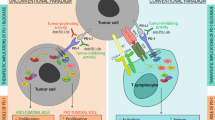
Abstract
Tumor stromal remodeling is an obstacle for immune checkpoint inhibitors (ICI). A stroma modifying small interfering RNA (siRNA) to carbohydrate sulfotransferase 15 (CHST15) was recently shown to enhance tumor-infiltrating T cells, yet its impact on antitumor response of ICI remains unexplored. In mouse pancreatic cancer KPC and Pan02 subcutaneous syngeneic tumor models, mice were divided into 4 groups for treatment; (1) control, (2) CHST15 siRNA monotherapy, (3) anti-programmed death receptor 1 (PD-1) monotherapy, and (4) combination therapy with CHST15 siRNA and anti-PD-1 antibody. Mice were sacrificed after 2 week-treatments and anti-tumor effects were evaluated by immunohistochemistry for KPC and flow cytometry for Pan02 model, respectively. In the KPC model, combination treatment with intratumoral CHST15 siRNA (0.9–1.0 mg/kg) and systemic anti-PD-1 antibody (5 mg/kg) synergistically and robustly suppressed tumor growth with a significant increase of tumor-infiltrating CD4+ and CD8+ T cells compared to anti-PD-1 monotherapy. In the Pan02 model, combination treatment with CHST15 siRNA and anti-PD-1 showed anti-tumor effect with significant increases in % necrosis area of the tumor, and tumor-infiltrating T cells compared to the control. Notably, the combination therapy dramatically diminishes Ly6C+Ly6G+ granulocytic myeloid-derived suppressor cells (MDSCs) compared to anti-PD-1 monotherapy. The present study demonstrated the robust synergy between systemic anti-PD-1 antibody and a single stroma modifying agent. Combination usage of intratumoral CHST15 siRNA would provide a novel therapeutic option to trigger the remarkable effect of ICI on this most hard-to-treat solid tumor.
Similar content being viewed by others
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of the most hard-to-treat solid tumors. Chemotherapy combinations do not always improve survival and quality of life compared to other cancer types. Although immunotherapy has become a major approach in clinical development, treatment with immune checkpoint inhibitors (ICI) alone is largely ineffective. Poor infiltration of primed T cells is one of the causes of ineffectiveness, and the stroma-rich pancreatic tumor microenvironment is considered an obstacle1,2,3,4,5,6,7,8. Stroma-modifying agents would thus be a promising strategy to improve the efficacy of ICI in order to enhance T cell infiltration. Dozens of clinical trials for combination therapy of ICI with stromal-modifying agents targeting cancer-associated fibroblasts (CAF) or extracellular matrix (ECM) are currently underway5,6,7,8.
Carbohydrate sulfotransferase 15 (CHST15) is a type II transmembrane Golgi protein that produces chondroitin sulfate E (CS-E) by biosynthesising sulfated disaccharide units (E-units) of chondroitin sulfate (CS), which is responsible for tumor matrix remodeling9,10,11,12,13,14. We previously reported that RNA interference-mediated specific knockdown of tumor-intrinsic CHST15 enhanced tumor-infiltrating CD4+ and CD8+ T cells and eliminated tumors in a T cell-dependent manner in a mouse model of PDAC15. In addition, intratumoral injection of synthetic human CHST15 (hCHST15) siRNA inhibited mouse CHST15 mRNA, repressed stromal remodeling, and augmented infiltration of CD4+ and CD8+ T cells in mouse PDAC, while did not have direct cell killing activity16. Therapeutic potential of hCHST15 siRNA monotherapy was suggested by converting immunosuppressive toward immunopermissive microenvironment.
Tumor-infiltrating lymphocytes (TIL)-enhancement by hCHST15 siRNA was also observed in patients with PDAC. In a phase I/IIa clinical study for chemotherapy-refractory, unresectable PDAC, intratumoral administration of STNM01, the RNA oligonucleotide to suppress the hCHST15 gene through siRNA mechanism17,18,19,20,21, significantly increased tumor-infiltrating CD3+ and CD8+ T cells as second-line therapy in combination with oral S-117. One possibility is considered that STNM01 broke the physiological matrix barrier, leading to accelerated recruitment of anti-tumor T cells into the tumor.
Based on these previous nonclinical and clinical study results, it is intriguing to investigate whether CHST15 siRNA-mediated T cell enhancement improves the efficacy of ICI. Thus, in the present study, we evaluated the antitumor and T cell-enhancing effects of combination therapy of CHST15 siRNA with anti-programmed death receptor 1 (PD-1) antibody in two mouse models of PDAC15,16,22.
Results
In vivo synergistic antitumor effect of hCHST15 siRNA by combination with anti-PD-1 antibody in KPC-implantation model in immunocompetent mice
To investigate whether a stromal modifying agent, CHST15 siRNA, impacts the antitumor effect of anti-PD-1 antibody in pancreatic cancer, we first tested murine KPC-implantation model, because increased tumor-infiltrating T cells were previously demonstrated in this model by monotherapy of human CHST15 siRNA (hCHST15 siRNA) through intratumoral route of administration16. In a previous study, we have already confirmed that hCHST15 siRNA significantly downregulates the level of mouse CHST15 protein in tumor cells and repressed stromal density and neutrophil extracellular traps in vivo15,16. Mice were divided into 4 treatment groups; (1) control, (2) hCHST15 siRNA monotherapy, (3) anti-PD-1 monotherapy and (4) combination therapy with hCHST15 siRNA and anti-PD-1 antibody at baseline (Day 6) where all mice sufficiently developed macroscopic tumors (Fig. 1A). Intratumoral injections with hCHST15 siRNA (0.9 mg/mL) or control vehicle and intraperitoneal administrations of anti-PD-1 antibody (5 mg/kg) or control were then performed twice a week for 2 weeks (Fig. 1A). At Day 20, mean tumor growth inhibition (TGI) rates were 33.5% and 64.0% in the hCHST15 siRNA and anti-PD-1 antibody group, respectively (Fig. 1B). Mean TGI rate of the combination therapy group was 80.3%, which was significantly lower than that of the anti-PD-1 antibody monotherapy group (Fig. 1B). Additionally, we analyzed the enhancement of PD-1 and PD-L1 binding strength by the addition of CS-E (Supplemental Fig. S1). There was no significant difference in PD-L1 expression between KPC WT and KPC-CHST15 KD (Supplemental Fig. S1A). The addition of CS-E enhanced the binding of PD-1 to PD-L1 in a dose-dependent manner at concentrations above 1 mg/mL. Compared to the condition without CS-E, a statistically significant enhancement was observed at 5 mg/mL (Supplemental Fig. S1B). The addition of an anti-PD-1 antibody (positive control) inhibits the binding of PD-1 to PD-L1 (Supplemental Fig. S1C).
Antitumor effect of combination therapy with hCHST15 siRNA and anti-PD-1 antibody in KPC-implantation model. (A) Experimental design. Mouse KPC cells (5 × 104 cells/mouse) were implanted subcutaneously into syngeneic C57/BL6 mice on day 0. Four groups were conducted: (1) Control (KPC inoculation only) (n = 5), (2) hCHST15 siRNA monotherapy (0.9 mg/mouse) (n = 5), (3) anti-PD-1 antibody monotherapy (aPD-1, RMP1-4, 5 mg/kg/mouse) (n = 5), (4) Combination therapy of hCHST15 siRNA and anti-PD-1 antibody (n = 5). Dosing was performed: Local (intratumoral) injection BIW from day 6 to day 17 (1) Control (KPC inoculation only) (n = 5), (2) hCHST15 siRNA monotherapy (0.9 mg/mouse) (n = 5), (3) anti-PD-1 antibody monotherapy (5 mg/kg/mouse) (n = 5), (4) Combination therapy of hCHST15 siRNA and anti-PD-1 antibody (n = 5). Local [Intratumoral] injections for saline or hCHST15 siRNA were performed BIW from day 6 to 17 (2 weeks), × 4 times in total. Systemic injections for anti-PD-1 antibody of control antibody were performed BIW from day 6 to 17 (2 weeks), × 4 times in total. (B) (left) The HE staining for tumor tissues of 4 groups at day 20. Scale bar = 2.5 mm. Dotted circles show the tumor area. (right) Tumor volume (mm3) was calculated by histology on day 20. CTL: Control (n = 5), siRNA: hCHST15 siRNA monotherapy (n = 5), aPD1: anti-PD-1 antibody monotherapy (n = 5), siRNA + aPD1: hCHST15 siRNA + aPD1 combination therapy (n = 5). One-way ANOVA Bonferroni’s multiple comparison test, *p < 0.05, ***p < 0.001. Mann–Whitney test for 2 groups (aPD1 vs combination), # < 0.05. Tumor growth inhibition (TGI) was calculated as follows; TGI (%) = (1-Ti/Vi) × 100. Ti as the mean tumor volume of the treatment group on day 20. Vi as the mean tumor volume of control group at day 20.
Combination of hCHST15 siRNA with anti-PD-1 antibody significantly enhanced tumor-infiltrating CD4+ and CD8+ T cells compared to anti-PD-1 monotherapy in KPC model
hCHST15 siRNA monotherapy significantly increased tumor-infiltrating CD4+ and CD8+ T cells as in the previous report15, while a significant increase by anti-PD-1 antibody monotherapy was shown in tumor-infiltrating CD8+ T cells (Fig. 2A). In contrast, combination therapy markedly enhanced tumor-infiltrating CD8+ and CD4+ T cells, both of which were significantly higher than those by anti-PD-1 antibody monotherapy (Fig. 2A,B).
hCHST15 siRNA showed enhancement of tumor-infiltrating T cells in KPC model. (A,B) Immunohistology and Quantitative analysis for immune-stained tissues was shown. The percentage of positive areas for CD4 and CD8 is shown. Mean ± SD (n = 5). (A) (Left) images of hematoxylin (purple) and immunohistochemistry with anti-CD8 antibody (brown) in each treatment. Scale bar = 250 μm. Yellow arrows show CD8+ signals. (Right) % CD8-positive areas at day 20. CTL: Control (n = 5), siRNA: hCHST15 siRNA monotherapy (n = 5), aPD1: anti-PD-1 antibody monotherapy (n = 5), siRNA + aPD1: hCHST15 siRNA + aPD1 combination therapy (n = 5). One-way ANOVA Bonferroni’s multiple comparison test, #p < 0.05. Welch’s t-test for 2 groups. *p < 0.05, **p < 0.01. (B) (Left) images of hematoxylin (purple) and immunohistochemistry with anti-CD4 (brown) antibody in each treatment. Scale bar = 250 μm. Yellow arrows show CD4+ signals. (right) % CD4-positive areas at day 20. CTL: Control (n = 5), siRNA: hCHST15 siRNA monotherapy (n = 5), aPD1: anti-PD-1 antibody monotherapy (n = 5), siRNA + aPD1: hCHST15 siRNA + aPD1 combination therapy (n = 5). One-way ANOVA Bonferroni’s multiple comparison test, **p < 0.01, ***p < 0.001, ****p < 0.0001. Welch’s t-test for CTL vs aPD1, ns: not significant.
In vivo synergistic tumor elimination of hCHST15 siRNA by combination with anti-PD-1 antibody in Pan02-implantation model in immunocompetent mice
We next tested the murine Pan02-implantation model, because the anti-PD-1 antibody alone does not show an anti-tumor effect in this model22. Four treatment groups were conducted; (1) control, (2) hCHST15 siRNA monotherapy, (3) anti-PD-1 monotherapy and (4) combination therapy with hCHST15 siRNA and anti-PD-1 antibody at baseline (Day 28) (Fig. 3A). Intratumoral injections with hCHST15 siRNA (1.0 mg/mL) or control vehicle and intraperitoneal administrations of anti-PD-1 antibody (5 mg/kg) or control were then performed twice a week for 2 weeks (Fig. 3A). At day 42, mean TGI rates were 24.2% and 26.2% in the hCHST15 siRNA and anti-PD-1 antibody group, respectively (Fig. 3B). Mean TGI rate of the combination therapy group was 30.8%, which was significantly lower than that of the control group (Fig. 3B). Additionally, tumor elimination was evaluated by % necrosis area of tumor. Only combination therapy showed a significant increase in tumor necrosis, which was never achieved by monotherapies (Fig. 3B).
Antitumor effect of combination therapy with hCHST15 siRNA and anti-PD-1 antibody in Pan02-implantation model. (A) Experimental design. Mouse Pan02 cells (3 × 106 cells/mouse) were implanted subcutaneously into syngeneic C57/BL6 mice on day 0. Four groups were conducted: (1) Control (Pan02 inoculation only) (n = 5), (2) hCHST15 siRNA monotherapy (1.0 mg/mouse) (n = 5), (3) anti-PD-1 antibody monotherapy (5 mg/kg/mouse) (n = 5), (4) Combination therapy of hCHST15 siRNA and anti-PD-1 antibody (n = 5). Local [Intratumoral] injections for saline or hCHST15 siRNA were performed BIW from day 28 to 38, × 3 times in total. Systemic injections for anti-PD-1 antibody of control antibody were performed BIW from day 28 to 38, × 4 times in total. (B) (left panels) The macroscopic images of the tumor in 4 groups at day 42. Scale bar = 10 mm. Dotted circles show the tumor area. Triangles indicate the margin of hemorrhagic tumor necrosis. (middle panel) Tumor volume (mm3) was calculated by macroscopically on day 42. (left panel) Macroscopic necrotic area (%) at day 42. CTL: Control (n = 5), siRNA: hCHST15 siRNA monotherapy (n = 5), aPD1: anti-PD-1 antibody monotherapy (n = 4), siRNA + aPD1: hCHST15 siRNA + aPD1 combination therapy (n = 5). One-way ANOVA Bonferroni’s multiple comparison test, *p < 0.05, ns: not significant, Welch’s t-test for CTL vs aPD1 + siRNA (middle), and aPD1 vs aPD1 + siRNA (right), #p < 0.05. TGI was calculated as follows; TGI (%) = (1-Ti/Vi) × 100. Ti as the mean tumor volume of the treatment group on day 42. Vi as the mean tumor volume of control group at day 42. (C) % CD3-positive (left panel) and CD19-positive (right panel) cells in tumor-infiltrating lymphocyte (TIL) at day 42. CTL: Control (n = 5), siRNA: hCHST15 siRNA monotherapy (n = 4), aPD1: anti-PD-1 antibody monotherapy (n = 4), siRNA + aPD1: hCHST15 siRNA + aPD1 combination therapy (n = 5). One-way ANOVA Bonferroni’s multiple comparison test, *p < 0.05, ns: not significant.
Changes in T cell component by combination therapy with hCHST15 siRNA and anti-PD-1 antibody in Pan02 model
The tumor cell component was evaluated by flow cytometry. One sample from the control group was shown (Supplemental Fig. S2). Combination therapy significantly increased tumor-infiltrating CD3+ T cells but not CD19+ B cells (Fig. 3C). hCHST15 siRNA monotherapy significantly increased tumor-infiltrating CD4+ and CD8+ T cells, while a significant increase by anti-PD-1 antibody monotherapy was shown in tumor-infiltrating CD8+ T cells (Fig. 4A) as observed in the KPC model.
hCHST15 siRNA showed enhancement of tumor-infiltrating T cells in Pan02 model. (A) % CD4 (middle panel) and CD8 (right panel)-positive cells at day 42. CTL: Control (n = 5), siRNA: hCHST15 siRNA monotherapy (n = 4), aPD1: anti-PD-1 antibody monotherapy (n = 4), siRNA + aPD1: hCHST15 siRNA + aPD1 combination therapy (n = 5). One-way ANOVA Bonferroni’s multiple comparison test, *p < 0.05, Welch’s t-test for CTL vs siRNA or qPD1, #p < 0.05. (B) % FoxP3+ Treg (middle panel) and the ratio of CD8/FoxP3 (right panel) at day 42. CTL: Control (n = 5), siRNA: hCHST15 siRNA monotherapy (n = 4), aPD1: anti-PD-1 antibody monotherapy (n = 4), siRNA + aPD1: hCHST15 siRNA + aPD1 combination therapy (n = 5). Mann–Whitney test, *p < 0.05, ns: not significant.
In contrast, combination therapy markedly enhanced tumor-infiltrating CD4+ and CD8+ T cells (Fig. 4A). There was no significant difference in % of FoxP3+ fraction between all treatment groups (Fig. 4B). The ratio of CD8/FoxP3 was significantly higher only in the combination therapy group (Fig. 4B).
Combination of hCHST15 siRNA with anti-PD-1 antibody significantly diminished tumor-infiltrating MDSCs compared to anti-PD-1 monotherapy in Pan02 model
hCHST15 siRNA monotherapy significantly reduced tumor-infiltrating granulocytic, but not monocytic MDSCs, while anti-PD-1 antibody monotherapy did not induce any changes in MDSCs (Fig. 5). The combination therapy group showed a significant reduction in tumor-infiltrating granulocytic MDSCs (gMDSC), compared to the control as well as the anti-PD-1 antibody monotherapy groups (Fig. 5).
hCHST15 siRNA showed enhancement of tumor-infiltrating T cells in Pan02 model. % mMDSC (middle panel) and gMDSC (right panel)-positive cells at day 42. CTL: Control (n = 5), siRNA: hCHST15 siRNA monotherapy (n = 4), aPD1: anti-PD-1 antibody monotherapy (n = 4), siRNA + aPD1: hCHST15 siRNA + aPD1 combination therapy (n = 5). One-way ANOVA Bonferroni’s multiple comparison test, **p < 0.01, *p < 0.05, Mann–Whitney test, aPD1 vs aPD1 + ST01, #p < 0.05.
Discussion
In the present study, we investigated whether locoregional injection of hCHST15 siRNA improves the efficacy of anti-PD-1 antibody using two syngeneic mouse models of PDAC. In the KPC model, anti-PD-1 antibody alone showed a decrease of tumor growth and an increase in CD8+ T cell infiltration. The antitumor effects were significantly improved by combination with intratumoral hCHST15 siRNA. In the Pan02 model, anti-PD-1 antibody alone showed an increase of CD8+ T cell infiltration but necrotic tumor elimination was not induced. Combination usage of hCHST15 siRNA achieved anti-tumor effect with significant tumor necrosis and an increased ratio of CD8 to FoxP3. Current study is thus the first report illustrating that stroma-modifying agent hCHST15 siRNA can improve the antitumoral efficacy of anti-PD-1 antibody as ICI with increased % of tumor-infiltrating CD8+ T cells in PDAC.
We found differences in CD4+ T cells between hCHST15 siRNA and anti-PD-1 antibody monotherapies in both models. While CD4+ T cells did not show significant change in the anti-PD-1 monotherapy group compared to the control group, hCHST15 siRNA significantly increased CD4+ T cells. As tumoral CHST15 silencing did not affect the infiltration of regulatory T cells (Tregs) in the KPC model15 as well as the Pan02 model (Fig. 4B), the increased CD4+ T cells are considered to contain helper T cell types that mediate antitumor immunity rather than Treg. The CD8+ T cell boosting effect by combination therapy may be attributable to more effective CD4+ T cell help to generate CD8+ cytotoxic T lymphocytes (CTL) when compared to anti-PD-1 monotherapy.
In addition, FACS analysis demonstrated that hCHST15 siRNA significantly reduced gMDSCs but not monocytic MDSCs in the Pan02 model. This is consistent with our previous report that hCHST15 siRNA diminished Ly6C/G+ MDSCs in both tumor and tumor-draining lymph nodes (TDLN) in the KPC model16. As anti-PD-1 monotherapy did not affect gMDSC, hCHST15 siRNA-mediated reduction of gMDSCs is thought to contribute to enhanced antitumor effect by combination therapy. We consider that hCHST15 siRNA acts directly on gMDSCs in tumor and TDLN and reactivates CD4+ helper T cells, leading to effective CTL generation when used in combination with anti-PD-1 antibody.
Several numbers of nonclinical and clinical studies have been conducted on stroma-modifying agents alone or in combination with ICI, chemotherapy, and radiation therapy in PDAC5,6,7,8. Systemic administration of recombinant hyaluronidase, PEGPH20, has not been found to improve OS as the first-line therapy when combined with mFOLFIRINOX partly due to toxicity2,23. Modified types of hyaluronidase or other stromal targeted compounds including focal adhesion kinase (FAK), TGF-β, CTGF, vitamin D, and CXCR4 are now under investigation5,6,7,8,24,25,26,27, and it is of interest whether these stroma-modifying agents enhance TIL in actual patient tissues and antitumor efficacy of ICI in clinical trials. In addition, the safety profile is of another interest since stroma-modifying agents might induce structural tissue damage especially when used systemically. In this respect, we choose CHST15 siRNA rather than chondroitinase which broadly and strongly disrupts matrix CS. Although we think CHST15 siRNA is relatively safe as CHST15 knockout mice did not show any abnormality distinct from other genes involved in proteoglycan synthesis28, we took the intratumoral approach to further increase the safety. In addition, the intratumoral route of administration effectively activates immunity compared to the systemic route29.
There are several limitations in our study. First, the silencing efficacy of hCHST15 siRNA on mouse CHST15 mRNA was not strong16. This might influence the extent of antitumor efficacy in the mouse PDAC model. Second, molecular mechanisms underlying the interaction between CHST15/CS-E and PD-1/PD-L1 were not investigated in the present study, because we focused on the in vivo findings of combination treatment of hCHST15 siRNA with anti-PD-1 antibody. However, we consider that extracellular CS-E competitively interferes the biding of anti-PD-1 antibody to PD-1 molecules on T lymphocytes, which may lead to decrease in th efficacy of anti-PD-1 antibody. In this respect, glycan modifications on PD-1/PD-L1 have been reported to impact on therapeutic effect of anti-PD-1/PD-L1 antibodies30. One possibility is thus considered that CHST15 sRNA-mediated reduction of CS-E may enhance activity of anti-PD-1 antibody and contribute to augment T cell-mediated tumor cell killing. Future investigation if matrix CS-E affects PD-1 molecule will be required. Third, our models did not show visible metastasis, so the effect of the combination on metastatic lesions needs to be investigated in other animal models. Forth, although CHST15 is also expressed by fibroblasts and MDSCs within tumor stroma16, the role of host fibroblast-derived CHST15 was still unexplored in the present study. Further investigations using CHST15 knockout mouse as a host will be needed to clarify the role of tumor fibroblast and/or MDSC-derived CHST15 in the modulation of PD-1 action in vivo, which would provide important insights into anti-PD-1-based therapy.
In conclusion, our study represents the first report of robust antitumor effects of combination treatment with stroma modifying hCHST15 siRNA and anti-PD-1 immune checkpoint antibody in PDAC in mice. We have also documented immune cell components and showed that hCHST15 siRNA-induced change in gMDSCs and CD4+ T cells would be a key finding to enhance the CD8+ T cell-mediated antitumor effect of anti-PD-1 antibody. As safety and TIL-enhancing findings were achieved by intratumoral hCHST15 siRNA injection in the past phase I/IIa clinical trial, combination therapy would be a clinically feasible approach for this ICI-resistant cancer.
Materials and methods
KPC syngeneic model in immunocompetent mice
The mouse PDAC cell line of KPC cells (C57BL/6J background) were purchased from Ximbio (London, UK), maintained, and used as previously described15,16. C57BL/6J mice (5 weeks old male) were purchased from CLEA Japan, Inc., Tokyo, Japan. KPC cells (5 × 104 cell/mouse) were subcutaneously injected into the left hind footpad of C57BL/6J mice. hCHST15 siRNA16,17,18,19,20,21 (0.9 mg/mL/mouse, n = 5) or physiological saline as control vehicle (n = 5) was intratumorally injected (100 μl/mouse) twice a week from day 7 to 17 (× 4 times in total). The concentration of injection solution (0.9 mg/mL) and the dosing interval was determined by in situ hybridization and stem-loop PCR as reported previously16. The volume of injection (100 μl) was fixed during the study period without adjusting the size of tumor. Anti-PD-1 antibody (clone RMP1-14, Bio X cell, 5 mg/kg/mouse, n = 5) or saline as control was intraperitoneally injected twice a week from day 7 to 17 (× 4 times in total). This study was approved by the Institutional Animal Care and Use Committee (Approval No. HKD49008) and was performed in accordance with the animal welfare laws of HOKUDO, Co., Ltd and regulations. The study is reported with in accordance with ARRIVE guidelines. At the end of the experiment, mice were euthanized by cervical sidlocation under deep anesthesia induced with isoflurane (4–5% for induction, 2% for maintenance in oxygen), and tumors were excised.
Pan02 syngeneic model in immunocompetent mice
The mouse PDAC cell line of Pan02 cells (C57BL/6 background) were derived from well-established mouse pancreatic ductal adenocarcinoma (PDAC) model developed by chemical induction with 3-methylcholanthrene (3-MCA)31 and purchased from ATCC. Pan02 cells were maintained in vitro with RPMI-1640 medium supplemented with 10% fetal bovine serum at 37ºC in an atmosphere of 5% CO2 in air. The cells growing in an exponential growth phase were harvested and counted for tumor inoculation. C57BL/6 mice (6 weeks old female) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. Pan02 cells (3 × 106 cell/mouse) were subcutaneously injected into the right front flank region of mice. hCHST15 siRNA16,17,18,19,20,21 (1.0 mg/mL/mouse, n = 5) or physiological saline as control vehicle (n = 5) was intratumorally injected (100 μl/mouse) twice a week from day 28 to 38 (× 3 times in total) and mice were sacrificed at day 42. The concentration of dosing solution (1.0 mg/mL) and the dosing interval was also determined with reference to the previous report16. The volume of injection (100 μl) was fixed during the study period. Anti-PD-1 antibody (clone RMP1-14, Crown BioScience, 5 mg/kg/mouse, n = 5) was intraperitoneally injected twice a week from day 28 to 38 (× 4 times in total). The tumor volume (mm3) was determined using the formula, width2 × length × 0.5. Hemorrhagic tumor necrosis was macroscopically identified32. Macroscopic tumor necrosis was measured using ImageJ (Version: 2.1.0/1.53c; https://imagej.net/). with the formula % macroscopic necrosis area = (Necsoris area/tumor mass area) × 10033. The protocol and any amendments or procedures involving the care and use of animals in this study were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of CrownBio before execution. During the study, the care and use of animals were conducted following the regulations of the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). The study is reported with in accordance with ARRIVE guidelines. At the end of the experiment, mice were euthanized by cervical sidlocation under deep anesthesia induced with isoflurane (4–5% for induction, 2% for maintenance in oxygen), and tumors were excised.
Immunohistochemistry
Hematoxylin–eosin (HE) and immunohistochemical staining were performed as described previously15,16,34,35. In brief, the tissues were fixed in 10% neutral buffered formalin, embedded in paraffin and were sliced serially into sections (3 μm thick) for HE, sirius red, immunohistochemical staining and immunofluorescence staining. Immunohistochemical staining was performed using CD4 (clone EPR19514, Abcam) and CD8α (clone EPR20305, Abcam). For quantification of immunostained areas, bright field images were captured using a digital camera (DFC280, Leica Microsystems) at 400-fold magnification, and the positive areas or cell counts in 5 fields/section were measured using ImageJ (Version: 2.1.0/1.53c; https://imagej.net/).
Mouse tissue isolation and Flowcytometry
Fluorescence-activated cell sorting (FACS) was performed as described previously15,34. Briefly, for T cell/MDSC staining of tumor-derived lymphocytes, add Fc-Block to the cells (2 × 106/ml per sample) for 15 min on ice. For surface stain, add surface antibodies mixture of anti-CD45 (clone 30-F11, BioLegend), anti-CD3 (clone 17A2, BioLegend), anti-CD4 (clone RM4-5, BioLegend), anti-CD8a (clone 53-6.7, BioLegend), anti-CD11b (clone M1/70; BioLegend), anti-Ly6G (clone 1A8, BioLegend), anti-Ly6C (clone HK1.4, BioLegend), and ani-CD19 (clone 6D5, BioLegend). For FoxP3 (clone FJK-16a, eBioScience), add an antibody in permeabilization buffer to each sample and incubate at RT for 30 min in the dark. Samples were then analyzed by flowcytometer.
The gating strategy was designed to logically define specific immune cell populations (Supplemental Fig. S2). For example, CD4+ and CD8+ cells were gated on CD3+ cells, and Tregs were gated on CD4+ cells. The statistical analysis normalized the data to a common reference level, such as the CD45 leukocytes, to enable meaningful comparisons between different cell populations and samples. Therefore, while the gating strategy presents the raw cell populations within their respective logical hierarchies, the statistical data were converted to the same reference level (CD45⁺) for consistency and comparability.
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 9.0 software (GraphPad Software, Inc.). For comparison variables, data was analyzed by Wilcoxon rank sum test or Welch independent t-test. A p-value of less than 0.05 was considered to indicate statistical significance. Results were expressed as mean ± SD.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Ho, W. J., Jaffee, E. M. & Zheng, L. The tumour microenvironment in pancreatic cancer - clinical challenges and opportunities. Nat. Rev. Clin. Oncol. 17, 527–540. https://doi.org/10.1038/s41571-020-0363-5 (2020).
Hosein, A. N., Brekken, R. A. & Maitra, A. Pancreatic cancer stroma: an update on therapeutic targeting strategies. Nat. Rev. Gastroenterol. Hepatol. 17, 487–505. https://doi.org/10.1038/s41575-020-0300-1 (2020).
Halbrook, C. J., Lyssiotis, C. A., Pasca di Magliano, M. & Maitra, A. Pancreatic cancer: Advances and challenges. Cell 186, 1729–1754. https://doi.org/10.1016/j.cell.2023.02.014 (2023).
Flies, D. B., Langermann, S., Jensen, C., Karsdal, M. A. & Willumsen, N. Regulation of tumor immunity and immunotherapy by the tumor collagen extracellular matrix. Front. Immunol. 14, 1199513. https://doi.org/10.3389/fimmu.2023.1199513 (2023).
Ju, Y. et al. Barriers and opportunities in pancreatic cancer immunotherapy. NPJ Precis. Oncol. 8, 199. https://doi.org/10.1038/s41698-024-00681-z (2024).
Farhangnia, P., Khorramdelazad, H., Nickho, H. & Delbandi, A. A. Current and future immunotherapeutic approaches in pancreatic cancer treatment. J. Hematol. Oncol. 17, 40. https://doi.org/10.1186/s13045-024-01561-6 (2024).
Chick, R. C. & Pawlik, T. M. Updates in immunotherapy for pancreatic cancer. J. Clin. Med. https://doi.org/10.3390/jcm13216419 (2024).
Lv, D. et al. Crosstalk between T lymphocyte and extracellular matrix in tumor microenvironment. Front. Immunol. 15, 1340702. https://doi.org/10.3389/fimmu.2024.1340702 (2024).
Habuchi, O., Moroi, R. & Ohtake, S. Enzymatic synthesis of chondroitin sulfate E by N-acetylgalactosamine 4-sulfate 6-O-sulfotransferase purified from squid cartilage. Anal. Biochem. 310, 129–136. https://doi.org/10.1016/s0003-2697(02)00277-4 (2002).
Mizumoto, S., Yamada, S. & Sugahara, K. Molecular interactions between chondroitin-dermatan sulfate and growth factors/receptors/matrix proteins. Curr. Opin. Struct. Biol. 34, 35–42. https://doi.org/10.1016/j.sbi.2015.06.004 (2015).
Matsuda, Y. et al. Overexpression of carbohydrate sulfotransferase 15 in pancreatic cancer stroma is associated with worse prognosis. Oncol. Lett. 18, 4100–4105. https://doi.org/10.3892/ol.2019.10764 (2019).
Ito, Z. et al. Prognostic impact of carbohydrate sulfotransferase 15 in patients with pancreatic ductal adenocarcinoma. Oncol. Lett. 13, 4799–4805. https://doi.org/10.3892/ol.2017.6071 (2017).
Sugahara, K. N. et al. Chondroitin sulfate E fragments enhance CD44 cleavage and CD44-dependent motility in tumor cells. Cancer Res. 68, 7191–7199. https://doi.org/10.1158/0008-5472.Can-07-6198 (2008).
Agoulnik, I. U. et al. Role of GalNAc4S-6ST in astrocytic tumor progression. PLoS ONE https://doi.org/10.1371/journal.pone.0054278 (2013).
Ye, J. et al. Silencing of tumoral carbohydrate sulfotransferase 15 reactivates lymph node pancreatic cancer T cells in mice. Eur. J. Immunol. https://doi.org/10.1002/eji.202250160 (2023).
Ye, J. et al. Intra-tumoral administration of CHST15 siRNA remodels tumor microenvironment and augments tumor-infiltrating T cells in pancreatic cancer. Mol. Ther. Oncol. https://doi.org/10.1016/j.omton.2024.200812 (2024).
Fujisawa, T. et al. STNM01, the RNA oligonucleotide targeting carbohydrate sulfotransferase 15, as second-line therapy for chemotherapy-refractory patients with unresectable pancreatic cancer: an open label, phase I/IIa trial. eClinicalMedicine https://doi.org/10.1016/j.eclinm.2022.101731 (2023).
Nishimura, M. et al. Effects of EUS-guided intratumoral injection of oligonucleotide STNM01 on tumor growth, histology, and overall survival in patients with unresectable pancreatic cancer. Gastrointest. Endosc. 87, 1126–1131. https://doi.org/10.1016/j.gie.2017.10.030 (2018).
Kiryu, H. et al. A detailed investigation of accessibilities around target sites of siRNAs and miRNAs. Bioinformatics 27, 1788–1797. https://doi.org/10.1093/bioinformatics/btr276 (2011).
Batra, S. K. et al. Inhibition of cell proliferation and growth of pancreatic cancer by silencing of carbohydrate sulfotransferase 15 in vitro and in a xenograft model. PLoS ONE https://doi.org/10.1371/journal.pone.0142981 (2015).
Siegmund, B. et al. Pivotal role of carbohydrate sulfotransferase 15 in fibrosis and mucosal healing in mouse colitis. PLoS ONE https://doi.org/10.1371/journal.pone.0158967 (2016).
Kanaya, N. et al. Immune modulation by telomerase-specific oncolytic adenovirus synergistically enhances antitumor efficacy with anti-PD1 antibody. Mol. Ther. 28, 794–804. https://doi.org/10.1016/j.ymthe.2020.01.003 (2020).
Ramanathan, R. K. et al. Phase IB/II randomized study of FOLFIRINOX plus pegylated recombinant human hyaluronidase versus FOLFIRINOX alone in patients with metastatic pancreatic adenocarcinoma: SWOG S1313. J. Clin. Oncol. 37, 1062–1069. https://doi.org/10.1200/jco.18.01295 (2019).
Jiang, H. et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat. Med. 22, 851–860. https://doi.org/10.1038/nm.4123 (2016).
Lander, V. E. et al. Stromal reprogramming by FAK inhibition overcomes radiation resistance to allow for immune priming and response to checkpoint blockade. Cancer Discov. 12, 2774–2799. https://doi.org/10.1158/2159-8290.Cd-22-0192 (2022).
Blair, A. B. et al. Dual stromal targeting sensitizes pancreatic adenocarcinoma for anti-programmed cell death protein 1 therapy. Gastroenterology 163, 1267–80.e7. https://doi.org/10.1053/j.gastro.2022.06.027 (2022).
Wang, S., Li, Y., Xu, C., Dong, J. & Wei, J. An oncolytic vaccinia virus encoding hyaluronidase reshapes the extracellular matrix to enhance cancer chemotherapy and immunotherapy. J. ImmunoTher. Cancer. https://doi.org/10.1136/jitc-2023-008431 (2024).
Mizumoto, S., Yamada, S. & Sugahara, K. Human genetic disorders and knockout mice deficient in glycosaminoglycan. Biomed. Res. Int. 2014, 1–24. https://doi.org/10.1155/2014/495764 (2014).
Topalian, S. L., Taube, J. M. & Pardoll, D. M. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science https://doi.org/10.1126/science.aax0182 (2020).
Liu, J. et al. Glycosylation and its role in immune checkpoint proteins: from molecular mechanisms to clinical implications. Biomedicines https://doi.org/10.3390/biomedicines12071446 (2024).
Corbett, T. H. et al. Induction and chemotherapeutic response of two transplantable ductal adenocarcinomas of the pancreas in C57BL/6 mice1. Can. Res. 44, 717–726 (1984).
Kircheis, R. et al. Tumor-targeted gene delivery of tumor necrosis factor-alpha induces tumor necrosis and tumor regression without systemic toxicity. Cancer Gene Ther. 9(8), 673–680. https://doi.org/10.1038/sj.cgt.7700487 (2002).
Martín-Ruiz, A. et al. Effects of anti-PD-1 immunotherapy on tumor regression: insights from a patient-derived xenograft model. Sci. Rep. https://doi.org/10.1038/s41598-020-63796-w (2020).
Yoneyama, H. et al. Regulation by chemokines of circulating dendritic cell precursors, and the formation of portal tract-associated lymphoid tissue, in a granulomatous liver disease. J. Exp. Med. 193, 35–50. https://doi.org/10.1084/jem.193.1.35 (2000).
Kitazawa, Y. et al. Novel targeting to XCR1+ dendritic cells using allogeneic T cells for polytopical antibody responses in the lymph nodes. Front. Immunol. https://doi.org/10.3389/fimmu.2019.01195 (2019).
Acknowledgements
This work was supported in part by Grant-in-Aid for Scientific Research on Innovative Areas from the Japan Society for the Promotion of Science (JSPS) KAKENHI (Grant Number JP: 19H03447, 23H02702 to F. S.).
Author information
Authors and Affiliations
Contributions
Data curation, J. Y., F. S., H. Y, K. K.; Funding acquisition, F. S, K. K.; Investigation, J. Y., F. S., K. Y., M. K., H. Y.; Methodology, J. Y., F. S., K. Y., H. Y., J. K., M. K., A. N., N. Y., K. K.; Supervision, N. Y., F. S., K. K.; Writing – original draft, J. Y., H. Y., F. S., K. K.; Writing – review & editing, J. Y., H. Y., F. S., A. N., K. K.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ye, J., Suizu, F., Yamakawa, K. et al. Stromal modifying CHST15 siRNA enhances antitumor effect synergistically with anti-PD-1 immune checkpoint antibody in murine pancreatic cancer. Sci Rep 15, 20365 (2025). https://doi.org/10.1038/s41598-025-09445-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-09445-6