Abstract
In high-risk newly diagnosed multiple myeloma (HRNDMM), two different induction regimens were evaluated for safety and efficacy: Bortezomib-Pomalidomide-Dexamethasone (VPd) and Bortezomib-Lenalidomide-Dexamethasone (VRd). Newly diagnosed high-risk MM patients(age > 18) were included in this retrospective study, who received VRd and VPd induction therapy between January 2021 and November 2023. All methods of this experiment were performed in accordance with relevant guidelines and regulations. Informed consent has been obtained from all subjects and/or their legal guardians. The Ethics Association of Anqing City Hospital has approved the study on pomadomide in this experiment (Medical Review (2021) No. 27). The evaluation of OS (overall survival), PFS (progression free survival), AE (adverse event) was the secondary endpoints, while the primary endpoint of the study was the overall response rate (ORR) after four cycles of VRd and VPd. Ultimately, 25 patients with VRd and 21 patients with VPd were enrolled. After four cycles of induction, stringent complete, complete, very good partial, partial and overall response rates were 16%/24%/12%/40%/92% with VRd, and 23.81%/33.33%/33.33%/9.52%/100% with VPd. VGPR or better was achieved in 52% of patients receiving VRd compared to 90.47% in those receiving VPd, with a p-value of 0.003. VPd was linked to more cases of skin rash (p = 0.02). For VRd and VPd, the average overall survival time was 27-months and 21-months, respectively (p = 0.801). The PFS was 27-months for VRd and 20-months for VPd (p = 0.116). Median OS and PFS were not defined in both groups. According to our research, the induction of VPd has been found to elicit more profound and superior responses in HRNDMM compared to VRd, thereby establishing its safety. Our results underscore the potential clinical advantage of pomalidomide as first-line therapy and offer more proof of the beneficial efficacy of VPd in the treating of HRNDMM patients.
Similar content being viewed by others
Introduction
Multiple myeloma (MM) is a disease that affects plasma cells. It is the second most common blood cancer1. New treatments for MM have helped people live longer. and has resulted in more robust responses and enhanced survival outcomes2. There is no known cure for MM. The standard treatment for individuals recently diagnosed with MM typically involves initiating chemotherapy, followed by potential consideration of autologous stem cell transplant (ASCT) if deemed suitable, and ultimately incorporating ongoing maintenance therapy3. However, treating high risk newly diagnosed multiple myeloma (HRNDMM) remains challenging with still markedly impaired survival and a higher likelihood of relapse4.
The aim of treating newly diagnosed multiple myeloma (NDMM) is to control the disease and achieve the best response possible. According to current international guidelines, it’s recommended to use a combination of three different drugs at the beginning of treatment. This includes using bortezomib and dexamethasone as the main treatment, along with adding another drug called an immunomodulatory drug (IMiD)5. Lenalidomide belongs to the second generation of IMiDs. For standard-risk patients, the implementation of VRd induction therapy, followed by the administration of lenalidomide for maintenance purposes has shown long-term positive results and is currently the accepted way to treat this condition. The problem of lenalidomide resistance means that we can’t improve survival much more6. Pomalidomide is a third-generation IMiD, exhibits no cross-resistance to lenalidomide and its dose does not require adjusted according to renal function (RI)6, and it has a unique way of activating genes and breaking down substances7. It provides treatment for patients with relapsed and refractory multiple myeloma (RRMM).
Currently available triplet induction treatment options comprise bortezomib-thalidomide/ lenalidomide/ cyclophosphamide/ doxorubicin-dexamethasone (VTd/ VRd/ VCD/ PAD). Although numerous studies have investigated VRd vs. VCD, VTd vs. VRd, and VTd vs. VCd, there is not enough data from trials to compare VRd and VPd for safety and efficacy.
Building on this foundation, recent studies have evaluated the potential of an induction regimen comprising bortezomib-pomalidomide-dexamethasone (VPd) to augment response rates and enhance outcomes in NDMM8. After four cycles of VPd induction, the Phase II Study found that: 32% of the subjects achieved a stringent complete response (sCR), while 29% attained a complete response (CR). Additionally, 26% exhibited a very good partial response (VGPR), and 13% demonstrated a partial response (PR). This regimen demonstrated higher rates of VGPR, leading to the conclusion that induction chemotherapy utilizing VPd is both safe and efficacious in patients with NDMM.
We studied whether bortezomib-lenalidomide-dexamethasone (VRd) or bortezomib-pomalidomide- dexamethasone (VPd) was more effective in treating HRNDMM. The data from the pomalidomide group was obtained from a clinical trial that we registered in 2021 (ChiCTR2100050710) and approved by Anqing Municipal Hospital Ethics Committee. We primarily enrolled newly diagnosed patients with high-risk multiple myeloma, in comparison to the current first-line treatment regimen VRd, to see if pomalidomide is safe and works for HRNDMM.
Methods
Patients
This was a study of patients with high-risk multiple myeloma who were at least 18 years of age and met the diagnostic criteria of the International Myeloma Working Group (IMWG)9, male or female, between January 2021 and November 2023. All patients signed a consent form. All methods of this experiment followed relevant guidelines and regulations. The study on pomadomide in this experiment has been approved by the Ethics Association of Anqing City Hospital (Medical Review (2021) No. 27). See Table 1 for a summary of their characteristics. All patients had at least one of the following high-risk factors: (1) high-risk cytogenetics, cytogenetics was defined as high risk if it showed at least one of these abnormalities: deletion 17p, t (4;14), t (14;20), t (14;16); (2) extramedullary disease (EMD).
The exclusion criteria included: (1) Patients who have had allergic reactions to pomalidomide or the ingredients contained in the drug; (2) Patients with active new thrombosis or unwilling to undergo antithrombotic therapy; (3) Patients who need long-term use of immunosuppressants or steroids; (4) Those who refuse to take reliable contraceptive methods during pregnancy, lactation or appropriate age; (5) The patient has other tumors at the same time or has a history of tumors, or has undergone anti-tumor treatment (including major surgery) within the last 4 weeks, except for the following tumor diseases: skin basal cell carcinoma, skin squamous cell carcinoma, cervical cancer Incidental histological findings of carcinoma in situ, carcinoma in situ of the breast, prostate cancer (TNM clinical stage T1a or T1b) or treated prostate cancer; (6) Patients suffering from central nervous system diseases and needing treatment; (7) Patients with peripheral neuropathy ≥ grade 3; (8) Severe mental illness; (9) Patients who are judged unsuitable for inclusion after the investigator’s evaluation.
Study design
The patients were centrally allocated to receive 4 cycles of VRd or VPd. Both VRd and VPd induction consisted of either a 21-day or 28-day cycle. Bortezomib was given under the skin every 21 days (on days 1, 4, 8, 11) or every 28 days (on days 1, 8, 15, 22), with a dose of 1.3 mg/m2. Concomitant with the subcutaneous bortezomib, dexamethasone 40 mg is administered orally or intravenously (IV). The daily dose of lenalidomide is 25 mg and is given over a 21-day (oral 14 days) or 28-day (oral 21 days) treatment cycle. The dose of pomalidomide was either 4 mg daily for 21-days (oral 14 days) or 28-days (oral 21 days); 48% (12/25) and 52.4% (11/21) of patients in the VRd and VPd groups were treated with the 21-day regimen, respectively. The 4-week regimen was 52% (13/25) and 47.6% (10/21), P = 0.767. Patients eligible for transplantation, who have achieved a very good partial response or better (≥ VGPR), are able to undergo ASCT after finishing at least four cycles. After undergoing ASCT, these patients have started taking oral medication as part of their maintenance treatment. For those not eligible for a transplant, they received a three-drug induction regimen followed by ongoing maintenance treatment.
Assessment
This study aimed to find out how many people overall respond to four courses of VRd and VPd. The secondary endpoints encompassed the assessment of progression free survival (PFS), overall survival (OS), adverse events (AE). The PFS was the duration between the start of treatment and either the disease worsening, the death of the patient, or the last follow-up. OS was the time between the start of treatment and the death of the patient or the last follow-up.
In addition to routine inspections such as blood count, all patients underwent comprehensive testing including light chain assay in serum and urine (SFLC), immunofixation (IF), serum protein electrophoresis (SPEP), estimation of β2 microglobulin, bone marrow cytology, fluorescent in situ hybridization (FISH) for cytogenetic abnormalities detection, chromosome karyotype analysis, skeletal imaging through CT or X-ray scanning, and assessment of extramedullary infiltration.
At the conclusion of four cycles, comprehensive response evaluations were conducted, encompassing assessments of complete blood count, renal and hepatic function, electrolyte levels, SPEP, IF, SFLC, bone marrow cytology, minimal residual disease (MRD) status and CT scans. The response was evaluated based on the IMWG Uniform Response Criteria. A quantitative analysis was conducted on the adverse events recorded in the captured electronic medical record (EMR), include hematological adverse events (neutropenia, anemia, thrombocytopenia, myelosuppression, etc.), nonhematological adverse effects (Peripheral sensory neuropathy, fatigue, diarrhea, infection nonneutropenic, etc.). The data cutoff date for this report was August 31, 2024.
Statistical analysis
Response evaluation was conducted based on the test results, while the analysis was conducted utilizing the SPSS software version 22. The Kaplan–Meier methodology was utilized to estimate PFS and OS. The rates of response and toxicity were calculated as percentages. The responses were analyzed utilizing a chi-squared statistical test. A p-value of < 0.05 was deemed to be statistically significant.
Results
Patients’ characteristics
In all patients who met the eligibility criteria, VRd induction was administered to 25 participants, while VPd induction was given to 21 participants. According to the data presented in Table 1, both groups of patients exhibited similar status of performance at the time of diagnosis, indicating no significant differences across these factors. The patients in the VRd and VPd cohorts exhibited comparable age distribution, the median age of the subjects was 64 and 69 years, respectively (p = 0.218) as well as sex distribution (males: 40% and 57%, p = 0.245). A similar lack of statistical significance was observed in the ISS stage III (96% vs. 100%, p = 0.266). Furthermore, a comparison of the R-ISS stage III between patients in the VRd and VPd groups revealed no statistically significant difference (96% vs. 95%, p = 0.9). The 16% and 19% incidence of patients with a complex karyotype observed in the VRd and VPd cohorts (p = 0.786). The patient population under study included those with extramedullary disease (EMD), with observations made in 4% and 9.5% of patients in the VRd and VPd groups, p = 0.449. None of the patients had a double or triple hit. The entire study population had been followed for a median period of 22 months (with an observed range of 9 to 43 months). 15% of all participants (n = 7) underwent ASCT, 6 (24%) in VRd arm and 1(4.8%) in VPd arm. Median time to transplant from induction start was 7 months (5–9months).
Their responses to Chemotherapy
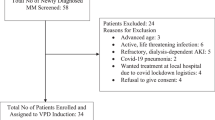
The induction response evaluation was conducted on all 46 patients who underwent VRd or VPd treatment (Fig. 1). In the VRd arm, 92% (23) of the patients achieved an overall response rate compared to 100% (21) in the VPd group (p = 0.113), which represents the study’s primary endpoint. Furthermore, 52% (13) of the patients in the VRd arm achieved at least VGPR, while this was observed in 90.47% (19) of those receiving VPd treatment (p = 0.003). In the VPd group, five patients (23.81%) achieved sCR, seven (33.33%) achieved CR, seven (33.3%) achieved VGPR, and two (9.52%) achieved PR. In the VRd group, four patients (16%) achieved sCR, six (24%) achieved CR, three (12%) achieved VGPR, ten patients (40%) achieved PR, and two (8%) achieved NR (no response).
Outcome
The mean follow-up duration was 22 months for the entire cohort, with a range of 9 to 43 months. The VRd survivors were monitored for an average period of 27 months (11 to 43 months), whereas it was 21 months (range: 9–33 months) for those in the VPd group. The median OS and PFS of VRd group and VPd group are not defined. The 22-month follow-up period was selected as the median follow-up period, three patients succumbed to mortality (VRd, n = 2; VPd, n = 1), while six instances of disease progression occurred (VRd, n = 5; VPd, n = 1). The 1-year OS was 92% for VRd and 90.5% for VPd (p = 0.801), 1-year PFS was 84% for VRd and 90.5% for VPd (p = 0.116). In order to exclude the effect of ASCT on survival, we excluded these patients and obtained 1-year OS: VRd: 89.5%, VPd: 90% (p = 0.425), 1-year PFS: VRd: 78.9%, VPd: 90% (p = 0.047). The corrected data provide evidence that a deep response to pomalidomide prolongs PFS, Patients continue on long term follow up for overall survival. During the induction phase, two patients deceased during the course of treatment with VRd. One of these patients died as a result of disease progression, while the other was lost to follow-up, and one patient was lost to follow-up (Fig. 2).
AE
The incidence of hematologic adverse events did not differ significantly across the two groups, as indicated in Table 2. For hematologic AEs, the most common in VRd and VPd group was bone marrow suppression (VRd, n = 4 vs. VPd, n = 2, p = 0.511). The most prevalent non-hematologic AE observed in the VRd group was peripheral neuropathy (n = 5, 20%), followed by fatigue (n = 1, 4%), infection (n = 1, 4%), and gastrointestinal symptoms (n = 1, 4%). In the VPd group, peripheral neuropathy (n = 4, 19%) was identified as the most prevalent non-hematologic adverse event, followed by fatigue (n = 1, 4.8%), infection (n = 1, 4.8%), and gastrointestinal symptoms (n = 1, 4.8%). The VPd group exhibited a significantly higher incidence of skin rashes compared to the VRd group (p = 0.024). No patients receiving VRd or VPd experienced AEs necessitating treatment discontinuation. Additionally, no incidences of second primary cancers were observed in either the VRd or VPd treatment arms.
Discussion
In recent years, the advent of novel targeted therapeutics, including immunomodulator (IMiD), proteasome inhibitors (PIs), histone deacetylase inhibitors (panobinostat) and monoclonal antibodies. The introduction of these novel agents has enabled a more effective treatment regimen, leading to a greater number of patients experiencing deeper responses and, consequently, a longer survival period10. However, HRNDMM patients still succumb to premature mortality as a result of disease progression11.
In this study, we compared the current standard triplet induction VRd with triplet induction VPd. Even though there wasn’t significant disparity in statistical terms in ORR amidst the two cohorts, patients in the VPd group achieved a significantly higher proportion of ≥ VGPR compared to those in the VRd cohort (90.47% vs. 52%, p = 0.003). Taking a retrospective approach, the response rates for both the VRd and VPd groups were found to be comparable to those reported in previous studies, thus affirming the credibility of our data. Studies have reported that the response rates of patients who have achieved a ≥ VGPR following the induction phase with VRd range from 57.1%12 to 70.4%13, showcasing a range of impressive outcomes across different studies.
However, it should be noted that these patients do not exclusively belong to the high-risk category. In the Gaballa MR et al. study14, high-risk patients exhibited remarkable response rates with the VRd regimen after four cycles of induction, demonstrating an ORR of 93.1% and a ≥ VGPR rate of 65.6%. In our study, a remarkable 52% of sufferers in the VRd achieved levels of ≥ VGPR following post-induction, while 92% of patients in the VRd group achieved an ORR, consistent with the aforementioned findings.
The existing data regarding the utilization of pomalidomide in HRNDMM is notably scarce. In the POMACE Phase II Study8, a total of 31 patients who had completed at least 4 cycles of induction were included in the analysis in order to assess response rates. The ORR rate of 100% was observed, with 87% of patients achieving a ≥ VGPR, the data indicates that the utilization of VPd in induction chemotherapy is both secure and effective for the treatment of NDMM. Li CC et al.15 enrolled twelve high-risk NDMM patients using VPd induction, an ORR was 100%, 77.8% patients achieved ≥ VGPR, think that VPd is efficacious and safe in HRNDMM. In this study, the ORR was achieved by 92% (23) of patients receiving VRd compared to 100% (21) of patients receiving VPd (p = 0.113). Furthermore, a significant difference was observed in achieving ≥ VGPR, with 52% (13) of patients achieving ≥ VGPR in the VRd group versus 90.47% (19) in the VPd group (p = 0.003). These findings suggest that induction therapy with VPd leads to superior and more profound responses than VRd induction in HRNDMM.
At a median follow-up of 22 months, the two treatment regimens demonstrated comparable PFS rates, with 27 months observed in the VRd group and 20 months in the VPd group (P = 0.116) (Fig. 3). The OS in the VRd group was 27 months, while it was 21 months in the VPd group (p = 0.801) (Fig. 2). The median OS and PFS were not defined. This observation may be attributed to the retrospective nature of this study and the relatively short duration of follow-up. During the follow-up period, a higher number of deaths/ loss to follow-up occurred in the VRd group compared to the VPd group (VRd, n = 2; VPd, n = 1), and six patients experienced disease progression (VRd, n = 5; VPd, n = 1).
The prevalence of severe or moderate renal impairment (RI) at the time of diagnosis was more prevalent in the VPd cohort as compared to patients undergoing VRd induction, which corresponds to a greater proportion of patients exhibiting elevated levels of creatinine within this particular cohort (creatinine level ≥ 110µmol/L: VPd 47.6% (n = 10) vs. VRd 12% (n = 3), P = 0.02). The therapeutic options for MM are constrained by RI, with approximately 15–50% of MM patients presenting with varying degrees of renal failure at the time of diagnosis13. Additionally, approximately 25–50% of patients diagnosed with MM are known to encounter varying degrees of renal impairment over the course of their illness16, which is the outcome of chronic nature and progressive advancement of the disease. The elimination of lenalidomide in urine occurs in its unchanged form, and may increase nonrenal toxicities16. Therefore, dose adjustments are necessary in patients with RI17.
The primary metabolic pathway of Pomalidomide takes place in the liver, with a mere 2% being excreted in an unaltered state via the urinary system. Therefore, there is no necessity to adjust the treatment dosage based on renal function17. Pomalidomide has been shown to have consistent clearance and plasma exposure in multiple myeloma patients with varying degrees of renal impairment, even in cases of moderate to severe renal dysfunction. This suggests that the presence of renal impairment does not significantly impact the pharmacokinetics of pomalidomide18. The MM-013 study19, enrolled in this study were three distinct patient cohorts: those with moderate RI (cohort A; estimated glomerular filtration rate (eGFR) of 30 to less than 45 mL/min/1.73 m2), individuals with severe RI (cohort B; eGFR < 30 mL/min/1.73 m2), and patients with severe RI necessitating hemodialysis (cohort C). The ORR was 39.4% in cohorts A, 32.4% in cohorts B, and only 14.3% in cohorts C. It can be concluded that the administration of POM + LoDEX is both administered with utmost safety and demonstrated to exhibit remarkable efficacy in patients diagnosed with RRMM and experiencing moderate to severe RI, including patients undergoing hemodialysis. In our study, approximately one half of the patients in the VPd group exhibited renal insufficiency (creatinine level ≥ 110µmol/L), and they achieved a remarkable ORR of 100%, which is comparable to that observed in the entire trial population. These findings provide support for the efficacy of pomalidomide treatment in patients with both normal and impaired r kidney function.
Another challenge in treating patients with NDMM is that high-risk MM patients exhibit chemotherapy resistance, easy to progress, and poor prognosis, approximately 10–20% of these patients are susceptible to premature mortality within 2–3 years following their diagnosis20. In our study, high-risk was delineated by the existence of at least one of the following anomalies: deletion 17p, t (4;14), t (14;20), t (14;16), or EMD, We enrolled 46 patients with HRNDMM; Among them, twenty-eight patients had 17p deletion (VRd, n = 14; VPd, n = 14), and sixteen patients had t (4;14): VRd, n = 10; VPd, n = 6, three patients had EMD (VRd, n = 1; VPd, n = 2); It is reported that the translocation (4;14) is found in about 15% of patients with NDMM, while Del(17p) is observed in 5–10% of patients upon initial diagnosis11. Within VRd group, 52% of the patients achieved at least VGPR vs. 90.47% receiving VPd. Therefore, it is hypothesized that pomalidomide may enhance the likelihood of achieving deep remission. Fu W J et al.6, twenty-five high-risk patients were enrolled, after 2 cycles of pomalidomide-containing regimens, the ORR stood at 36.0% among patients with RRMM who exhibited high-risk cytogenetics, may be the patients were relapsed and refractory, ORR was significantly lower than the high-risk patients in our study. These findings imply that pomalidomide may confer benefits to patients with MM, and potentially mitigate the adverse effects of high-risk cytogenetics in this patient population.
Extramedullary disease (EMD) remains a formidable challenge from both a biological perspective as well as a therapeutic standpoint21, due to the fact that the underlying pathogenetic pathways remain somewhat unclear. Moreover, patients with EMD are classified as high-risk MM patients, presenting more negative results and prognoses. Among a cohort of 46 patients diagnosed with NDMM, only three individuals presented with EMD at initial diagnosis, exclusively involving soft tissue sites. Unfortunately, no subgroup analysis was conducted. When compared to traditional chemotherapy (VAD: vincristine-doxorubicin-dexamethasone), new medicines (thalidomide/lenalidomide-bortezomib-based regimens) have been shown to work better in treating new EMD patients, with higher rates of complete response (12/23 vs. 2/21, P < 0.02)22. It has been shown by the Medical Research Council XI Group23 that patients at high risk do not benefit from lenalidomide maintenance at the same rate as patients at normal risk. Short KD et al.24, demonstrated in their study that pomalidomide, a novel immunomodulatory drug, elicits a response rate of approximately 30% for EMD patients. Our findings further support the notion that pomalidomide can provide partial benefits to patients with EMD.
As for AEs, it should be noted that the same level of rigor was not employed for their collection as would be the case in clinical trials due to the retrospective nature of the investigation. Analysis was limited to AEs that were recorded in the electronic medical record (EMR). Between the two groups, there were no appreciable variations in hematologic AE (Table 2). The most common hematological AE observed was bone marrow suppression (16% vs. 9.6%, p = 0.511). Peripheral sensory neuropathy (20% vs. 19%, p = 0.935) were the most common nonhematological AE, none of these adverse events had an impact on patients’ quality of life. Patients receiving VPd treatment exhibited a higher rate of skin rashes (p = 0.024), but none required dose modification of pomalidomide. One case of infection occurring during VRd therapy was considered to be caused by myelosuppression following chemotherapy, while one infection during VPd therapy could not be definitively attributed to neutropenia.
Conclusion
In conclusion, our results suggest that both VRd and VPd induction regimens may be effective in treating HRNDMM. However, our data indicate that VPd induction leads to superior and more profound responses compared to VRd induction. There were minimal discrepancies in terms of PFS and OS across the two groups. This study provides further corroboration of the efficacy of VPd as a front-line therapeutic intervention for patients diagnosed with HRNDMM, highlighting the potential clinical benefits of pomalidomide. Importantly, this study offers clinically relevant information for physicians and patients dealing with HRNDMM. One of the most evident limitations of this study is the potential for selection bias, which arises from the retrospective nature of the research and insufficient number of samples included. Consequently, in order to ascertain the true survival outcomes, it is necessary to conduct studies utilizing larger samples and longer follow-up periods.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Siegel, R. L. et al. Cancer statistics, 2022. Cancer J. Clin. 72 (1), 7–33 (2022).
Kumar, S. K. et al. Multiple myeloma. Nat. Rev. Dis. Primers. 3 (1), 17046 (2017).
Mikhael, J. et al. Treatment of multiple myeloma: ASCO and CCO Joint Clinical Practice Guideline. Clin. Oncol. 37 (14), 1228–1263 (2019).
Sonneveld, P. et al. Treatment of multiple myeloma with high-risk cytogenetics: A consensus of the International Myeloma Working Group. Blood 127 (24), 2955–2962 (2016).
Dimopoulos, M. A. et al. Multiple myeloma: EHA-ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 32 (3), 309–322 (2021).
Fu, W. J. et al. Efficacy and safety of pomalidomide and low-dose dexamethasone in Chinese patients with relapsed or refractory multiple myeloma: a multicenter, prospective, single-arm, phase 2 trial. BMC Cancer. 22 (1), 722 (2022).
Lopez-Girona, A. et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia 26 (11), 2326–2335 (2012).
Saj, F. et al. Efficacy and safety of pomalidomide, bortezomib, and dexamethasone combination chemotherapy for newly diagnosed multiple myeloma: POMACE phase II study. Blood Cancer. 13 (1), 45 (2023).
Kumar, S. et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 17 (8), e328–e346 (2016).
Joseph, N. S., Gentili, S., Kaufman, J. L., Lonial, S. & Nooka, A. K. High-risk multiple myeloma: Definition and management. Clin. Lymphoma Myeloma Leuk. 17S, S80–S87 (2017).
Hanamura, I. Multiple myeloma with high-risk cytogenetics and its treatment approach. Int. J. Hematol. 115 (6), 762–777 (2022).
Attal, M. et al. Lenalidomide, Bortezomib, and dexamethasone with transplantation for Myeloma. N. Engl. J. Med. 376 (14), 1311–1320 (2017).
Rosiñol, L. et al. Bortezomib, lenalidomide, and dexamethasone as induction therapy prior to autologous transplant in multiple myeloma. Blood 134 (16), 1337–1345 (2019).
Gaballa, M. R. et al. KRD vs. VRD as induction before autologous hematopoietic progenitor cell transplantation for high-risk multiple myeloma. Bone Marrow Transpl. 57 (7), 1142–1149 (2022).
Li, C. C., Zhang, W. Y. & Zhang, R. J. Early Efficacy Observation of Pomalidomide-based Regimen in the treatment of high-risk multiple myeloma. Zhongguo Shi Yan xue ye xue Za Zhi. 31(5):1432–1436. (2023).
Chanan-Khan, A. A., San Miguel, J. F., Jagannath, S., Ludwig, H. & Dimopoulos, M. A. Novel therapeutic agents for the management of patients with multiple myeloma and renal impairment. Clin. Cancer Res. 18 (8), 2145–2163 (2012).
Hoffmann, M. et al. Absorption, metabolism and excretion of [14 C]pomalidomide in humans following oral administration. Cancer Chemother. Pharmacol. 71 (2), 489–501 (2013).
Fotiou, D., Gavriatopoulou, M., Terpos, E. & Dimopoulos, M. A. Pomalidomide- and dexamethasone-based regimens in the treatment of refractory/relapsed multiple myeloma. Ther. Adv. Hematol. 13, 20406207221090089 (2022).
Sonneveld, P. et al. MM-013 phase 2, Multicenter Study of Pomalidomide (POM) plus low-dose dexamethasone (LoDEX) in patients (pts) with Relapsed/Refractory multiple myeloma (RRMM) and renal impairment (RI): pharmacokinetics (PK) analysis. Blood 130, 1847 (2017).
Rajkumar, S. V. Multiple myeloma: 2020 update on diagnosis, risk-stratification and management. Am. J. Hematol. 95 (5), 548–567 (2020).
Bansal, R., Rakshit, S. & Kumar, S. Extramedullary disease in multiple myeloma. Blood Cancer J. 11 (9), 161 (2021).
Kumar, L. et al. Multiple myeloma with extramedullary disease: impact of autologous stem cell transplantation on outcome. Bone Marrow Transpl. 52 (10), 1473–1475 (2017).
Jackson, G. et al. Outcomes of transplant-eligible newly diagnosed Ultra-high Risk Myeloma patients treated in the NCRI Myeloma XI Trial Indicate the need for early treatment stratification and Novel Treatment approaches. Blood 134 (Supplement_1), 604–604 (2019).
Short, K. D. et al. Incidence of extramedullary disease in patients with multiple myeloma in the era of novel therapy, and the activity of pomalidomide on extramedullary myeloma. Leukemia 25 (6), 906–908 (2011).
Acknowledgements
The authors gratefully acknowledge Bengbu Medical University, Anqing Municipal Hospital their irreplaceable support in this study. Additionally, we thank all study contributors, data collectors.
Funding
This work was supported by Anqing City Science and Technology Bureau (2021Z2005).
Author information
Authors and Affiliations
Contributions
L.C.Y.: Original draft, Validation, Data curation; Z.L.: Writing – review & editing, Project administration, Supervision, Validation; C.D.G.: Writing – review & editing, Formal analysis, Validation; Y.F.S.: Writing – review & editing, Formal analysis, Validation; Y.H.: Writing – review & editing, Conceptualization, Resources;
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, C.Y., Zhong, L., Chen, D.G. et al. VRd vs. VPd as induction therapy in high risk newly diagnosed multiple myeloma. Sci Rep 15, 9946 (2025). https://doi.org/10.1038/s41598-025-85398-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-85398-0