Abstract
The purpose of this study was to present the surgical technique of Unilateral Biportal Endoscopic (UBE) decompression combined with percutaneous pedicle screws for the treatment of thoracolumbar burst fractures with secondary spinal stenosis. Thoracolumbar burst fracture is a common traumatic disease in spinal surgery. In the Arbeitsgemeinschaft für Osteosynthesefragen (AO) classification of thoracolumbar fractures, Type A fractures have the highest incidence, accounting for about 70%, with A1 and A3 types being the most common1. In Type A3 fractures, there is often a displacement of fracture fragments into the spinal canal, leading to secondary spinal stenosis. The traditional approach is posterior open surgery pedicle screws combined with direct visualization for decompression, which requires extensive stripping of paravertebral muscles and resection of more bone, and is more traumatic2, which is not in line with the current development concept of minimally invasive spine. The UBE technique in spinal endoscopy is currently a hot spot in the development of minimally invasive spine3, and we attempted to utilize UBE decompression combined with percutaneous pedicle screws to treat thoracolumbar burst fracture with spinal stenosis, which provides a new option for the surgical treatment of thoracolumbar burst fracture with secondary spinal stenosis. We included five patients with thoracolumbar burst fractures with secondary spinal stenosis admitted to our hospital between January 2023 and January 2024, who were treated with UBE decompression combined with percutaneous pedicle screw internal fixation by our team. The degree of correction of spinal deformity was assessed using the sagittal Cobb angle and the percentage of height of the anterior margin of the vertebral body, the rate of canal encroachment was used to assess the decompression of the spinal canal, and the recovery of the patients’ ability to live was assessed using the Visual Analogue Scale (VAS) and Japanese Orthopaedic Association (JOA) Score. The results showed that the average operative length of the patients was 154.2 min, and the average intraoperative bleeding was 90 ml; the sagittal Cobb angle averaged 22.23° preoperatively, and 6.10° at 3 days postoperatively; the anterior vertebral body height ratio averaged 36.77% preoperatively, and 91.16% at 3 days postoperatively; and the residual spinal canal volume averaged 52.01% preoperatively, and 91.58% at 3 days postoperatively; VAS score averaged 7 preoperatively and 2 at 3 days postoperatively; JOA score averaged 8.4 preoperatively and 22.4 at 3 days postoperatively. UBE decompression combined with percutaneous pedicle screws is effective in the treatment of thoracolumbar burst fractures with secondary spinal stenosis and is a safe, minimally invasive surgical option for this patient population.
Similar content being viewed by others
Introduction
Thoracolumbar burst fracture is a common traumatic disease in spinal surgery, and its pathogenesis is mainly due to fall injuries, traffic injuries, and heavy object injuries, and the incidence of the population is mainly for young adults, which often leads to short-term loss of labor, and some patients are even left with long-term neurological impairments, which has brought a heavy economic burden to the patient’s family and society4,5. Some patients with thoracolumbar burst fractures often have spinal stenosis secondary to displacement of the fracture fragment into the spinal canal or injury to the ligamentum flavum, and often require surgical treatment to re-establish spinal stability because of their severely impaired spinal stability.
The traditional surgical approaches for thoracolumbar fractures with secondary spinal stenosis are posterior approach surgery, anterior approach surgery, or combined anterior and posterior approaches6. If reconstruction of the anterior column is not involved, posterior approach pedicle screw internal fixation combined with spinal canal decompression is usually chosen. Traditional open surgery has a long incision, more bleeding, and more damage to the paravertebral muscles, and some patients have long-term residual low back pain7. In order to avoid the shortcomings of traditional open surgery, spine surgeons are now gradually applying minimally invasive spinal techniques to the treatment of thoracolumbar fractures, such as percutaneous pedicle screw technique, Wiltse approach nailing technique in the thoracolumbar spine, microscope-assisted spinal decompression technique, and spinal endoscopic decompression technique8.
The UBE technique is currently a hot technique for minimally invasive spine surgery, and it has been gradually accepted and favored by spine surgeons worldwide since it was proposed in 2003 due to its high flexibility and safety. Currently, the UBE technique has been routinely used to manage degenerative diseases of the cervical, thoracic and lumbar spine, such as cervical spondylosis, thoracic ligamentum flavum ossificans, lumbar disc herniation, lumbar spinal stenosis, etc9. With the gradual maturation of UBE technology, its indications have been gradually expanded, and in recent years, UBE technology can be seen to be applied to spinal tumors, spinal infections, and traumatic diseases of the spine10.
Percutaneous pedicle screw technique is another minimally invasive technique in spinal surgery, which avoids extensive stripping of the posterior spinal muscles, reduces blood loss, decreases complications, and shortens recovery time compared with the traditional open pedicle screw nailing technique11. We treated some patients with thoracolumbar burst fractures with secondary spinal stenosis using UBE decompression combined with percutaneous pedicle screw technique, and here we performed a retrospective analysis to evaluate its clinical efficacy and summarize the technical points.
Materials and methods
Participants
This study was a single center, retrospective study. Cases were collected from January 2023 to January 2024. The study complied with the basic principles of medical ethics set forth in the Declaration of Helsinki and was reviewed and approved by the Human Research Ethics Committee of the Fourth Affiliated Hospital of Zhejiang University School of Medicine (approval number: K2024131). All patients were informed in detail about their medical conditions and available surgical options, and enrolled patients voluntarily chose the surgical options of UBE decompression and percutaneous pedicle screw internal fixation, and signed an informed consent for surgery. Inclusion criteria: (1) Thoracolumbar burst fracture due to trauma, Thoracolumbar injury classification and severity score (TLICS) > 4, Load Sharing Classification (LSC) score < 7. (2) Fracture secondary to significant stenosis of the spinal canal, i.e. significant ventral compression of the nerve due to displacement of the fracture fragments or dorsal compression of the nerve due to injury to the facet joint or folding of the ligamentum flavum. (3) Those with complete follow-up data. Exclusion criteria: (1) pathological fractures or the presence of severe osteoporosis. (2) the presence of severe cardiac, pulmonary, cerebral and renal dysfunction that could not tolerate surgery. (3) the presence of localized or systemic infections. (4) incomplete follow-up data or loss to follow-up. Based on the above criteria, five patients were enrolled, and we summarize the main points of this technique.
Surgical procedures
All the patients were put under tracheal intubation combined with general anesthesia, and after successful anesthesia, the patients were placed in prone position on the surgical bed. Silicone pads were used to keep the patient’s abdomen in suspension, with the aim of reducing intra-abdominal pressure and minimizing bleeding in the surgical area; the patient was kept in a mildly flexed hip and knee position, with the aim of decreasing the tension of the muscles of the lumbar and back in order to facilitate the placement of pedicle screws. The surgical procedure is shown in Fig. 1.
General view of the surgical procedure. A: The patient was lying prone on the surgical bed. B: Bilateral vertebral pedicle projection points were localized and marked under C-arm fluoroscopy. C: Percutaneous pedicle screws were placed under C-arm fluoroscopy, and decompression was performed under the UBE endoscopic system. D: Drainage was placed in the decompressed area, and the incision was sutured.
Contralateral placement of pedicle screws
After the patient was positioned, 5 Kirschner’s needles were placed in the surgical area, and the C-arm fluoroscopic anteroposterior X-ray of the surgical area was performed to confirm the bilateral pedicle projections of the target segment. If the pedicle projection was considered as a clockwork disk, the body positioning point was located at about 1.5 cm lateral to the pedicle projections of the “2 points and 10 points” (Fig. 2A).
Procedure of percutaneous pedicle screw placement. A: Kirschner’s needle was used for surface localization of the pedicle screw entry point. B: Puncture was performed under C-arm surveillance; this was an anteroposterior X-ray image. C: Puncture was performed under C-arm surveillance; this was a lateral X-ray image. D: Pedicle screws were screwed along the guidewire on the non-decompressed side, the guidewire was fixed on the decompressed side, and subsequent endoscopic decompression operation was carried out.
The puncture was performed with the assistance of C-arm fluoroscopy, the guidewire was placed (Fig. 2B, C), and the pedicle screw of the appropriate length was screwed in after flaring along the guidewire. It should be noted that the pedicle screws on the side where the decompression is planned should not be screwed in first, and the guidewire should be secured to the surgical sheet using a small patch so that it does not interfere with subsequent endoscopic decompression of the spine (Fig. 2D).
Decompression of the spinal canal under UBE, direct repositioning of the fracture fragment
After the insertion of contralateral pedicle screws, a connecting rod is placed, and after slight distraction, the tail caps are tightened to maintain a certain height of the injured vertebra.
C-arm fluoroscopy was used to determine the two incisions of the UBE decompression: the observation incision and the operation incision, which were set at the intersection of the medial margin tangent and the inferior margin tangent of the pedicle projection. Typically, the incisions for UBE decompressions are closer to the midline than the incisions for percutaneous pedicle screws. The operative channel was prepared with the use of a sequential dilator with the assistance of the C-arm, followed by the connection of a spinal endoscopic imaging system, a continuous irrigation system, and a plasma radiofrequency tip.
The following procedure was performed under spinal endoscopy.
Step one soft tissue clean-up
The plasma radiofrequency tip was used to remove soft tissue on the lamina and in the interlaminar space, with sufficient clean-up to facilitate subsequent bony decompression.
Step two spinal decompression
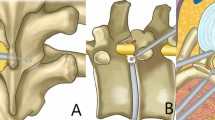
The junction of the lamina and spinous process of the superior vertebra was used as the starting point for bony decompression (marked by a star in Fig. 3A). The lower edge of the lamina and the inner edge of the inferior articular process of the superior vertebral body were partially abraded in a counterclockwise direction using an endoscopic grinding (Fig. 3A), and the lower edge of the upper lamina was exposed until the cephalic end of the ligamentum flavum was reached, and the inner edge of the inferior articular process was exposed until the inner edge of the superior articular process was revealed below. Subsequently, we returned to the starting point and abraded the base of the spinous process, the lower edge of the contralateral lamina, and part of the bone of the inner edge of the contralateral lower articular process in a clockwise direction (Fig. 3B), with the same extent of abrasion on the ipsilateral; then we removed the superficial ligamentum flavum and abraded the upper edge of the lower lamina with a endoscopic grinding (Fig. 3C) until the caudal stop of the ligamentum flavum was exposed, and then we finished the bony decompression.
Schematic diagram of the process of spinal decompression. A: Used the junction of the ipsilateral lamina and the spinous process as the starting point, the bone was abraded in a counterclockwise direction. B: Returned to the starting point, the root of the spinous process and the contralateral portion of the bone were abraded in a clockwise direction. C: Abraded a portion of the upper edge of the lower lamina.
The ligamentum flavum was gradually bitten off with laminectomy forceps to access the spinal canal.
Step three repositioning of the fracture fragment
The dural sac was moderately pulled to the midline by the nerve root retractor, at this time, the fracture fragment invading into the vertebral canal could be directly visualized, and the “L”-shaped fracture reset device was placed against the fracture fragment, and the end of the reset device was struck by the hammer, and the fracture fragment was gradually struck ventrally, and the reset device was at the same height with the posterior edge of the vertebral body under C-arm fluoroscopy in the lateral position, the reset device was taken out, and the nerve root retractor was released, and no obvious fracture fragment pressing on the ventral side of the dura, and the repositioning of the fracture fragment was finished.
Step four ipsilateral placement of pedicle screws
The spinal endoscopy system was removed, the appropriate length pedicle screws were screwed along the pre-positioned guide wires, the connecting rod was put in, and the tail caps were screwed in for fixation. C-arm fluoroscopy was used to confirm that the screw position was satisfactory and that the fracture fragment had not been displaced again, and one drainage tube was placed in the decompression operation incision, and the incisions were closed layer by layer, and the operation was completed.
Step five post-operative treatment
After the operation, the patient was given oral non-steroidal drugs for analgesia, bed rest until the drainage tube was removed, wearing a waist girdle and gradually moving down to the ground, followed by a review of the X-ray, CT and MRI of the operation area.
Data collection
General data of the patients included gender, age, fracture segment, cause of injury, AO typing of the fracture, duration of surgery, intraoperative blood loss, and postoperative drainage. The patients were scored on VAS and JOA scales 1 day preoperatively and 3 days postoperatively. Sagittal Cobb angle and vertebral anterior margin height ratio were measured on preoperative and postoperative X-rays, and residual spinal canal volume was measured on preoperative and postoperative fracture level CT cross-sections of the patients. All of the above data were collected by 2 specialized spine surgeons.
Statistical analysis
All values are expressed as mean ± standard deviation, and comparisons between means were made using paired t-tests, with P < 0.05 considered statistically significant. Imaging images were intercepted by RSVSviewer software.
Results
Demographic data
A total of 5 cases were included in this study, 3 males and 2 females, aged 28–64 years, fracture segments T11-L3, type of injuries were high fall and car accident injuries, and AO typing was A3 and B2 + A3 (Table 1).
Perioperative data
We statistically obtained the duration of surgery for the patients included in the study to be 122–188 min, intraoperative bleeding to be 50–120 ml, postoperative drainage to be 35–152 ml, postoperative drain placement time to be 22–41 h, length of hospitalization to be 7–10 days, and follow up time to be 4–8 months (Table 2).
Evaluation of spinal deformity correction
The preoperative vertebral anterior margin height ratio was (36.77 ± 3.23)% and the postoperative vertebral anterior margin height ratio was (91.16 ± 3.69)% (for measurements, see Fig. 4A, B), and the postoperative vertebral anterior margin height ratio was greater than that of the preoperative period, P < 0.0001, and the difference was statistically significant (see Fig. 6A for results); the preoperative vertebral cobb angle was (22.23 ± 2.7)°, the postoperative vertebral cobb angle was (6.10 ± 1.24)° (for measurements, see Fig. 4C, D), the postoperative cobb angle was significantly lower than the preoperative one, P < 0.0001, the difference was statistically significant (see Fig. 6B for results).
Preoperative and postoperative residual vertebral canal volume ratios. A: Vertebral canal volume of the superior vertebrae of the injured vertebrae. B: Vertebral canal volume of the inferior vertebrae of the injured vertebrae, with the expected vertebral canal volume of the injured vertebrae calculated from the vertebral canal volumes of the superior and inferior vertebrae of the injured vertebrae shown in the upper right corner. C: Preoperative residual vertebral canal volume and residual canal volume ratios. D: Postoperative residual vertebral canal volume and residual canal volume ratios.
Vertebral volume improvement results
We used residual spinal canal volume to assess the enlargement of the spinal canal before and after surgery (see Fig. 5 for measurements). The results showed that the preoperative predicted residual spinal canal volume ratio was (52.01 ± 4.83) % and the postoperative residual spinal canal volume ratio was (91.58 ± 2.18) %, and the postoperative residual spinal canal volume ratio was greater than the preoperative one, with a statistically significant difference of P < 0.0001 (see Fig. 6C for results).
Pre- and postoperative imaging and functional scoring results. A: Pre- and postoperative changes in vertebral anterior margin height ratio. B: Pre- and postoperative changes in cobb angle. C: Pre- and postoperative changes in residual volume ratio of the spinal canal. D: Preoperative and 3-day postoperative VAS scoring results. E: Preoperative and 3-day postoperative JOA scoring results.
Improved outcomes in spinal function
We assessed the patients’ pain improvement using the VAS scale and spinal function improvement using the JOA scale. The results showed that the patients’ preoperative VAS score was (7.0 ± 1.22), and the 3-day postoperative VAS score was (2.0 ± 0.70), which was lower than that of the preoperative one, with P < 0.0001, and the difference was statistically significant (see Fig. 6D); the preoperative JOA score was (8.4 ± 1.14), and the 3-day postoperative JOA score was (22.4 ± 2.07), which was higher than that of the preoperative one, with P < 0.0001, and the difference was statistically significant (see Fig. 6E).
Typical case
A 56-year-old male with L3 vertebral body fracture caused by a fall from a height of 3 m, AO classification of B2 + A3, underwent UBE decompression + percutaneous pedicle screw internal fixation 3 days after the injury, with a drainage tube placed in the operative area for 35 h after the surgery, and he went down to the ground gradually after the removal of the drainage tube, and the CT of the operative area was rechecked 3 days after the surgery (see Fig. 7 for preoperative and postoperative images), and he was discharged from the hospital 7 days after the surgery, and is currently under follow-up for 7 months (see Fig. 8 for follow-up images).
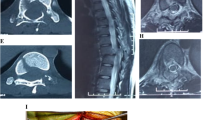
56-year-old man with L3 vertebral body fracture secondary to spinal stenosis, early results of UBE decompression with percutaneous pedicle screw internal fixation. A: Preoperative CT sagittal image showed a burst fracture of the anterior column of the vertebral body. B: Preoperative CT axial image did not show a fracture fragment encroaching on the spinal canal. C: Preoperative MRI axial image showed spinal stenosis due to injury to the facet joints, and folding of the ligamentum flavum. D: Preoperative MRI sagittal image showed an injury to the interspinous ligaments of the L3/4 and a stenosis of the spinal canal at the L3/4 level. E: Recovery of the height of the anterior column of the vertebral body was seen on 3-day postoperative CT sagittal images. F: Significant enlargement of the spinal canal volume was seen on 3-day postoperative CT axial images.
Imaging results of the patient’s postoperative review at 2 and 6 months. A: Anteroposterior lumbar spine X-ray image at 2 months postoperatively. B: Lateral lumbar spine X-ray image at 2 months postoperatively, which showed that the vertebral body heights were well maintained, and the physiological anterior convexity of the lumbar spine was basically normal. C: 6-month postoperative lumbar MRI sagittal image showed good recovery of the interspinous ligament of L3/4 and patency of the spinal canal. D: Anteroposterior lumbar spine X-ray image at 6 months postoperatively. E: Lateral X-ray image of the lumbar spine 6 months after surgery, which showed that the fracture fragment of the anterior column of the vertebral body had healed and there was no loss of vertebral height. F: MRI axial image of the lumbar spine 6 months after surgery, which showed that the anterior and posterior diameters of the spinal canal were close to 1.2 cm, and there was no obvious spinal canal stenosis.
Discussion
Thoracolumbar fracture is a common traumatic disease in spinal surgery, the thoracolumbar vertebra is at the junction of thoracic kyphosis and lumbar anterior kyphosis, the stress is more concentrated12; at the same time, the vertebral body here loses the supportive and stabilizing effect of ribs, and the mobility is larger, so the thoracolumbar segment is a high incidence part of spinal fracture, accounting for about 40% of the overall spinal fracture. The type of injury is often traffic accident injury, high fall injury, and the injured people are often young adults, and the short-term loss of labor ability after injury brings a heavy burden to the family and the society13,14.
Currently, there is no uniform standard for the treatment of thoracolumbar fractures, and spine surgeons often develop treatment plans for thoracolumbar fractures based on fracture typing. Currently, the commonly used typing of thoracolumbar fractures includes Denis typing, AO thoracolumbar fractures typing, Thoracolumbar Injury Classification and Injury Level Scoring System (TLICS), and Load Sharing Classification (LSC)15. Denis typing was proposed by Denis in 1984 based on the three-column theory of the spine, combined with the fracture pattern, which categorizes thoracolumbar fractures into compression fracture, burst fracture, flexion distraction fracture, and fracture with subluxation. Denis typing is relatively simple and has limited clinical significance16. In 1994, Magerl et al. proposed the AO typing of thoracolumbar fractures based on the fracture stress mechanism and fracture stability, which is now widely used worldwide, but the typing is more complicated, dividing thoracolumbar fractures into 27 types, which is more difficult to master in practical clinical application17. In 1994, Thomas et al. published the LSC for spinal injuries, a scoring system based on the degree of vertebral compression, the degree of burst separation, and the degree of kyphosis, which is a good guideline for choosing surgical approach18. In 2005, the U.S. Spine Injury Study Group developed the TLICS based on fracture morphology, posterior longitudinal ligament complex (PLL) integrity, and neurologic status, which determines surgical or non-surgical treatment based on specific scores, and has good reproducibility and operability19.
In terms of the choice of treatment for thoracolumbar fractures, some of the current literature chooses to combine the TLICS score and the LSC score system to decide, in the TLICS score system < 4 points suggests that the patient be treated conservatively, ≥ 5 points suggests that the patient be treated surgically, and the score of 4 points selects surgical or conservative treatment according to the patient’s wishes. The LSC score was further used to determine the surgical approach, with a score of ≤ 6 choosing posterior short-segment fixation and a score of ≥ 7 choosing anterior surgery, and a combined anterior and posterior approach was feasible in cases of LSC ≥ 7 and posterior longitudinal ligament complex injury20,21. This surgical selection strategy is appropriate for most patients, and most spine surgeons agree with this strategy. However, some scholars believe that for TLICS score > 4, LSC score < 7, burst fracture of the anterior and middle column of the vertebrae, with significant displacement of the fracture fragment into the spinal canal resulting in significant neurological compression, the choice of posterior surgery in accordance with the criteria of the appeal is not appropriate. The reason is as follows: the anterior and middle vertebral columns bear about 80% of the axial stress transmission of the spine, and the mechanical transmission of the spine will change accordingly after the burst fracture of the anterior and middle vertebral columns, and the compressive stress that would have been transmitted axially along the anterior and middle columns of the vertebral body are converted into tension transmitted down the PLL with the facet joints as fulcrums, so the change of such mechanical conduction puts forward a higher requirement for the integrity of the PLL22. Open posterior surgery in this group of patients, if performed according to the traditional approach, will result in more or less damage to the posterior structures, causing a partial loss of PLL stability. Burst fractures of the anterior and middle columns of the vertebral body have already deprived the vertebral body of some of its support, and secondary injuries to the PLL have resulted in injuries to the posterior traction conduction pathways, so that the mechanical stability of the spine will be severely impaired. Therefore, some scholars advocate for an anterior approach to treat anterior and mid-column burst fractures with significant neural tissue compression, regardless of whether the LSC score exceeds 6 or not23.
However, the anterior approach to the thoracic and lumbar spine is more traumatic, with the presence of important blood vessels and nerves in the operative area, which is a higher risk24, and the anterior approach to the thoracolumbar segment needs to be considered as a thoracic obstruction. Therefore, in patients with thoracolumbar fractures with TLICS score > 4, LSC score < 7, burst fractures of the anterior and middle columns of the vertebral body, and significant compression of the neural tissues, we explored a minimally invasive posterior approach to minimize the harassment of the PLL and to preserve as much as possible the tension stability of the PLL.
The percutaneous pedicle screw technique is now widely accepted and utilized by spine surgeons globally. Currently, the methods for pedicle screw placement in posterior thoracolumbar spine surgery include open screw placement, the Wiltse approach, and percutaneous pedicle screws25. In cases of thoracolumbar fractures that do not require decompression of the spinal canal, the Wiltse approach can be considered to minimize the damage to the back muscle tissue. For cases that require spinal canal decompression, traditional open surgery involves open pedicle screw placement, which necessitates extensive stripping of the paraspinal muscles to expose the screw entry points. There is a possibility of damaging the joint capsule of the facet joints during the procedure, and postoperatively, there is a high risk of myofibrosis and fat degeneration, leading to a decline in local muscle function and a propensity for long-term low back pain26,27. The percutaneous pedicle screw technique for the treatment of traumatic spinal injuries was initially reported by Magerl in 1982. Later, Foley reported the use of percutaneous pedicle screws with the Sextant system in 2001. This method, known for its shorter learning curve and higher safety, became a good choice for spine trauma and gradually gained popularity28. It has been shown that percutaneous pedicle screws can achieve the same fixation and deformity correction as open screw placement and are less traumatic than open screw placement29. The choice between unidirectional and universal screws for percutaneous pedicle screws in the treatment of thoracolumbar fractures is currently a matter of debate. The Sextant system’s percutaneous pedicle screws are universal screws, which feature a “ball-and-socket structure” between the screw tail and the rod, allowing for a certain range of mobility. Studies have indicated that universal screws have a weaker resistance to flexion compared to unidirectional screws and are limited in their effectiveness in distraction reduction maneuvers, but their advantage lies in the ease of rod connection operations30.
We utilized unidirectional screws above and below the fractured vertebrae, and universal screws in the fractured vertebrae, achieving satisfactory correction of the deformity. The preoperative vertebral anterior margin height ratio increased from (36.77 ± 3.23) % to (91.16 ± 3.69) %, indicating a good recovery of the anterior vertebral body height, with no loss of height observed in the follow-up cases. The preoperative Cobb angle of the vertebral body decreased from (22.23 ± 2.7) ° to (6.10 ± 1.24) °, demonstrating a good correction of the kyphotic deformity.
Unidirectional screws are used above and below the injured vertebrae. During distraction reduction, the force of distraction can be transmitted from the screw tail to the screw body and the screw tip, completing the distraction reduction. If universal screws are used, due to the possibility of a certain angle between the screw body and the screw tail, when the screw tail is distracted, the screw body forms an angle with the screw tail. As a result, the distraction distance at the screw tip is less than that at the screw tail. Therefore, the distraction reduction effect of universal screws is not as effective as that of unidirectional screws. The unidirectional screws used above and below the injured vertebrae, once their tails are secured to the connecting rods, ensure that the tangent between the screws and the rods is perpendicular. This configuration maintains a constant tension at the tips of the screws, which is beneficial for the long-term maintenance of the anterior height of the injured vertebrae. The purpose of inserting universal screws into the injured vertebrae is to enhance the mechanical strength of the spine and provide additional support during the fracture healing process.
Currently, there are open decompression, microscopic decompression, and endoscopic decompression for spinal canal decompression, among which endoscopic decompression is a hot topic in spinal surgery31. Some studies have shown that endoscopic decompression can achieve the same effect as microscopic decompression. In 2011, Komp utilized total spinal endoscopic decompression for the first time to treat lumbar spinal stenosis, and achieved good therapeutic results32. Javier Quillo-Olvera reported a case of UBE decompression combined with vertebroplasty and percutaneous pedicle screw technique for the treatment of L5 vertebral fracture with good therapeutic results33. Huiming Yang utilized coaxial spinal endoscopy combined with percutaneous pedicle screws to treat 32 patients with thoracolumbar burst fractures with neurological injury symptoms and achieved good results34. Spinal endoscopes currently include coaxial endoscopes and non-coaxial endoscopes, and the non-coaxial endoscope is currently the main UBE technique. Decompression using coaxial endoscopy and UBE has been reported in the literature and both achieve good decompression results35,36, and the choice of endoscopic technique depends on the operator’s habits. Our center currently primarily utilizes UBE technique for decompression treatments. UBE decompression surgery has its unique advantages: (1) The working channel and observation channel are separate, which means that the surgical maneuvers are not limited by the channels or the endoscope, allowing for more flexible and natural operations. (2) Bilateral hand coordination, which is closer to conventional surgical procedures, results in a shorter learning curve. (3) The use of a water channel leads to a lower infection rate in the surgical area37. We utilized UBE decompression, and the residual spinal canal volume ratio of the cases included in the study increased from (52.01 ± 4.83) % preoperatively to (91.58 ± 2.18) %, indicating that decompression was effective.
The lessons learned from this study are summarized as follows: (1) Patient selection is recommended for those with TLICS score > 4 and LSC score < 7, as well as those with significant neurological compression due to displacement of the bone fragment after burst fractures of the anterior and middle columns of the vertebral body, or those with spinal stenosis secondary to facet joint injuries, ligamentum flavum injuries, or ligamentum flavum folds. (2) For the selection of percutaneous pedicle screws, it is recommended to use unidirectional screws for the cephalic and caudal ends and universal screws for the injured vertebrae. (3) The order of percutaneous pedicle screws and UBE decompression should be chosen according to the operator’s habit. (4) Precise decompression is selected at the site of the most severe spinal stenosis. The smaller the decompression range while ensuring the effectiveness, the more stability of the posterior column of the spine is preserved, with particular attention to not destroying more than 1/2 of the facet joint. (5) There is no pursuit of complete reduction of the fracture fragments. The spinal canal has a certain degree of remodeling capability38. Intraoperative exploration reveals that if there is no significant compression of the ventral side of the spinal cord, cauda equina, or nerve roots by the fracture fragments, it is sufficient. Over-pursuing the complete reduction of the posterior vertebral body fragments prolongs the surgery time and increases the risk of vascular and nerve damage.
The shortcomings of this study and the points worthy of further investigation are summarized as follows: (1) The injured vertebra was restored to its normal height after the repositioning, but a cavity was formed in the middle of the vertebral body, which was not filled with bone, forming an “eggshell-like” change. Some scholars believe that bone grafting through the pedicle can fill the cavity in the vertebral body, reduce the loss of the corrective angle of the spine in the later stage, and reduce the incidence of broken screws and connecting rods39; Some scholars have also argued that intravertebral bone grafting is unnecessary40. We found that the “eggshell-like” changes were still visible from 2 to 6 months after surgery (Fig. 8B, E), and longer follow-up may be needed to determine whether the intravertebral cavities have an effect on spinal function. Therefore, we will further follow up the changes of intravertebral cavities and conduct a controlled study with and without bone grafting at a later stage. (2) In the process of fracture healing, hematoma and inflammatory mediators at the fracture site in the early stage play an important role41, UBE uses water as the working medium, and continuous intraoperative water flushing reduces the inflammatory mediators in the operative area, and whether this affects fracture healing in any way needs to be explored further. (3) Since we did not perform bone graft fusion between the facet joints, transverse processes, or intervertebral spaces, we generally advise patients against the removal of pedicle screws in the later stage. The presence of pedicle screws in the body can cause stress shielding, and the long-term stability of the spine and the possibility of screw or rod breakage require further validation with larger sample sizes and longer follow-up periods.
In conclusion, the UBE decompression combined with percutaneous pedicle screw technique in the treatment of patients with thoracolumbar burst fractures with spinal canal stenosis has been confirmed to have good deformity correction ability, adequate spinal canal decompression, and excellent results in the near-term and mid-term follow-up, making it a minimally invasive and effective treatment for this type of disease.
Data availability
The datasets generated during and/or analysed during the current study are available throughout the manuscript.
References
Tanasansomboon, T. et al. Thoracolumbar Burst fracture without neurological deficit: review of controversies and current evidence of treatment. World Neurosurg. 162, 29–35. https://doi.org/10.1016/j.wneu.2022.03.061 (2022). Epub 2022 Mar 19. PMID: 35318156.
Kapoen, C., Liu, Y., Bloemers, F. W. & Deunk, J. Pedicle screw fixation of thoracolumbar fractures: conventional short segment versus short segment with intermediate screws at the fracture level-a systematic review and meta-analysis. Eur Spine J. 29(10), 2491–2504. (2020). https://doi.org/10.1007/s00586-020-06479-4. Epub 2020 Jun 11. PMID: 32529525.
Chu, P. L. et al. Global and Current Research Trends of Unilateral Biportal Endoscopy/Biportal Endoscopic Spinal Surgery in the treatment of lumbar degenerative diseases: a bibliometric and visualization study. Orthop. Surg. 14 (4), 635–643. https://doi.org/10.1111/os.13216 (2022). Epub 2022 Mar 16. PMID: 35293686; PMCID: PMC9002063.
Meyblum, J. et al. Management of thoracolumbar fracture in France. Analysis of practices and radiologic results of a cohort of 407 thoracolumbar fractures. Orthop Traumatol Surg Res. 106(6), 1203–1207. https://doi.org/10.1016/j.otsr.2020.02.023. Epub 2020 Aug 3. PMID: 32763012. (2020).
Giotta Lucifero, A., Bruno, N. & Luzzi, S. Surgical management of thoracolumbar junction fractures: an evidence-based algorithm. World Neurosurg. X. 17, 100151. https://doi.org/10.1016/j.wnsx.2022.100151 (2023). PMID: 36793355; PMCID: PMC9923224.
Lee, N. H., Kim, S. K., Seo, H. Y., Park, E. T. & Jang, W. Y. How should patients with a Thoracolumbar Injury classification and severity score of 4 be treated? J. Clin. Med. 10 (21), 4944. https://doi.org/10.3390/jcm10214944 (2021). PMID: 34768463; PMCID: PMC8584330.
Lu, Q., Kang, H., Xiong, C., Sun, C. & Xu, F. Percutaneous Uniplanar Pedicle Screw-Rod System with Injured Vertebra Fixation for Thoracolumbar Burst Fracture and Technique Notes. J Coll Physicians Surg Pak. 32(10), 1295–1299. (2022). https://doi.org/10.29271/jcpsp.2022.10.1295. PMID: 36205274.
Chen, L., Liu, H., Hong, Y., Yang, Y. & Hu, L. Minimally invasive decompression and Intracorporeal Bone Grafting Combined with Temporary Percutaneous short-segment pedicle screw fixation for treatment of Thoracolumbar Burst fracture with neurological deficits. World Neurosurg. 135, e209–e220 (2020). Epub 2019 Nov 28. PMID: 31786380.
Yuan, C., Wen, B. & Lin, H. Clinical analysis of minimally invasive Percutaneous treatment of severe lumbar disc herniation with UBE two-Channel Endoscopy and Foraminal single-Channel Endoscopy technique. Oxid. Med. Cell. Longev. 2022, 9264852. https://doi.org/10.1155/2022/9264852 (2022). PMID: 36275895; PMCID: PMC9584735.
Junjie, L., Jiheng, Y., Jun, L., Haixiong, L. & Haifeng, Y. Comparison of unilateral Biportal Endoscopy Decompression and microscopic decompression effectiveness in lumbar spinal stenosis treatment: a systematic review and Meta-analysis. Asian Spine J. 17 (2), 418–430. https://doi.org/10.31616/asj.2021.0527 (2023). Epub 2023 Feb 6. PMID: 36740930; PMCID: PMC10151631.
Momin, A. A. & Steinmetz, M. P. Evolution of minimally invasive lumbar spine surgery. World Neurosurg. 140, 622–626. https://doi.org/10.1016/j.wneu.2020.05.071 (2020). Epub 2020 May 17. PMID.
Gomez, G. I. et al. Thoracic and lumbar spine Injury: evidence-based diagnosis, management, and outcomes. Am. Surg. 90 (4), 902–910 (2024). Epub 2023 Nov 20. PMID: 37983195.
Anania, C. D. et al. Single-stage posterior transpedicular corpectomy and 360-Degree Reconstruction for thoracic and Lumbar Burst Fractures: technical nuances and outcomes. J. Neurol. Surg. Cent. Eur. Neurosurg. 84 (5), 489–497. https://doi.org/10.1055/s-0042-1743515 (2023). Epub 2022 Apr 6. PMID: 35388449.
Spiegl, U. J. et al. The conservative treatment of traumatic Thoracolumbar Vertebral fractures. Dtsch. Arztebl Int. 115 (42), 697–704. https://doi.org/10.3238/arztebl.2018.0697 (2018). PMID: 30479250; PMCID: PMC6280041.
Prajapati, H. P. & Kumar, R. Thoracolumbar fracture classification: evolution, merits, demerits, updates, and concept of stability. Br. J. Neurosurg. 35 (1), 92–97 (2021). Epub 2020 Jun 19. PMID: 32558596.
Vaněk, P., Kaiser, R., Saur, K. & Beneš, V. History, development and use of classification of thoracolumbar spine fractures. Rozhl Chir. English. (2020). Winter;99(1):15–21 https://doi.org/10.33699/PIS.2020.99.1.15-21. PMID: 32122135.
Santander, X. A. & Rodríguez-Boto, G. Retrospective evaluation of Thoracolumbar Injury classification system and Thoracolumbar AO Spine Injury Scores for the Decision Treatment of Thoracolumbar Traumatic Fractures in 458 consecutive patients. World Neurosurg. 153, e446–e453. https://doi.org/10.1016/j.wneu.2021.06.148 (2021). Epub 2021 Jul 6. PMID: 34237449.
Muratore, M. et al. Surgical treatment of traumatic thoracolumbar fractures: a retrospective review of 101 cases. Musculoskelet Surg. 105(1), 49–59. (2021). https://doi.org/10.1007/s12306-020-00644-0. Epub 2020 Feb 5. PMID: 32026381.
Park, C. J., Kim, S. K., Lee, T. M. & Park, E. T. Clinical relevance and validity of TLICS system for thoracolumbar spine injury. Sci. Rep. 10 (1), 19494. https://doi.org/10.1038/s41598-020-76473-9 (2020). PMID: 33177557; PMCID: PMC7658963.
Karaali, E., Ciloglu, O., Duramaz, A., Kusvuran Ozkan, A. & Ekiz, T. Management of thoracolumbar injury classification and severity score of 4 (TLICS = 4) thoracolumbar vertebra fractures: Surgery versus conservative treatment. Ulus Travma Acil Cerrahi Derg. 26(5), 805–810. English. (2020). https://doi.org/10.14744/tjtes.2020.30524. PMID: 32946086.
Gonzales-Portillo, G. S. et al. Evaluation of the Thoracolumbar Injury classification and severity (TLICS) score over a two-year period at a Level one Trauma Center. Cureus 15 (8), e43762. https://doi.org/10.7759/cureus.43762 (2023). PMID: 37600439; PMCID: PMC10439826.
Langrana, N. A., Harten, R. D. R. D., Lin, D. C., Reiter, M. F. & Lee, C. K. Acute thoracolumbar burst fractures: a new view of loading mechanisms. Spine (Phila Pa 1976). 27(5), 498–508. (2002). https://doi.org/10.1097/00007632-200203010-00010. PMID: 11880835.
Deqing, L., Kejian, L., Teng, L., Weitao, Z. & Dasheng, L. Does the fracture fragment at the anterior column in thoracolumbar burst fractures get enough attention? Med. (Baltim). 96 (6), e5936. https://doi.org/10.1097/MD.0000000000005936 (2017). PMID: 28178133; PMCID: PMC5312990.
Jiang, Y. et al. A comparative study on functional recovery, complications, and changes in inflammatory factors in patients with Thoracolumbar spinal fracture complicated with nerve Injury treated by anterior and posterior decompression. Med. Sci. Monit. 25, 1164–1168. https://doi.org/10.12659/MSM.912332 (2019). PMID: 30753178; PMCID: PMC6380160.
Zhao, Q. et al. Complications of percutaneous pedicle screw fixation in treating thoracolumbar and lumbar fracture. Med. (Baltim). 97 (29), e11560. https://doi.org/10.1097/MD.0000000000011560 (2018). PMID: 30024554; PMCID: PMC6086516.
Jiang, F. et al. The Mini-open Wiltse Approach with pedicle screw fixation Versus Percutaneous pedicle screw fixation for treatment of neurologically intact Thoracolumbar fractures: a systematic review and Meta-analysis. World Neurosurg. 164, 310–322 (2022). Epub 2022 Jun 1. PMID: 35659586.
Kocis, J., Kelbl, M., Kocis, T. & Návrat, T. Percutaneous versus open pedicle screw fixation for treatment of type a thoracolumbar fractures. Eur. J. Trauma. Emerg. Surg. 46 (1), 147–152. https://doi.org/10.1007/s00068-018-0998-4 (2020). Epub 2018 Aug 23. PMID: 30167741.
Ishii, K. et al. The history and development of the Percutaneous Pedicle Screw (PPS) System. Med. (Kaunas). 58 (8), 1064. https://doi.org/10.3390/medicina58081064 (2022). PMID: 36013531; PMCID: PMC9414999.
Zhao, X. B., Ma, H. J., Geng, B., Zhou, H. G. & Xia, Y. Y. Early clinical evaluation of percutaneous full-endoscopic transforaminal lumbar Interbody Fusion with pedicle screw insertion for treating degenerative lumbar spinal stenosis. Orthop. Surg. 13 (1), 328–337. https://doi.org/10.1111/os.12900 (2021). Epub 2021 Jan 10. PMID: 33426744; PMCID: PMC7862160.
Li, K. et al. Pedicle screw fixation combined with intermediate screw at the fracture level for treatment of thoracolumbar fractures: a meta-analysis.Medicine(Baltimore). 95(33):e4574. https://doi.org/10.1097/MD.0000000000004574. (2016). PMID: 27537586; PMCID: PMC5370812.
Li, C. et al. Efficacy and safety of unilateral biportal endoscopy compared with microscopic decompression in the treatment of lumbar spinal stenosis: a protocol for systematic review and meta-analysis. Med. (Baltim). 100 (50), e27970. https://doi.org/10.1097/MD.0000000000027970 (2021). PMID: 34918647; PMCID: PMC8678029.
Ruetten, S. & Komp, M. Endoscopic Lumbar Decompression. Neurosurg Clin N Am. 31(1), 25–32. (2020). https://doi.org/10.1016/j.nec.2019.08.003. Epub 2019 Oct 15. PMID: 31739926.
Quillo-Olvera, J., Quillo-Olvera, D., Quillo-Reséndiz, J. & Barrera-Arreola, M. Unilateral Biportal endoscopic-guided Transcorporeal Vertebroplasty with neural decompression for treating a traumatic lumbar fracture of L5. World Neurosurg. 144, 74–81 (2020). Epub 2020 Aug 22. PMID: 32841799.
Yang, H., Han, D. & Li, X. Endoscopic decompression combined with percutaneous pedicle screw fixation for treating Thoracolumbar Burst fractures with neurological deficits: technical note and early outcomes. World Neurosurg. 173, e521–e531. https://doi.org/10.1016/j.wneu.2023.02.088 (2023). Epub 2023 Feb 24. PMID: 36841532.
Ruetten, S. & Komp, M. Der Trend zu vollendoskopischen Dekompressionen: Aktuelle Möglichkeiten und Grenzen bei Bandscheibenvorfall und Spinalkanalstenose [The trend towards full-endoscopic decompression : Current possibilities and limitations in disc herniation and spinal stenosis]. Orthopade. 48(1), 69–76. German. (2019). https://doi.org/10.1007/s00132-018-03669-3. PMID: 30535764.
Bai, G., Wu, J. & Hu, Q. Unilateral biportal endoscopic treatment of complications in a patient with lumbar burst fracture with pedicle screw repositioning and fixation: a case report. Interdisciplinary Neurosurg. 34, 101837. https://doi.org/10.1016/j.inat.2023.101837 (2023).
Chang, H. et al. Comparison of full-endoscopic foraminoplasty and lumbar discectomy (FEFLD), unilateral biportal endoscopic (UBE) discectomy, and microdiscectomy (MD) for symptomatic lumbar disc herniation. Eur. Spine J. 32 (2), 542–554. https://doi.org/10.1007/s00586-022-07510-6 (2023). Epub 2022 Dec 26. PMID: 36571643.
Moon, Y. J. & Lee, K. B. Relationship between clinical outcomes and spontaneous canal remodeling in Thoracolumbar Burst fracture. World Neurosurg. 89, 58–64. https://doi.org/10.1016/j.wneu.2016.02.010 (2016). Epub 2016 Feb 9. PMID: 26872515.
Dong, H. et al. Clinical outcomes of Thoracolumbar Burst Fracture treated by Trans-Kambin triangle versus Transpedicular Bone Grafting combined with posterior internal fixation. World Neurosurg. 156, e130–e138 (2021). Epub 2021 Sep 8. PMID: 34508909.
Nguyen, N. Q. & Phan, T. H. The Radiological complications of short-segment pedicle screw fixation combined with Transforaminal Interbody Fusion in the treatment of unstable Thoracolumbar Burst fracture: a retrospective Case Series Study in Vietnam. Orthop. Res. Rev. 14, 91–99 (2022). PMID: 35378735; PMCID: PMC8976479.
Hayoun, T., Siboni, R., Ohl, X. & Bredin, S. Treatment of thoracolumbar fractures: comparison of the clinical and radiological outcomes of percutaneous versus open surgery. Eur. J. Orthop. Surg. Traumatol. 33 (6), 2393–2397. https://doi.org/10.1007/s00590-022-03444-3 (2023). Epub 2022 Nov 29. PMID: 36446956.
Acknowledgements
None.
Funding
No funding.
Author information
Authors and Affiliations
Contributions
Q.H. contributed conception and design of the study; X.Q. organized the figures; G.W. organized the tables; X.J. completed the statistical analysis of the data; G.B. wrote the first draft of the manuscript and critically revised the final manuscript draft. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bai, G., Qiu, X., Wei, G. et al. Unilateral biportal endoscopic decompression combined with percutaneous pedicle screw fixation offers new treatment option for thoracolumbar burst fractures with secondary spinal stenosis. Sci Rep 15, 877 (2025). https://doi.org/10.1038/s41598-025-85543-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-85543-9