the victim. To date, antivenom is the only SBE treatment that has been recognized by the WHO and Ministries of Health worldwide for SBE7,9. Antivenoms efficiently prevent death in most cases and thus reduce mortality rates. However, the use of antivenoms has disadvantages, such as high production costs, side effects (rash, severe malaise, fever, and allergic reactions), the inability to block tissue necrosis, and the potential for untimely administration or incorrect dosages20. Victims must be quickly transported to a health center and administered antivenom early to maximize its effects. Despite these adverse reactions, antivenoms are still considered the only available and effective treatment for SBE. In recent years, the WHO has become concerned about the use of antivenoms, including their safety, efficacy, adverse effects, distribution, and production. A roadmap was developed to improve the quality of antivenoms and mechanisms to develop complementary solutions for the treatment and prevention of SBE21,22.
Natural resources are a source of active compounds of pharmacological interest to industry and communities because of their availability, cost-effectiveness, cultural acceptability, and fewer side effects versus antivenoms23. Plants have long been used to treat symptoms of SBE, especially in remote areas24,25,26,27. In addition to medicinal plants, seaweeds and microalgae are rich sources of biologically active substances28,29. Although abundant in the marine environment, microalgae remain poorly understood as sources of compounds with pharmacological and biological relevance, particularly for use as antivenoms. No antivenom in SBE of exopolysaccharides from microalgae has been reported in the literature; however, they are a potential source of valuable chemicals with biological activities30,31. Several eukaryotic and prokaryotic microalgae species produce and release large volumes of polysaccharides into the environment32,33. The biological properties of exopolysaccharides produced by microalgae include anti-inflammatory, immunomodulatory, antitumor, antiviral, antiparasitic, antioxidant, hypoglycemic, and cholesterol-lowering activities29,30. Microalgae are good candidates for inhibiting the main active enzymes of snake venoms, including SVMPs, SVSPs, and PLA2s, and should be prioritized in future pharmacological investigations15,16,17.
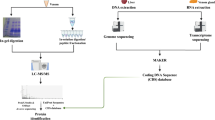
In this study, the abilities of four exopolysaccharides extracted from the microalgae Chlorella sorokiniana (C. sorokiniana), Scenedesmus obliquus (S. obliquus), Nannochloris sp. Naumann, and Scenedesmus acuminatus (S. acuminatus) to inhibit the PLA2, proteolytic, and coagulant activities of B. jararaca, B. jararacussu, and B. neuwiedi venoms, as well as their physicochemical properties and in vitro toxicity, were assessed.
Results
Molecular weight and viscosity of exopolysaccharides from microalgae
Table 1 shows that among the C1, S302, 1509, and S329, samples S329 and S302 from S. acuminatus (8.04 mg/mL) and S. obliquus (7.15 mg/mL) had the highest viscosities, whereas exopolysaccharide 1509 from Nannochloris sp. Naumann had the lowest value (1.35), and the lowest molecular weight (13.25 × 104 Da). C1 from C. sorokiniana had the highest molecular weight (17.71 ×·104 Da).
Determination of the sugar composition of exopolysaccharides from microalgae via HPLC
The chromatograms of the exopolysaccharides from the microalgae are shown in Fig. 1, including C1 from C. sorokiniana (Panel A), 1509 from Nannochloris sp. Naumann (Panel B), S302 from S. obliquus (Panel C), and S329 from S. acuminatus (Panel D). Table 2 provides the carbohydrate composition on the basis of the peak areas presented in the chromatograms. The error in calculating the concentration of the exopolysaccharides did not exceed 10%.
Chromatographic separation revealed that the exopolysaccharide S302 from S. obliquus is composed of arabinose, glucose, maltose, and trehalose (Table 2). The sugar composition of S329 from S. acuminatus was mainly fucose, xylose, arabinose, glucose, sucrose, and maltose. The exopolysaccharide C1 from C. sorokiniana did not contain xylose or sucrose but contained arabinose, maltose, and the highest amount of glucose and trehalose content among the samples (Table 2). The sugars galactose and raffinose were not present in the polysaccharides C1, S302, 1509, or S329 (Table 2).
Effect of exopolysaccharides on the proteolytic activity of B. jararaca, B. jararacussu, and B. neuwiedi venom
One arbitrary unit designed effective concentration (EC) of B. jararaca and B. neuwiedi venom (30 µg/mL) or B. jararacussu (50 µg/mL) venom was incubated for 5 min at 37 °C with saline solution (positive control group) or with the exopolysaccharides C1, S302, 1509, and S329 at a 1:10 (wt/wt) venom-to-polysaccharide ratio. C1 and S329 did not inhibit the proteolytic activity of B. neuwiedi venom (Fig. 2C) but inhibited approximately 20% of the proteolysis of B. jararaca (Fig. 2A) and B. jararacussu venom (Fig. 2B). The exopolysaccharides S302 and 1509 completely inhibited (100%) the B. jararaca venom-induced proteolytic activity, 50% of the B. jararacussu venom activity (Fig. 2B), and 72% of the B. neuwiedi venom activity (Fig. 2C). In the absence of venom, none of the four exopolysaccharides hydrolyzed azocasein and, thus, were devoid of proteolytic activity (data not shown).
Effect of exopolysaccharides on the proteolytic activity of B. jararaca, B. jararacussu, and B. neuwiedi venom. B. jararaca (A), B. jararacussu (B), and B. neuwiedi (C) venoms were incubated with saline solution or C1, S302, 1509, or S329 for 5 min at 37 °C. Venom plus saline yielded 100% of proteolytic activity. The results are expressed as the means ± SD (n = 6). * p < 0.05 compared with venom plus saline.
Effect of exopolysaccharides on the coagulant activity of B. jararaca, B. jararacussu, and B. neuwiedi venom
B. jararaca, B. jararacussu, and B. neuwiedi venom clotted plasma in a concentration-dependent manner, and the concentration of venom able to clot plasma at approximately 60 s was considered the minimum coagulant concentration (MCC). The MCCs of B. jararaca (20 µg/mL), B. jararacussu (30 µg/mL), and B. neuwiedi (15 µg/mL) were incubated with the exopolysaccharides C1, S302, 1509, or S329 at the ratio of 1:10 venom/polysaccharide for 5 min at 37 oC. The plasma coagulation assay was performed as described in the methods. The exopolysaccharide C1 did not inhibit coagulation caused by B. jararaca (Fig. 3A), B. jararacussu (Fig. 3B), or B. neuwiedi (Fig. 3C) venom, and S329 was ineffective against B. jararaca (Fig. 3A) and B. jararacussu venom (Fig. 3B). S302 and 1509 inhibited plasma coagulation caused by all the venoms (Fig. 3) and more efficiently inhibited B. jararacussu venom-induced coagulation (Fig. 3B). The exopolysaccharides C1, S302, 1509, and S329 did not clot the plasma in the absence of venom (data not shown). Notably, for the venom of B. jararaca, the time of 82 s was chosen because higher concentrations of this venom would have been needed to clot the plasma at 60 s.
Effect of exopolysaccharides on plasma coagulation caused by B. jararaca, B. jararacussu, and B. neuwiedi venom. 20 µg/mL B. jararaca (A), 30 µg/mL B. jararacussu (B), and 15 µg/mL B. neuwiedi (C) venom samples were incubated with saline (S) or C1, S302, 1509, or S329 at a 1:10 ratio for 5 min at 37 °C, and then added to the medium. The coagulation of the plasma was then monitored. The results are expressed as the means ± SD (n = 6). * p < 0.05 compared with venom plus saline.
Effect of exopolysaccharides on the PLA2 activity of B. jararaca, B. jararacussu, and B. neuwiedi venom
Venoms of B. jararaca, B. jararacussu, and B. neuwiedi venom incubated at a concentration of 50 µg/mL with saline produced readouts of approximately 0.8 at an absorbance of 850 nm, which was considered as 100% PLA2 activity. Then, the same concentration of venom was incubated with 500 µg/mL of the exopolysaccharides C1, S302, 1509, or S329 (1:10 venom/polysaccharide, wt/wt) for 5 min at 37 °C. As shown in Fig. 4, C1 and S329 did not inhibit the PLA2 activity of B. jararaca, B. jararacussu, or B. neuwiedi venom. S302 and 1509 inhibited PLA2 activity by 17% and 30%, respectively, regardless of the venom tested (Fig. 4). In the absence of venom, C1, S302, 1509, and S329 did not exhibit PLA2 activity (data not shown).
Effect of exopolysaccharides on the PLA2 activity of B. jararaca, B. jararacussu, and B. neuwiedi venom. B. jararaca (A), B. jararacussu (B), and B. neuwiedi (C) venom (50 µg/mL) was incubated with saline or with 500 µg/mL C1, S302, 1509, or S329 for 5 min at 37 °C. PLA2 activity was measured as described. The results are expressed as the means ± SD (n = 6). * p < 0.05 compared with venom plus saline.
Discussion
C. sorokiniana, S. obliquus, Nannochloris sp. Naumann, and S. acuminatus exopolysaccharides inhibited the main toxic activities of some Bothrops sp. venom, including that from B. jararaca, B. jararacussu, and B. neuwiedi. The exopolysaccharides neutralized the coagulant, proteolytic, and PLA2 activities of venom from these Brazilian pit vipers. These activities are considered the most important toxic effects following SBE, resulting in severe hemorrhaging, inflammation, blood clotting disturbances, and tissue necrosis. We also demonstrated that the exopolysaccharides lysed only 5%–10% of human red blood cells; thus, they can be considered non- hemotoxic compounds. According to literature34hemolysis below 20% is acceptable in molecules being considered as candidates as medicine. Therefore, plant products may inhibit the toxic activity of envenomation, prevent death and physical sequelae caused by snake bites, and may complement current antivenom therapy with safely. On the other hand, other toxicity assessments must be done to postulate the non-toxicity of exopolysaccharides from C. sorokiniana, S. obliquus, Nannochloris sp. Naumann, and S. acuminatus, including: acute, sub-acute, and chronic toxic tests. However, microalgae have been used safely as food to humans and animals.
The exopolysaccharides demonstrated carbohydrate composition differences. The four microalgae contain neutral carbohydrates, including fucose, arabinose, and glucose, but not galactose, rhamnose, or raffinose. The carbohydrate composition may influence the inhibitory potential of polysaccharides against the toxic activities of B. jararaca, B. jararacussu, and B. neuwiedi venom. The trehalose concentration was low in 1509 and S302 but high in C1. C1 failed to inhibit the proteolytic, coagulant, and PLA2 activities of all the venoms; however, 1509 and S302 neutralized these activities. Thus, the presence of trehalose in the exopolysaccharides of these microalgae may interfere with their binding with the dominant active enzymes (as SVMPs, SVSPs, and PLA2) that are responsible for proteolysis, coagulant activity, and PLA2 activity or cofactors of such enzymes (such as Ca+2 and Zn+2). The complex structure and steric inhibition process (conformational changes) of exopolysaccharides may prevent their binding to the predominant active enzymes. Different glucose contents were observed among the exopolysaccharides of the microalgae. C1 and 1509 had the highest contents, whereas S302 and S329 had the lowest. However, despite having the highest concentration of glucose, C1 and 1509 had opposite inhibitory effects against proteolytic, coagulant, and PLA2 activities. Notably, C1 has a twofold lower glucose content than 1509, S302 has twofold lower glucose than S329, and S302 has the lowest content of glucose of all exopolysaccharides from the microalgae. S302 was the most efficient antagonist of the toxic activities assessed in this study. Nonetheless, the contents of all carbohydrates from C1, 1509, S302, and S329 should be analyzed to determine the relationship between their composition and biological activity. Similar content has been reported in the literature where microalgae have been shown to synthesize monosaccharide units of hexoses and pentoses linked to form complex sugars29.
Polysaccharides are found in all living organisms and a wide range of biological and pharmacological effects have been attributed to them29beyond their use in cosmetics and foods and as medicines for the treatment for several ailments28,34. Seaweeds are the most prominent producers of polysaccharides, and annually approximately 26,500 tons of carrageenan, 50,000 of alginate, and 9,600 of agar are produced on the market, yielding a profit of billions of dollars35,36. Alginate and agar demonstrate no or minimal29,37are biocompatible at high concentrations in microalgae, are ecologically sustainable, and have stable structures32. Carrageenans are the main thickening, emulsifying, or stabilizing additives used in milkshakes, ice creams, beers, hams, and typically have molecular weights of 400–600 kDa35. However, the Food and Drug Administration (FDA) has established a minimum value for the molecular weight of carrageenan (100 kDa) for human consumption due to reports of colon ulceration resulting from highly degraded carrageenans35. The World Health Organization and Agriculture Organization of the United Nations Expert Committee on Food Additives revised the studies on carrageenans and established that a concentration of up to 1,000 mg/L is “not of concern” in infants. Thus, carrageenans are not toxic or associated with adverse health effects and are generally recognized as safe (GRAS) food additives. Some microalgae species have been included in the FDA list as GRAS for human consumption37,38 including Chlorella vulgaris, Arthrospira platensis, and Euglena gracilis29. The composition of polysaccharides differs in structure, molecular weight, and sugar composition. Glucose is generally the most abundant component, and fructose is found in exopolysaccharides from cyanobacteria30,39. Exopolysaccharides from Charophyta have mainly fucose and uronic acids, whereas exopolysaccharides from Rhodophyta are predominantly composed of xylose and galactose30.
Biological and pharmacological effects, including antioxidant and antibacterial activities are attributed to the protein and lipid contents of microalgae40. Nevertheless, polysaccharides from seaweed are of great interest because of their unique structures and pharmacological properties. In addition to biologically active proteins and lipids, sulfate groups are essential for many polysaccharides to display pharmacological activities in many cases.
The viscosity of C1, 1509, S302, and S320 does not appear to be a crucial parameter for blocking the proteolytic, coagulant, and PLA2 activities of venoms; C1 and 1509 have the lowest viscosity, but the latter is a more effective as inhibitor than the former. Furthermore, S302 and S329 have the highest viscosity values, but significant differences on the inhibitory of the activitivies. Overall, S302 showed greatest percentage of venom-induced toxic activities, whereas S329 was not an efficient antagonist.
The molecular weights of the exopolysaccharides ranged from 13 to 18 × 104 Da. C1 had the highest molecular weight (17.71 × 104 Da) and was the least efficient inhibitor of the toxic effects of snake venom. Exopolysaccharides 1509, S302, and S329 had molecular weights of approximately 14 × 104 Da. Therefore, one may speculate that the molecular weight is not a crucial feature for distinguishing the inhibitory efficacy of exopolysaccharides derived from microalgae. High-molecular-weight fucoidan polysaccharides from the brown macroalgae Fucus vesiculosus and Undaria pinnatifida have been reported to inhibit some toxic activities of B. jararaca, B. jararacussu, and B. neuwiedi venom41and sulfate groups are required for their inhibition because of the high content of negatively charged groups within their chemical structure41. The major active enzymes found in most viper venoms, e.g. the Bothrops genus, are SVMPs, SVSPs, and PLA2s, which are key contributors to the toxicity of these venoms. SVMPs are zinc-dependent endopeptidases containing Zn2+ at the catalytic center and Ca2+ in the C-terminal region, which are involved in structural stabilization of the protein, and basic residues, e.g., lysine at the molecule´s surface. Thus, molecules with a net negative charge can bind to these venom enzymes, leading to inhibition41,42. Varespladib42,43 CP47147443, and suramin44 are inhibitors of PLA2s, SVSPs, and SVMPs, respectively, and their net charge is vital for their interaction with specific amino acid residues or cofactors of such enzymes45,46. However, further studies should be performed to elucidate the mechanism of action of exopolysaccharides using purified enzymes from venoms. Docking studies should also be performed. Inhibiting the different enzymes of snake venoms that have multiple toxic effects via one molecule or extract is challenging. Combining molecules or extracts may be a strategy to efficiently block venom toxicity.
Conclusions
Samples of exopolysaccharides isolated by precipitation from the culture fluid of the microalgae C. sorokiniana, S. obliquus, Nannochloris sp. Naumann, and S. acuminatus inhibited the PLA2, proteolytic, and plasma coagulant activities of venom from B. jararaca, B. jararacussu, and B. neuwiedi. Exopolysaccharide samples from S. acuminatus and Nannochloris sp. Naumann completely suppressed the assessed toxic actions of B. jararaca venom. The chemical components of these exopolysaccharides are mainly carbohydrates, such as fucose, arabinose, glucose, sucrose, maltose, and trehalose, which may be highly important for neutralizing venom toxicity. These findings may inspire scientists to explore the enormous potential of exopolysaccharides from microalgae as a new source of valuable chemicals that inhibit snake venom proteases and PLA2 enzymes, which are responsible for the most toxic effects of SBE.
Materials and methods
Reagents and snake venoms
Lyophilized venom of B. jararaca, B. jararacussu, and B. neuwiedi was provided by the serpentarium of the Ezequiel Dias Foundation (FUNED), Belo Horizonte, Minas Gerais, Brazil. Venom was diluted in physiological saline and stored at − 20 °C before analysis. Snake venom was collected with authorization from the Brazilian National System for the Management of Genetic Heritage and Related Traditional Knowledge (SISGEN), process number A39CTRI 04E. The venom was dissolved in physiological saline at a concentration of 1 mg/mL. Azocasein was purchased from Sigma Chemical Co. (St. Louis, Missouri, USA). All other reagents and solvents were of research grade.
Extraction of the exopolysaccharides from microalgae
Exopolysaccharides were extracted from C. sorokiniana (coded as C1) with an isopropyl alcohol to dry biomass ratio of 1:3 at room temperature for 700 min; from S. acuminatus (coded as S329) with an ethyl alcohol to dry biomass ratio of 1:3 at − 25 °C for 720 min; from Nannochloris sp. Naumann (coded as 1509) with an ethyl alcohol to dry biomass ratio of 1:2 at − 25 °C for 720 min; and from S. abundans (coded as S302) with an isopropyl alcohol to dry biomass ratio of 1:3 at 25 °C for 540 min. The resulting mixtures were centrifuged (20 min, 5,000 rpm), after which the alcohol was removed by decantation, and the exopolysaccharides were dissolved in sodium phosphate buffer, pH 7.0.
The exopolysaccharides were purified via deproteinization using the Sevag method, depigmented using hexane, and subjected to dialysis to remove salts, from the culture medium and DNA, RNA, and other molecules trapped in the sample extracts during precipitation with isopropyl alcohol. After preparation, the whole exopolysaccharide extract was lyophilized, and solution samples were prepared by dissolving in physiological saline solution at 25 mg/mL.
Determination of the molecular weight and viscosity of the exopolysaccharides from microalgae
The polysaccharides´ molecular weight (MW) was determined by gel permeation chromatography (GPC) using Sephadex G 150 gel as the filler47. Dextrans of different molecular weights (T-10, T-40, T-70, and T-500) were used as molecular standards. GPC was performed via elution with phosphate-salt buffer (PBS, 0.2 M) at a flow rate of 6 mL/h. The total carbohydrate content of each sample was estimated via the phenol‒sulfuric acid method48. The molecular weight of each fraction was determined via regression of a calibration plot of the molecular masses of standard dextran samples. The average molecular weight of the exopolysaccharides was calculated via the following equation:
,
where \(\:{M}_{i}\) is the molecular weight of each fraction, Da;
Ci is the total carbohydrate concentration in each fraction, mg/mL.
For viscosity studies, the polysaccharides were evaporated in a Hei-VAP Precision HL/G3 rotary evaporator (Heidolph, Germany) and lyophilized in a Triad lyophilic drying unit (Labconco, USA). A Ubbelohde ASTM glass capillary viscometer (Fungilab, Spain) was used to evaluate viscosity. The lyophilized exopolysaccharide fractions were dissolved in 0.2 M phosphate buffer at 25.0 ± 0.5 °C, centrifuged at 3,500 rpm, and filtered through a membrane filter with a pore diameter of 0.45 µm48. The relative viscosity (ηr) was calculated via the following equation:
where η0’ is the solvent viscosity, cP, and η is the solution viscosity at the same temperature, cP.
The specific viscosity (ηsp) was determined via the following formula:
Determination of the monosaccharide composition of exopolysaccharides from Microlgae via HPLC
The exopolysaccharide were prepared for HPLC by acid hydrolysis per the modified method described by Dische and Shettles47. Three milliliters of 20% trifluoroacetic acid (TFA) solution was added to a 10 mg sample of exopolysaccharide. The hydrolysis process was conducted for 1 h at 120 °C. TFA was distilled via a rotary evaporator and re-extracted by adding 500 µL of isopropyl alcohol to the reaction mixture. The purified hydrolysate was dissolved in 3 mL of distilled water, centrifuged for 5 min at 3,500 rpm, and filtered through a syringe filter with a pore diameter of 0.22 μm. The separation was performed at 30 °C in isocratic elution mode at a wavelength of 192 nm, with a flow rate of 1 mL/min. Acetonitrile and double-distilled water were used as the mobile phase. The components were identified using a refractometer detector on the basis of their retention times and the spectra of the individual standard substances, with which the instrument was precalibrated.
Effects of exopolysaccharides on the PLA2 activity of B. jararaca, B. jararacussu, and B. neuwiedi venom
The PLA2 activity of B. jararaca, B. jararacussu, and B. neuwiedi venom was measured as described by Marinetti et al.49. One fresh hen egg yolk obtained from a local supermarket was filtered (yielding, on average, 15 mL) and dissolved in physiological saline to a final volume of 100 mL. The solution was centrifuged at 15,000 x g for 60 min at 10 oC to clarify the solution and remove any insoluble material. The supernatant was transferred to tubes and stored at 4 oC prior to use. One volume of the supernatant was mixed with nine parts of saline solution to produce the egg yolk substrate mixture. Five hundred micrograms of exopolysaccharides C1, S302, 1509, and S329 or saline were incubated with 50 µg of each venom mixture for 5 min at 37 °C in a final volume of 220 µL. Then, 50 µL of this mixture was added to medium containing 132 µL of saline, 20 µL of 0.4% sodium taurocholate, 5 µL of Tris-HCl (200 mM), pH 7.5, and 2 µL of CaCl2 (0.5 M). The enzymatic reaction was triggered by adding 50 µL of the egg yolk substrate solution. After 30 min at 37 oC, the absorbance at 850 nm was read via a microplate reader (Versamax, Molecular Devices, California, USA). 100% of the PLA2 activity of venoms was achieved by incubating venom with saline in the absence of exopolysaccharides, whereas 0% PLA2 activity was obtained with saline alone. The negative control comprised exopolysaccharides incubated in saline solution without venom.
Effect of exopolysaccharides on the proteolytic activity of B. jararaca, B. jararacussu, and B. neuwiedi venom
The proteolytic activity of B. jararaca, B. jararacussu, and B. neuwiedi venom was evaluated as described by Garcia et al.50. Different concentrations of each venom mixture (10–50 µg/mL) were incubated for 90 min at 37 °C with 400 µL of azocasein (0.2% wt/vol. dissolved in 20 mM Tris-HCl, and 8 mM CaCl2, pH 8.8). The enzymatic reaction was stopped by adding 400 µL of trichloracetic acid (10%), and the tubes were centrifuged for 3 min at 12,000 rpm. Then, 1.0 mL of the supernatant was removed, transferred to tubes containing 500 µL NaOH 2 N, and incubated for 10 min at room temperature. The absorbance at 420 nm was read on a spectrophotometer. The concentration of venom (µg/mL) capable of producing a readout of 0.2 (i.e., 70–80% of the maximum activity) was defined as the arbitrary unit of effective concentration (EC). One EC of B. jararaca and B. neuwiedi venom (30 µg/mL) and B. jararacussu (50 µg/mL) venom was incubated with saline (positive control) or the exopolysaccharides C1, S302, 1509, or S329 for 5 min at 37 °C at a venom-to-exopolysaccharide ratio of 1:10 (wt/wt), resulting in a final volume of 220 µL. After incubation, an aliquot of 50 µL was removed and added to the mixture, and the proteolytic activity was measured. The negative controls comprised medium with saline or exopolysaccharides to the medium without venom.
Effect of exopolysaccharides on the coagulation caused by B. jararaca, B. jararacussu, and B. neuwiedi venom
The coagulant activity of B. jararaca, B. jararacussu, and B. neuwiedi venom was assessed using a pool of human plasma donated from the blood bank of the Hospital Antônio Pedro of Federal Fluminense University (HUAP) under the authorization of the Committee for Ethical in Experimentation (CEP-UFF, CAAE: 28941314.0.0000.5243). Two hundred microliters of diluted plasma (1:1 in saline solution) was incubated at 37 ◦C for 1 min, and then different concentrations of B. jararaca, B. jararacussu, and B. neuwiedi venom (10–100 µg/mL) were added to the medium. The coagulation time was monitored via a digital Amelung coagulometer, model KC4A (Labcon, Heppenheim, Germany). The concentration of venom (µg/mL) able to clot plasma at ~ 60 s was determined as the arbitrary unit minimum coagulant concentration (MCC). One MCC of each venom mixture was incubated for 5 min at 37 ◦C with saline (positive control) or with the exopolysaccharides C1, S302, 1509, and S329 at a ratio of 1:10 venom: polysaccharide (wt/wt), in a final volume of 220 µL. An aliquot of 50 µL was added to the plasma, and coagulation was monitored as described above. As negative controls, exopolysaccharides or saline were added to the plasma without venom. A similar procedure was described by Castro-Pinheiro et al.39.
Toxicity of exopolysaccharides
The in vitro toxicity of exopolysaccharides was evaluated via a hemocompatibility test, as previously described34. Exopolysaccharides (2 mg/mL, final concentration) and saline solution were incubated with a 13% (v/v) washed human red blood cell suspension for 3 h at 37 °C. The tubes were centrifuged for 3 min at 1,800 × g, and the volume of hemoglobin released from the washed red blood cells was measured at an absorbance of 578 nm. Hemolysis (100% and 0%) were obtained by adding water and saline, respectively, to the washed red cells.
Statistical analysis
The results are expressed as the mean ± standard deviation (SD) obtained with the number of experiments indicated in each result via the GraphPad Prism® program. The significance of differences between experimental groups was assessed via one-way ANOVA with a post hoc Dunnett’s test; p values of < 0.05 were considered to indicate significance.
Data availability
All the data generated or analyzed during this study are included in this published article.
References
Fry, B. G. & Snakebite When the human touch becomes a bad touch. Toxins 10, 1–24 (2018).
Harrison, R. A., Hargreaves, A., Wagstaff, S. C., Faragher, B. & Lalloo, D. G. Snake envenoming: A disease of poverty. PLoS Negl. Trop. Dis. 3, e569–e574 (2009).
Kasturiratne, A. et al. The global burden of snakebite: A literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 5, e218 (2008).
Herzig, V. Animal Venoms—Curse or cure?? Biomedicines 9, 413 (2021).
Oliveira, A. L. et al. The chemistry of snake venom and its medicinal potential. Nat. Rev. Chem. 6, 451–469 (2022).
Robert, N. L. S. et al. Global mortality of snakebite envenoming between 1990 and 2019. Nat. Comm. 13, 6160 (2002).
Gutiérrez, J. M. et al. Nat. Rev. Dis. Primers 14, 17063 (2017).
Chippaux, J. P. Estimating the global burden of snakebite can help to improve management. PLOS Med. 5, e221 (2008).
Williams, D. J. et al. Strategy for a globally coordinated response to a priority neglected tropical disease: Snake-bite envenoming. PLoS Negl. Trop. Dis. 13, e0007059 (2019).
WHO. Snakebite envenoming. Available from:https://www.who.int/news-room/fact-sheets/detail/snakebite-envenoming (Accessed on August 2024).
Chippaux, J. P. Incidence and mortality due to snakebite in the Americas. PLoS Negl. Trop. Dis. 11, e0005662 (2017).
Queiroz, G. P., Pessoa, L. A., Portaro, F. C. V., Furtado, M. F. D. & Tambourgi, D. V. Interspecific variation in venom composition and toxicity of Brazilian snakes from Bothrops genus. Toxicon 52, 842–851 (2008).
Gutiérrez, J. M. et al. Unresolved issues in the Understanding of the pathogenesis of local tissue damage induced by snake venoms. Toxicon 148, 123–131 (2018).
Weekers, D. J. C., Alonso, L. L., Verstegen, A. X., Slagboom, J. & Kool, J. Qualitative profiling of venom toxins in the venoms of several Bothrops species using High-Throughput venomics and coagulation bioassaying. Toxins (Basel). 16, 300 (2024).
Cavecci-Mendonça, B. et al. Santos, L.D.D. Preliminary insights of Brazilian snake venom metalloproteomics. Toxins (Basel). 15, 648–654 (2023).
de Oliveira, F. N., Sachetto, A. T. A. & Santoro, M. L. Two-dimensional blue native/sds polyacrylamide gel electrophoresis for analysis of Brazilian Bothrops snake venoms. Toxins (Basel). 14, 661–680 (2022).
Casewell, N. R., Jackson, T. N. W., Laustsen, A. H. & Sunagar, K. Causes and consequences of snake venom variation. Trends Pharmacol. Sci. 41, 570–581 (2020).
Alangode, A., Rajan, K. & Nair, B. G. Snake antivenom: challenges and alternate approaches. Biochem. Pharmacol. 181, 114135 (2020).
Espín-Angulo, J. & Vela, D. Exploring the venom gland transcriptome of Bothrops asper and Bothrops jararaca: de Novo assembly and analysis of novel toxic proteins. Toxins (Basel). 16, 511 (2024).
Warrell, D. A. Snake bite. Lancet 375, 77–88 (2010).
Rathore, A. S., Kumar, R. & Tiwari, O. S. Recent advancements in snake antivenom production. Int. J. Biol. Macromol. 240, 124478 (2023).
Williams, D. et al. The global snake bite initiative: an antidote for snake bite. Lancet 375, 89–91 (2010).
Singh, P., Yasir, M., Hazarika, R., Sugunan, S. & Shrivastava, R. A review on venom enzymes neutralizing ability of secondary metabolites from medicinal plants. J. Pharmacopunct. 20, 173–178 (2017).
Gómez-Betancur, I., Gogineni, V., Salazar-Ospina, A. & León, F. Perspective on the therapeutics of anti-snake venom. Molecules 24, 3276 (2019).
Mors, W. B., Nascimento, M. C., Pereira, B. M. & Pereira, N. A. Plant natural products active against snake bite - the molecular approach. Phytochemistry 5, 627–642 (2000).
Félix-Silva, J., Silva-Junior, A. A., Zucolotto, S. M. & Fernandes-Pedrosa, M. F. Medicinal plants for the treatment of local tissue damage induced by snake venoms: an overview from traditional use to pharmacological evidence. Evid. Based Complement. Alternat. Med. 5748256 (2017). (2017).
Puzari, U., Fernandes, P. A. & Mukherjee, A. K. Pharmacological re-assessment of traditional medicinal plants-derived inhibitors as antidotes against snakebite envenoming: A critical review. J. Ethnopharmacol. 28, 115208 (2022).
Hamidi, M. et al. Marine bacteria versus microalgae: who is the best for biotechnological production of bioactive compounds with antioxidant properties and other biological applications? Mar. Drugs. 29, 28 (2019).
Babich, O. et al. Dolganyuk, V. Algae: study of edible and biologically active fractions, their properties and applications. Plants (Basel). 11, 780 (2022).
Moreira, J. B. et al. Microalgae polysaccharides: an alternative source for food production and sustainable agriculture. Polysaccharides 2, 441–457 (2022).
Colusse, G. A., Carneiro, J., Duarte, M. E. R., Carvalho, J. C. & Noseda, M. D. Advances in microalgal cell wall polysaccharides: a review focused on structure, production, and biological application. Crit. Ver. Biotechnol. 42, 562–577 (2022).
Barsanti, L. & Gualtieri, P. Is exploitation of microalgae economically and energetically sustainable? Algal Res. 31, 107–111 (2018).
Chanda, M. J., Merghoub, N. & Arroussi, E. Microalgae polysaccharides: the new sustainable bioactive products for the development of plant bio-stimulants? World J. Microbiol. Biotechnol. 35, 177–190 (2019).
Bauer, M. et al. Poly(2-ethyl-2-oxazoline) as alternative for the stealth polymer poly(ethylene glycol): comparison of in vitro cytotoxicity and hemocompatibility. Macromol. Biosci. 12, 986–998 (2012).
Leandro, A., Pereira, L. & Gonçalves, A. M. M. Diverse applications of marine macroalgae. Mar. Drugs. 24, 17 (2019).
Chudasama, N. A., Sequeira, R. A., Moradiya, K. & Prasad, K. Seaweed polysaccharide based products and materials: an assessment on their production from a sustainability point of view. Molecules 26, 260 (2021).
Benalaya, I., Alves, G., Lopes, J. & Silva, L. R. A review of natural polysaccharides: sources, characteristics, properties, food, and pharmaceutical applications. Int. J. Mol. Sci. 25, 1322 (2024).
Ravindran, R. & Rajauria, G. Carbohydrates derived from microalgae in the food industry. Cult. Microalgae Food Ind. 2, 127–146 (2021).
Costa, J. A. V., Lucas, B. F., Alvarenga, A. G. P., Moreira, J. B. & Morais, M. G. Microalgae polysaccharides: an overview of production, characterization, and potential applications. Polysaccharides 2, 759–777 (2021).
Shevelyuhina, A. et al. Antioxidant and antimicrobial activity of microalgae of the Filinskaya Bay (Baltic Sea). Plants (Basel). 31, 2264 (2022).
Castro-Pinheiro, C. et al. Effect of Seaweed-derived fucoidans from Undaria pinnatifida and Fucus vesiculosus on coagulant, proteolytic, and phospholipase A2 activities of snake Bothrops jararaca, B. jararacussu, and B. neuwiedi venom. Toxins (Basel). 16, 188 (2024).
Salvador, G. H. M., Cardoso, F. F., Lomonte, B. & Fontes, M. R. M. Inhibitors and activators for myotoxic phospholipase A(2)-like toxins from snake venoms - A structural overview. Biochimie 30, S0300–S9084 (2024).
Lewin, M., Samuel, S., Merkel, J. & Bickler, P. Varespladib (LY315920) appears to be a potent, broad-spectrum, inhibitor of snake venom phospholipase A2 and a possible pre-referral treatment for envenomation. Toxins 8, 248 (2016).
Preciado, L. M., Pereañez, J. A. & Comer, J. Potential of matrix metalloproteinase inhibitors for the treatment of local tissue damage induced by a type P-I snake venom metalloproteinase. Toxins 12, 8 (2019).
Ullah, A. et al. Thrombin-like enzymes from snake venom: structural characterization and mechanism of action. Int. J. Biol. Macromol. 15, 788–811 (2018).
Quiroz, S. et al. Inhibitory effects of varespladib, CP471474, and their potential synergistic activity on Bothrops asper and Crotalus durissus cumanensis venoms. Molecules 6, 8588 (2022).
Xu, Y. et al. Chemically modified polysaccharides: synthesis, characterization, structure activity relationships of action. Int. J. Biol. Macromol. 132, 970–977 (2019).
Dische, Z. & Shettles, L. B. A specific color reaction of methylpentoses and a spectrophotometric micromethod for their determination. J. Biol. Chem. 175, 595–603 (1948).
Marinetti, G. V. The action of phospholipase A2 on lipoproteins. Biochim. Biophys. Acta Lipid Metab. 98, 554–565 (1965).
Garcia, E. S., Guimarães, J. A. & Prado, J. L. Purification and characterization of a sulfhydryl-dependent protease from Rhodnius prolixus midgut. Arch. Biochem. Biophys. 2, 315–322 (1978).
Acknowledgements
This work was supported by financial support and fellowships from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Grants 304719/2012-9 and 309823/2021-8; Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Grants E-26/201.163/2014 and E-26/01.001918/2015; Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Grants APQ-01724-18; Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). EFS and ALF are Research Members of CNPq. The work on the isolation of polysaccharides from microalgae and the study of their composition, conducted by IKBFU, was funded by the Ministry of Education and Science of the Russian Federation (Agreement No. 13.2251.21.0262).
Author information
Authors and Affiliations
Contributions
C.C.P. and A.L.F. designed the study, discussed the data, performed the experiments and drafted the manuscript. S.S., O.B., and S.I. analyzed the data, drafted and revised the manuscript, and acquired the data. E.F.S. received funding, discussed the data, drafted and revised the manuscript. A.L.F. conceptualized the research, analyzed and interpreted the data, received funding, wrote and drafted the manuscript, and supervised the work. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Castro-Pinheiro, C., Sukhikh, S., Babich, O. et al. Exploiting exopolysaccharides from microalgae to block the toxic in vitro effects of Bothrops sp. venom. Sci Rep 15, 22754 (2025). https://doi.org/10.1038/s41598-025-08825-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-08825-2