Abstract
Herbicide paraquat dichloride, a potent redox agent found its way to natural water bodies and influences their health; however, its impact on the reproductive health of fish is potentially less studied and requires clear investigation. This study was conducted to elucidate its effect on the gonadal health of female fish, Channa punctatus over 60 days. The 96-h LC50 of test herbicide was calculated as 0.24 mL/L for the fish under examination, subsequently, three sub-lethal concentrations were taken in addition to control for the study. The experimental methodology included assessment of oxidative stress markers, hormone levels, expression of interrelated genes, and histological analysis to ascertain the damage to the ovary. At each exposure period, a significant (p < 0.05) rise in endogenous reactive oxygen species in blood cells and activities of oxidative markers in the ovary tissue were observed in treated groups. The gonadosomatic index of the ovary and hormone concentration in plasma decreased at the highest treatment concentration. A significant (p < 0.05) change in the expression of target genes for ovary growth, inflammation, and apoptosis was observed in the treated fish. Histopathological and ultrastructural investigations of the ovary tissue revealed the occurrence of oophoritis and reduced growth of the ovary in herbicide-treated fish. The findings conclude that, herbicide paraquat dichloride causes inflammation in the ovary, in addition to its growth reduction that ultimately, poses a threat to the fish population.
Similar content being viewed by others
Introduction
Agrochemicals, though beneficial, pose significant threat to humans and animals, particularly those near application sites, including aquatic systems and their inhabitants, such as fish. Paraquat dichloride (PD) is used commercially as a herbicide in its soluble liquid (SL) form. In India, the recommended concentration is 24% SL, with the remaining 76% consisting of surfactants, adjuvants, and solvents1. Paraquat is known to disrupt redox cycles within living organisms and is associated with high toxicity, leading to its ban in many countries2.
Despite its use at lower concentrations in commercial formulations, it is crucial to understand the impact of paraquat-containing herbicides on various systems and functions of the body. PD acts as a systemic toxicant with immediate effects on the respiratory system3. Its application in agricultural areas and for aquatic weed control paves the path for its entry into aquatic ecosystems and thus, affects their resident species. The reproductive potential of fish, which is critical for maintaining their population and ecological roles, is of particular concern. The redox cycle, disrupted by PD, initiates the production of reactive oxygen species (ROS), leading to oxidative stress4. This oxidative stress is initially crucial for various functions of the body however, can become detrimental when exacerbated. While the harmful effects of PD have been studied in various vital organs of fish and rats, its impact on reproductive organs, particularly the ovary, has not been thoroughly explored5,6,7,8,9. ROS and apoptosis are essential for the growth and maturation of the ovary; any deviation from optimal levels can lead to adverse effects. Thus, examination of ROS and various antioxidant markers becomes essential to evaluate the impact of any redox toxicant on ovarian growth10.
Exposure to toxicant can lead to changes in molecular expressions within the body, alongside immediate systemic effects. The functions of various genes are influenced by both favourable and unfavourable conditions, making their responses crucial for development. Likewise, under normal conditions, key regulatory proteins such as phosphatase and tensin homolog B (PTENb), phosphoinositide 3-kinase (PI3K), AK transforming (AKT), mechanistic target of rapamycin (mTOR), ribosomal protein S6 kinase Beta-1 (S6K1), and forkhead box O3a (FOXO3a) play critical roles in ovarian development and proliferation. PI3K initiates this pathway through AKT/mTOR/S6K1 to promote ovarian proliferation, while AKT regulates FOXO3a expression to control natural apoptosis during ovarian growth. PTENb counteracts this signaling by dephosphorylating PI3K substrates, required for activating the signaling pathway and its activity is also regulated by ROS. Altered expression of these genes is associated with irregular and compromised ovarian development, leading to increased atretic conditions in response to toxicant exposure11,12,13,14,15,16,17,18,19,20,21,22.
Further, conditions of oxidative stress trigger the organ to activate antioxidant defences, which being protective; can lead to compromised health and inflammation. The inflammation, characterized by the infiltration of inflammatory cells into the ovary, is recognised as oophoritis and may be exacerbated by chemical exposure. Inflammatory genes such as tumour necrosis factor alpha (TNFα), NOD-, LRR-, and pyrin ___domain-containing protein 3 (NLRP3), apoptosis-associated speck-like protein containing a CARD (ASC), caspase-1 (Cas1), interleukin 1β (IL-1β), and interleukin 18 (IL-18) are involved in a cascade of inflammatory responses in various tissues under stress conditions caused by toxicants. Previous studies have documented toxicant-induced inflammation in various organs, correlating with the expression of these inflammatory genes23,24,25,26.
Besides molecular changes, the growth and function of the ovary are also influenced by hormones, particularly the ovarian steroid hormones estradiol and progesterone. These hormones are indicators of ovarian function and their levels can vary with changes in light, temperature, reproductive and non-reproductive phases, ovarian health, and exposure to chemicals27,28.
In this way, this study was designed to examine the potential of prolonged PD (SL) exposure to induce oxidative toxicity, leading to oophoritis and alterations in the ovary of Spotted Snakehead fish (Channa punctatus; C. punctatus). The study also aims to evaluate the relationship between oxidative stress markers and the transcriptional expression of genes involved in ovarian growth, proliferation, apoptosis, and inflammation, to better understand the manifestations of oophoritis in ovarian tissue.
Results
96 h-LC50 of PD (SL) for C. punctatus
The results from probit analysis exhibited a 96 h-LC50 value of PD (SL) for C. punctatus as 0.24 mL/L with a 95% upper and lower limit of 0.25 mL/L and 0.23 mL/L, respectively. Corresponding to this, test fish was subjected to three sub-lethal concentrations of PD (SL), 0.01 mL/L (1/20 of 96 h-LC50 value), 0.02 mL/L (1/10 of 96 h-LC50value) and 0.05 mL/L (1/5 of 96 h-LC50value) with the untreated control group for assessing the impact of prolonged exposure to PD (SL) on ovary growth and functions.
Gonadosomatic index (GSI) value for PD (SL) exposed C. punctatus
The GSI value for C. punctatus showed noticeable variation among the experimental groups. The values were found to decrease significantly (p < 0.05) with the increasing concentration of PD (SL), the lowest value of GSI was observed in group IV when compared to group I (control) at 60d (Table 1).
PD (SL) generated ROS in test fish blood cells
The statistically significant (p < 0.05) dose-dependent increase in ROS was observed in nucleated blood cells of the test fish C. punctatus of all intoxicated groups as compared to control following an exposure period of 30 and 60d. Figure 1a, b show quantified ROS levels and fluorescence intensity of cells revealing the presence of ROS in blood cells, respectively.
ROS production in nucleated blood cells of fish C. punctatus under the exposure of PD (SL) for subsequent 30d and 60d. Graph (a) represents a significant (p < 0.5) rise in ROS at 30 and 60d of PD (SL) exposure in treated groups as compared to control. Microphotographs (b) at 40x of magnification showing PD (SL) induced ROS in groups II, III, and IV at both exposure periods as compared to group I (control) in erythrocytes of C. punctatus. (Values are calculated as mean ± S.E.M.; n = 3; significant (p < 0.05) change among groups is marked with*).
Activities of oxidative stress markers in the ovary of C. punctatus exposed to PD (SL)
The activity of oxidative stress markers Superoxide dismutase (SOD), Catalase (CAT), Glutathione (GSH), Glutathione peroxidase (GPx), Glutathione reductase (GR), and Glutathione-S-transferase (GST) (Fig. 2a–f) were found to increase significantly (p < 0.05) in the ovary tissue of PD (SL) treated groups as compared to control (group I) in a time-dependent manner, highest being recorded in group IV having maximum concentration of PD (SL) at 60d. SOD in group IV was 3.53 ± 0.05 units/mg protein as compared to control 2.09 ± 0.00 units/mg protein. In group IV, the level of CAT was observed as 77.0 ± 0.16 units/mg protein in comparison to control (58.81 ± 0.26 units/mg protein). The observed level of GSH in group IV was 197.35 ± 0.56 units/mg protein while the control group had 58.81 ± 0.26 units/mg protein. GPx values for group I and group IV were recorded as 0.24 ± 0.00 and 0.35 ± 0.00 units/mg protein, respectively while for GR it was 0.12 ± 0.00 and 0.26 ± 0.01 units/mg protein, respectively. The level of GST observed in group IV was 29.09 ± 0.07 units/mg proteins in comparison to the control group which was detected with 22.47 ± 0.02 units/mg protein.
Effect of PD (SL) on oxidative stress markers of ovary tissue. Graphs (a–f) represent significant (p < 0.05) increase in activities of SOD, CAT, GSH, GPx, GR, and GST respectively, in the ovary tissue of C. punctatus at both exposure periods in PD (SL) treated groups II, III and IV as compared to control group I. (Values are calculated as mean ± S.E.M.; n = 3; significant (p < 0.05) change among groups is marked with*).
Concentration of estradiol and progesterone in plasma of test fish
The level of gonadal hormone, estradiol, and progesterone, in the plasma of female fish C. punctatus significantly (p < 0.05) decreased with a higher concentration of PD (SL) in comparison to the control, in a time-dependent manner (Table 2).
Expression of target genes in the ovary of test fish
Figure 3 heat map shows the log-transformation with a base of 2 (log2) normalized relative expression of target genes across different groups at both exposure periods. The positive and negative log2 value indicates increase and decrease, respectively, in the relative expression of genes. The results observed disseminates that expression of genes PTENb, FOXO3a, TNFα, NLRP3, ASC, Cas1, IL-1β, and IL-18 leading to apoptosis and inflammation in ovary significantly (p < 0.05) up-regulated with the prolonged exposure to PD (SL) as compared to control. However, significant (p < 0.05) down-regulation was observed in the expression of PI3K, AKT, mTOR, and S6K1 leading to progressive development of ovary with the increasing concentration of PD (SL) in a time-dependent manner in C. punctatus under the influence of PD (SL) in comparison to control.
Analysis of stained and scanning electron microscope (SEM) microphotographs of ovary tissue
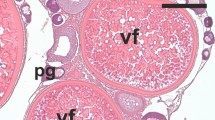
Figure 4a, b show stained and SEM microphotographs, respectively of sections of test fish’s ovary tissue of control group I and PD (SL) exposed group II, III, and IV at 60d. Ovary tissue of group I show normal vitellogenic oocytes (black arrow) while sections of group II and III show signs of few oocytes witnessing inflammation (encircled) and distortion. In group IV almost every section of ovary tissue showed inflammatory cell infiltration directing the chronic incidence of oophoritis in the test fish, ultimately reducing the healthy growth of the ovary. SEM image facilitates observation of altered ovary structure and improper growth in the ovary witnessing apoptosis and inflammation in groups II, III, and IV (white arrow) as compared to ovary section of control fish with normal development (blue arrow).
Histological representation. Microphotographs (40x) (a) represents inflammation (black arrow) in ovary tissue of groups II, III, and IV (complete inflammation) as compared to group I (control) of fish C. punctatus at the end of the exposure period of 60d. Scanning electron microscope photos (b) showing reduced/damaged structure of ovarian cells in PD (SL) intoxicated groups II, III, and IV as compared to group I at 60d.
Two-way analysis
To ascertain the significant (p < 0.05) impact of the sub-lethal concentration of PD (SL) and the duration of exposure to ROS, SOD, CAT, GSH, GPx, GR, GST, estradiol, and progesterone, a two-way analysis of variance (ANOVA) was conducted (Supplementary Table 1). The outcomes represented both concentration and exposure period have substantial (p < 0.05) impact on the ROS, markers of oxidative stress, and hormones. Findings have revealed their levels in group IV were highest at 60d. Results of Tukey’s test of multiple comparisons have recorded levels of ROS and oxidative stress markers are significantly (p < 0.05) greater at 60d than 30d. A statistically significant (p < 0.05) interaction exists between the exposure duration and the concentration of PD (SL).
Correlation between parameters of oxidative stress and target genes
The ovary tissue of the test fish exposed to PD (SL) observed a close positive relation at 60d among the parameters of oxidative stress (ROS, SOD, CAT, GSH, GPx, GR, and GST) and target genes (PTENb, FOXO3a, TNFα, NLRP3, ASC, Cas1, IL-1β, and IL-18) responsible for alteration in the ovary tissue. Additionally, a strong positive relation was also observed among the genes (PI3K, AKT, mTOR, and S6K1) that function for ovary growth in the ovary of test fish C. punctatus at 60d. Supplementary Table 2 represents the regression analysis of tested parameters.
Principal component analysis (PCA)
Using bilinear decomposition, the PCA method transforms the original multivariate data array into a new dataset where the variables are orthonormal and capture the maximum amount of variance. For the same purpose, a data matrix was created using four grouping factors and ROS, markers of oxidative stress, and target genes as independent variables. The ordination plots illustrated that every parameter assessed in this study corresponded to the F1 (87.46%) and F2 (11.95%) principal components with a total variance of 99.41% (Supplementary Fig. S1). The values of ROS, SOD, CAT, GSH, GPx, GR, GST, PTENb, FOXO3a, TNFα, NLRP3, Cas1, IL-1β, and IL-18 were found to have a significant degree of positive co-linearity with groups II and IV. This positive correlation reveals its involvement in causing damage to the ovary with a high concentration of PD (SL) exposure. Also, positive correlations were observed between group I and PI3K, AKT, mTOR, and S6K1, indicating growth of ovary in unexposed fish.
Discussion
Reproductive growth is essential for any species, especially in shifting environmental conditions. Persistent exposure to pesticides, especially those that generate ROS, can negatively impact the vital organs of the fish and can jeopardize their reproductive capabilities29,30. This prompted the authors to conjecture that an herbicide containing redox potential chemical, PD may influence the growth, development, and functions of reproductive organs in fish. The present study investigated PD (SL) generated oxidative stress that can result in oophoritis, which causes detrimental changes to the growth of the ovary and concentration of ovarian hormones in Channa punctatus.
ROS are by-products of cellular metabolic processes that involve redox reactions, essential for generating the energy required for life processes31. Recent advances suggest that ROS plays a crucial role in regulating various intracellular functions at optimal levels. However, when this balance is disturbed, elevated levels of ROS can have detrimental effects on cells and tissues, impacting growth, physiology, disease development, and ageing processes. Thus, during normoxia, there is a stable equilibrium between ROS and cellular antioxidant systems32. The constant exposure to PD (SL) in the present study demonstrated an elevated level of ROS, suggesting the onset of the anti-oxidant system in the fish, to neutralize the over-produced ROS, and to terminate the toxicity caused by the herbicide in ovary tissue. Superoxide ions generated endogenously in cells due to chemical exposure are converted into molecular oxygen and hydrogen peroxide (H2O2) by the first-line defence action of SOD. Subsequently, H2O2, another reactive species, is broken down into water by the enzyme CAT33. An increase in the activity of SOD and CAT has been observed in various organs following exposure to microplastics34, chemicals and pesticides, including paraquat. Aribisala et al.5 documented elevated levels of these enzymes in the gills and liver of Nile Tilapia (Oreochromis niloticus) exposed to glyphosate and paraquat, which aligns with the findings of the present study in ovarian tissue. These results suggest that PD (SL) induces oxidative stress in the ovary, similar to other organs, impairing its normal growth. Consequently, the frontline defense, involving SOD and CAT, becomes activated to counteract the damage caused by superoxide radicals. The most prevalent intracellular antioxidant, GSH, is a tripeptide consisting of glutamine, cysteine, and glycine. It can potentially be found in two states: oxidized (Glutathione disulphide, GSSG) and reduced (GSH). By donating electrons and changing into GSSG, GSH neutralizes radicals such as superoxide, peroxide, and free radicals. Further, the enzyme GR recycles GSSG back into GSH utilizing NADPH as an electron donor. This process preserves cellular redox equilibrium and guarantees a consistent supply of GSH necessary for controlling stress. Retaining the appropriate GSH/GSSG ratio is essential for efficient antioxidant protection against ROS. Furthermore, GPx, an enzyme that detoxifies endogenously generated H2O2, requires GSH as a cofactor. Additionally, an enzyme GST catalyzes the conjugation of GSH to toxic substances, xenobiotics, electrophilic chemicals, and environmental contaminants. This process renders the hazardous molecules water-soluble and facilitates their excretion from the body thus; the oxidative damage caused by these dangerous chemicals is lessened. Collectively, the glutathione family has significance in ovary development and proliferation10.In this study, significant (p < 0.5) increases in GSH levels were observed at both time points with rising concentrations of PD (SL) in the ovarian tissue of the test fish, C. punctatus. This finding aligns with a study on African Catfish (Clarias gariepinus) from Nigeria’s Ogun River, where elevated GSH levels were noted in the liver, kidney, and heart tissues in response to heavy metal toxicity. Similarly, the elevated levels of GPx, GR, and GST observed in the present study correspond with results from research on C. punctatus exposed to chromium and copper35,36, Zebrafish (Danio rerio) exposed to deltamethrin37, and Striped Dwarf Catfish (Mystus vittatus) exposed to lead38, where increased levels of these enzymes were recorded in various fish organs, including the liver, kidney, brain, and ovary. While low GSH levels generally indicate oxidative stress and high levels suggest healthy function, the elevated GSH levels observed in this study, along with other related enzymes, may reflect the ovary cells’ response to high oxidative stress induced by PD (SL). This upregulation of GSH likely represents an attempt to counteract excess ROS. However, despite this increase, the GSH system may not be functioning optimally due to the overwhelming levels of ROS production which could be the reason for damage in the ovary of exposed fish.
The limited understanding of oxidative stress-induced alterations in PI3K/AKT signaling and oophoritis affect fish ovaries after PD (SL) exposure, this emphasizes the significance of study in assessing the herbicide’s toxic potential. The research found that increasing concentrations of PD (SL) over extended periods significantly (p < 0.5) altered the mRNA expression of genes related to growth, proliferation, apoptosis, and inflammation in the ovary tissue. Fish play a crucial role in pharmaceuticals, cosmetics, and the food industry, as well as in the natural ecosystem. Damage to the ovaries, which are vital for reproduction, can lead to a decline in fish populations, impacting both natural ecosystems and human activities. PD (SL) triggers a cascade of cellular and molecular changes through ROS production upon exposure. PI3K and PTENb are activated and deactivated, respectively, at low ROS levels, which are crucial for intracellular signaling related to ovarian function13. In our study, treatment with PD (SL) led to a down-regulation of PI3K and an up-regulation of PTENb, coinciding with significantly (p < 0.5) elevated ROS levels compared to normal conditions. This suggests that high ROS levels negatively regulate PI3K and PTENb, impairing the PI3K/AKT downstream signaling pathway, crucial for ovarian growth13. Additionally, we observed a down-regulation of AKT, mTOR, and S6K1 expression in PD (SL)-treated tissues at each time point, indicating an inhibition of ovarian cell development. At low AKT levels and elevated ROS, FOXO3a—a regulator of ovarian atresia via apoptosis was highly expressed in the exposed tissues, suggesting that increased FOXO3a expression may lead to excessive cell death and ovarian atrophy13. Similar findings were noted in other studies: chlorpyrifos exposure in grass carp liver cells led to ROS-induced negative regulation of the PI3K/AKT and PTEN pathways16, and deoxynivalenol was shown to induce neutrophil apoptosis in carps through ROS-mediated inhibition of the PI3K/AKT pathway14. A study on kidney cells of Grass Carp (Ctenopharyngodon idellus) showed that exposure to paraquat activates the PTEN/PI3K/AKT pathway in a dose-dependent manner11, which aligns with our findings in ovary tissue. Zhang et al.22 reported that cyclophosphamide caused dysregulation of AKT and FOXO3a signalling, resulting in excessive follicular apoptosis in female C57BL mice. Furthermore, the lower expression of mTOR in the exposed tissue of our study suggests decreased phosphorylation of S6K1, leading to reduced cell growth and proliferation. Magnuson et al.39 reviewed that S6K1 activation promotes cell growth and proliferation, while its inhibition has the opposite effect. Similarly, Jiang et al.15 found that the AKT/TOR/S6K1 axis was upregulated in hybrid bagrid catfish (Pelteobagrus vachelli♀ × Leiocassis longirostris♂), enhancing muscle growth when provided with an isoleucine-rich diet. A study has shown that oxidative stress suppresses mTOR phosphorylation of S6K1 in rat pheochromocytoma (PC12) cells and primary murine neurons, leading to lower expression of these genes under high oxidative stress conditions40. Similar results were observed in PC12 cells exposed to rotenone41. Thus, the PTEN/PI3K/AKT/mTOR/S6K1 signaling pathway is essential for ovarian growth, and any disruption in this pathway can result in reduced growth and increased cellular death. In addition to the altered expression of genes involved in ovarian growth, genes responsible for inflammation (such as TNFα, NLRP3, ASC, Cas1, IL-1β, and IL-18) in the ovary were significantly (p < 0.5) upregulated following exposure to PD (SL). Elevated ROS and oxidative stress have been shown to trigger the assembly of TNFα and the NLRP3/ASC complex, leading to a cascade of inflammatory responses. High levels of TNFα not only produce additional inflammatory cytokines but also facilitate the adherence of other immune cells and promote further ROS production at the site. Under conditions of elevated ROS-mediated oxidative stress, the inflammasome complex, comprising NLRP3 and ASC, is formed. This complex then activates Cas1, IL-1β, and IL-18, contributing to localized inflammation and promoting the infiltration of immune cells. Paraquat-intoxicated Wistar male adult rats exhibited inflammation in the serum23 and lungs25, confirmed by the upregulation of inflammatory genes such as TNFα and IL-1β. Zebrafish exposed to paraquat showed high expression of inflammation-related genes, including TNFα, IL-1β, and IL-18, in the liver, along with inflammatory cell infiltration in the swim bladder26. Similarly, weaned piglets exposed to cadmium chloride developed inflammation in the brain due to the expression of the NLRP3, ASC, and Cas1 inflammasome complex24. These findings suggest that the roles of these inflammatory genes are interconnected and collectively contribute to inflammation upon exposure to toxicants, leading to the infiltration of various inflammatory cells in ovarian tissue. Thus, prolonged exposure to the herbicide PD (SL) results in elevated ROS and oxidative stress, causing molecular changes that lead to inflammation in the ovary, a condition known as oophoritis.
In the current study, ovary tissues exposed to PD (SL) for 60d showed reduction in size, weight, and inflammation. Additionally, plasma concentrations of estradiol and progesterone decreased in the exposed fish. The observed decline in ovarian growth and proliferation, attributed to oxidative stress, suggests that PD (SL) causes damage and reduces ovarian size. Concurrently, elevated expression of inflammatory genes contributed to ovarian damage and decreased growth. This aligns with similar findings in the literature, where oxidative stress and inflammation were shown to adversely affect ovarian growth10,13. For instance, fluoride exposure in female Danio rerio has been linked to increased oxidative stress and apoptosis, which impairs ovarian development and reduces GSI and hormone concentrations28. Similarly, beta-cypermethrin exposure negatively impacts male Zebrafish reproductive organs, resulting in lower GSI and sperm activity22. These findings underscore the impact of reduced GSI on reproductive organ development and function. Hormonal concentrations are crucial for ovarian health and development. Studies have shown that lower GSI is associated with decreased levels of gonadal hormones, indicating a positive relationship between GSI and hormone levels. For example, research on Oreochromis niloticus demonstrated that GSI and serum estradiol concentrations are positively correlated with optimal temperature and photoperiod combinations27. Similarly, the present study supports this positive correlation, showing that GSI and hormone concentrations decrease in a dose- and time-dependent manner following exposure to PD (SL).
Histological analysis and end-point biomarkers confirm the stress-induced effects of toxicants on organ development. In this study, the developmental changes in the ovary were assessed using classical staining techniques with hematoxylin and eosin, as well as electron microscopy. The results revealed the infiltration of inflammatory cells in the ovaries of C. punctatus exposed to PD (SL) and further structural damage observed through ultra-structural analysis. This damage was attributed to ROS, oxidative stress-induced apoptosis, inflammation, and reduced growth. Supporting these findings, Fu et al.42 demonstrated that glyphosate, an herbicide, causes oxidative stress and inflammatory cell infiltration in the reproductive systems (uterus and ovary) of weaned hybrid (Duroc × Landrace × Yorkshire) piglets. Similarly, cadmium exposure in swine has been shown to trigger NLRP3 inflammasome-mediated pyroptosis in brain tissue24. These findings suggest that PD (SL) has similar detrimental effects, highlighting its role as a toxicant that can disrupt organ development through oxidative and inflammatory stress.
A two-way analysis of ROS, SOD, CAT, GSH, GPx, GR, GST, estradiol, and progesterone in this study on ovary of C. punctatus revealed a significant interaction (p < 0.05) between the exposure duration and the concentration of PD (SL). This indicates that the effects of PD (SL) on the studied parameters are influenced by both the duration of exposure and the concentration levels, suggesting that changes in either factor can alter the overall impact. Further, it suggests that prolonged exposure to the tested herbicide can adversely affect ovarian development by increasing oxidative stress levels and decreasing gonadal hormone concentrations. These findings also indicate that chronic exposure may lead to additional detrimental effects on the ovary, further impeding its development.
The correlation analysis among oxidative stress parameters and target genes in the ovarian tissue of PD (SL)-exposed C. punctatus revealed a strong relationship. Increased ROS generation led to oxidative stress, which was reflected in the elevated levels of antioxidant markers such as SOD, CAT, GSH, GPx, GR, and GST in the ovarian tissue. This study found a strong correlation between ROS and these antioxidant markers. Moreover, elevated oxidative stress was associated with the activation of apoptotic and inflammatory pathways. A robust positive correlation was observed between ROS and the expression of genes involved in these pathways, including PTENb, FOXO3a, TNFα, NLRP3, ASC, Cas1, IL-1β, and IL-18. Additionally, there was a significant positive correlation between antioxidant markers (SOD, CAT, GSH, GPx, GR, GST) and target genes. The study highlights a significant increase in ROS levels, alterations in antioxidant markers, and changes in the expression of genes related to growth, apoptosis, and inflammation. These findings provide insights into the complex mechanisms of PD (SL) induced toxicity in ovarian tissue and highlight concerns for the health of fish, the environment, and potentially human health due to PD (SL) exposure in aquatic ecosystems. On the other hand, while the growth-promoting genes PI3K, AKT, mTOR, and S6K1 were observed to be strongly correlated, suggesting their synchronized expression to promote growth and proliferation in the ovary, their negative correlation with oxidative stress parameters and inflammation-related genes indicates that their expression is impaired in the presence of PD (SL).
The PCA results from this study demonstrate that healthy ovarian development relies on the synchronized activity of the genes PI3K, AKT, mTOR, and S6K1. These genes were found to be inversely correlated with ROS and oxidative stress markers, suggesting that increased oxidative stress leads to reduced expression of these genes, which impairs protein synthesis, necessary for cell growth and proliferation. This highlights that chemicals causing or exacerbating oxidative stress represent a significant barrier to ovarian development, thereby diminishing reproductive capability. Additionally, oxidative stress induced by PD (SL), along with the activation of apoptosis and inflammation-related genes in ovarian tissue, strongly contributes to reduced growth and oophoritis, which are associated with the principal components. PCA and regression correlation analyses reveal a strong relationship between oxidative stress markers and mRNA transcription factors related to apoptosis and inflammation, which are relevant to oophoritis. These findings support the notion that oophoritis is linked to elevated levels of these factors. Furthermore, the two-way analysis shows that PD (SL) is particularly effective at causing these detrimental effects when exposure occurs over extended periods and in a dose-dependent manner.
Conclusion
The results of the present study demonstrate that PD (SL)-induced oxidative stress triggers inflammation and apoptosis in the ovary of test fish, C. punctatus, by up-regulating expression of PTENb, FOXO3a, TNFα, NLRP3, ASC, Cas1, IL-1β and IL-18 while down-regulating expression of PI3K, AKT, mTOR, and S6K1, which ultimately leads to reproductive impairment (Fig. 5). Decreased GSI and hormonal concentration, further indicates the impaired or stunted growth, and the ovary also loses its physiological functions. It can be concluded that PD (SL) exposure in even low doses inhibits the development and functional ability of ovary in fish, C. punctatus. Consequently, reduced reproductive development can result from PD (SL) exposure in aquatic bodies, which may contribute to the extinction of some species. These findings underscore the need to explore measures to prevent the use of such chemicals near aquatic environments involved in fish production and to develop remedial therapies to mitigate their impact on ovarian health. Addressing these issues will contribute to maintain a healthy aquatic environment and ensuring a consistent, nutrient-rich supply for the food and pharmaceutical industries, thereby aligning with human needs.
Materials and methods
Test chemical
Paranex™, a PD herbicide (SL) manufactured by ADAMA India Private Limited was purchased from the pesticide retailer for the experimental study.
Other chemicals used in the study
2’, 7’-Dichlorodihydrofluoresceindiacetate (H2DCF-DA), 2,4-dinitrochlorobenzene, 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB), acetone, azide, Bouins fluid, bovine serum albumin, calcium carbonate, chloroform, isopropanol, copper sulphate, diethypyrocarbonate-treated water, dibutylphthalate polystyrene xylene (DPX), dimethylsulfoxide, dinucleotide phosphate hydrogen, disodium hydrogen phosphate, dithiothreitol (DTT), ethanol, ethylenediaminetetraacetic acid (EDTA), Folins reagent, glacial acetic acid, glutaraldehyde, GSSG, GSH, hematoxylin and eosin dye, hydrochloric acid, H2O2, methanol, nicotinamide adenine dinucleotide hydrogen (NADH), nicotinamide adenine dinucleotide phosphate hydrogen (NADPH), nitrobluetetrazolium (NBT), osmium tetraoxide, phenazinemetasulphate (PMS), pheny methyl sulfonyl fluoride (PMSF), phosphate buffer saline (PBS), potassium chloride, potassium permanganate, potassium phosphate, potassium sodium tartrate, sodium carbonate, sodium chloride (NaCl), sodium dihydrogen phosphate, sodium hydroxide, sucrose, SYBR green master mix, tricainemethanesulfonate, trichloroacetic acid (TCA), tris (hydroxymethyl) aminomethane hydrochloride (tris-HCl), triton X, trizma base, trizol, xylene.
Fish procurement and acclimatization
Healthy specimens of C. punctatus (34 ± 3.2 g; 16 ± 3.0 cm) were sourced from a local hatchery in Lucknow, Uttar Pradesh, India, and transported to the experimental room in aerated containers. The fish were acclimated to laboratory conditions for 15d in glass aquaria (100 × 40 × 40 cm3) filled with 10d aged water following a 2 min pre-treatment with 0.05% potassium permanganate. The fish were fed twice daily with commercial aquarium food pellets from the Perfect Companion Group Company Limited, Thailand, as described by Kumar et al.35. Aquaria were maintained by regular observation and removal of any debris or dead fish.
Median lethal toxicity of herbicide, PD (SL)
Standard methods and guidelines detailed in Baird et al. and OECD43,44, respectively were incorporated for evaluating the LC50 value in a semi-static bioassay system. Fish specimens of C. punctatus were randomly distributed to a series of 5 different concentrations of PD (SL) (0.0, 0.1, 0.2, 0.3, and 0.4 mL/L) for 96 h. Mortality recorded confirmed the toxic range between 0.2 and 0.3 mL/L. Subsequently, for next 96 h fish were exposed to eight different concentrations of PD (SL) (0.20, 0.22, 0.24, 0.26, 0.28, 0.30, 0.32, and 0.34 mL/L) to define the definite range. Well-adapted healthy female fish were starved for 24 h and 8 fish were loaded in each tank for the study. The dead fish were removed upon observation and mortality was recorded up to 96 h. 96 h-LC50 of PD (SL) for the test fish was calculated by probit analysis using Statistical Package for Social Science (SPSS) (IBM Corp, USA; version 27). Supplementary Fig. S2 determines the standard dose-response curve of PD (SL)-96 h-LC50 value.
Sample size
G*Power (3.1.9.4) software was used for determining the sample size of the designed study. For one-way ANOVA repeated measures, with effective size (f) 0.4, error prob (α) 0.05, and power (1-β) 0.90 the sample size was calculated as 48.
Experimental design
To assess the long-term effects of PD (SL) on the ovaries, 48 female fish of the test species C. punctatus were starved for 24 h and then randomly assigned to four groups, with each group consisting of four fish per aquarium, across three replicates. The experimental four groups I, II, III, and IV comprised of control, 1/20 of PD (SL)-96 h-LC50, 1/10 of PD (SL)-96 h-LC50, and 1/5 of PD (SL)-96 h-LC50, respectively. No natural deaths occurred during the experimental period. The study involved two sampling periods at 30d and 60d from the start of the experiment. The test medium was renewed after 15d. Water quality parameters in each tank were monitored and analyzed during each renewal, following standard methods outlined by Baird et al.43. The parameters were found to be pH—7.19 ± 0.06, temperature—26.93 ± 0.42 °C, dissolved oxygen (DO)—7.05 ± 0.25 mg/L, total hardness—79.87 ± 4.12 mg/L as CaCO₃, and alkalinity—83.08 ± 2.84 mg/L as CaCO₃. At the end of the specified exposure periods, two fish per replicate were euthanized by immersion in 0.2 g/L tricainemethanesulfonate (MS-222) for 2 min; subsequently weighed and used for further analysis45. Blood samples were collected from the caudal vein to measure ROS and plasma was used for measuring gonadal hormones. Additionally, the ovaries were dissected and weighed to assess oxidative stress parameters and transcriptional analysis of target genes at each sampling time. Histological alterations were examined at the conclusion of the experiment. The study was carried out following the ARRIVE guidelines. All the methods were performed in accordance with the relevant guidelines and regulations provided by Committee for Control and Supervision of Experiments on Animals (CCSEA), Ministry of Environment and Forests, Government of India under registration no.1861/GO/Re/S/16/CCSEA.
GSI
Sexual maturity of fish in correspondence to ovary development was calculated as.
\({\text{GSI}}\left( \% \right)={\text{ }}\left( {{\text{Ovary weight}}/{\text{Body weight}}} \right){\text{ }} \times {\text{ 1}}00\)
ROS
The intracellular oxygen species in fish blood were measured using the oxidation-sensitive fluorescent dye H2DCF-DA (Sigma Aldrich, USA). In short, H2DCF-DA dye was applied to blood samples, and incubated for 30 min. Slides were made and dried in the dark after incubation. H2DCF-DA oxidizes when exposed to ROS, resulting in the formation of green fluorescent 2′,7′-dichlorofluorescein (DCF) dye. To measure intracellular fluorescence in blood cells, images were captured using a fluorescent microscope (Nikon ECLIPSE Ti-S) with objectives set to 10/40X magnification at 485 nm excitation and 528 nm emission wavelength. Using the ImageJ tool (version 1.50, USA), the fluorescence intensity was measured as corrected total cell fluorescence (CTCF)36.
Oxidative stress markers
To evaluate the markers of oxidative stress, the ovary tissue from the sampled fish was processed as followed by36. Briefly, tissue was first rinsed with PBS. Following this, the tissue was homogenized using a homogenization buffer (1:1) that contained 32 M sucrose, 1 mM EDTA, and 10 mM tris HCl. The homogenate was then centrifuged twice at a temperature of 4 °C for 10 min at 3000 rpm. Subsequently, the resulting pellets were incubated in a solution composed of lysis buffer, PMSF, and DTT for 15 min at 4 °C. Finally, the mixture was centrifuged at 12,000 rpm for 15 min at 4 °C. The supernatant was collected for further analysis. The protein content was determined using the Lowry et al.46 method to normalize the activity of oxidative stress markers. Ultraviolet-visible (UV–VIS) spectrophotometer (Shimadzu, UV-1800) was used to measure the absorbance, and quantified values for each marker is expressed as units/mg protein.
SOD
The samples were initially incubated at 36 °C for 5 min. Following this, 0.1 mL of the sample supernatant was mixed with 0.6 mL of 0.052 M sodium pyrophosphate buffer (pH 8.3), 0.05 mL of 3 M PMS, and 0.15 mL of 1 M NBT. The reaction was initiated by adding 0.1 mL of 1 M NADH. This mixture was then incubated for an additional 5 min at 37 °C in a water bath. The reaction was stopped by adding 0.25 mL of 20 M glacial acetic acid. Subsequently, the color intensity and SOD activity were measured at 560 nm47.
CAT
For estimating CAT activity, 0.05 mL of tissue homogenate was mixed with 1 mL of 50 mM sodium phosphate buffer (pH 7.0) and 0.5 mL of 30mM H2O2, and the absorbance was taken at 240 nm48,49.
GSH
To 0.1 mL of tissue supernatant obtained, 1 mL tris-EDTA buffer (pH 8.5) was added and mixed. Then 0.05 mL color compound DTNB was added and the mixture was incubated for 10 min at room temperature to initiate a reaction between GSH and DTNB for color formation. Post incubation absorbance was recorded at 412 nm50,51.
GPx
Firstly 0.15 phosphate buffer (pH 7.4), 0.1 mL 2 mM GSH, 0.05 mL 10 mM azide, and 0.05 mL 1 mM H2O2 were sequentially added to 0.15 mL tissue homogenate and incubated at 37 °C for 15 min. Later, 0.25 mL 10% TCA was added to precipitate proteins and stop the enzymatic reaction. Then the mixture was centrifuged at 3000 rpm for 5 min. Further, 0.25 mL supernatant was collected to which 0.1 mL phosphate buffer was previously used and 0.35 mL DTNB (4 mg/mL) was added and absorbance was taken at 420 nm52.
GR
Briefly, 0.1 mL supernatant, 0.6 mL phosphate buffer (pH 7.5) 0.1 mL 0.1 mM NADPH, and 0.1 mL double distilled water were mixed and allowed to incubate for 5 min at room temperature. Then 0.1 mL 1 mM GSSG was added to the mixture and absorbance was recorded at 340 nm53.
GST
In short, the activity of GST was evaluated by adding 0.98 mL PBS, 0.01 mL 100mM CDNB, and 0.01 mL 100 mM GSH to 1 mL homogenate supernatant. Absorbance was immediately recorded at 340 nm54.
Estradiol and progesterone concentration in plasma
Plasma was collected using heparin coated syringe and placed in EDTA coated blood vials. Immediately, the sample was centrifuged for 15 min at 1000 rpm at 2 °C. Collected plasma was immediately used for further analysis. For determining the concentration of estradiol and progesterone in sample plasma, a fish enzyme linked immunosorbent assay (ELISA) kit was obtained from MyBioSource, Inc. (CA, USA). The assays were conducted following the manufacturer’s instructions. The optical density was determined by taking the absorbance at 450 nm using Epoch™ 2 Microplate Reader (BioTek, USA) and the concentrations of estradiol and progesterone in each sample were estimated.
Transcriptional analysis
The TRIzol method and Revert Aid H Minus Synthesis Kit (K1632; Thermo Scientific, USA) were used to obtain RNA from the ovary tissue and synthesize complementary deoxyribonucleic acid (cDNA), respectively, for transcriptional analysis as previously done by Kumar et al.35. cDNA was stored at − 80 °C until used for analysis. Primer sequences (forward and reverse) of the target genes were bought from Integrated DNA Technologies, Lowa, USA, and were diluted using nuclease-free water as per the instructions. The details of the primer are provided in Supplementary Information Table S3. The reverse transcription and quantitative polymerase chain reaction (qRT-PCR) analysis was done by CFX™ Real-Time PCR (C1000 Touch™ Thermal Cycler, Bio-Rad, USA).
β-actin and ELF1α were used as internal control genes for data normalization and an approach made by Vandesompele et al.55 was followed to assess the comparative expression of the target genes. Briefly, ΔCt value and primer efficiency were calculated for each gene. Following this, the relative quantity (RQ) value was calculated by raising primer efficiency to the power of ΔCt for each gene. Thereafter, RQ values of both internal control genes were used to calculate the geometric mean. Following this relative expression of target genes was calculated by dividing the RQ of the target gene by the geometric mean of internal control genes. Finally, the relative expression of genes are normalized and represented as log2.
Histology
Ovary tissue was cleaned with 0.9% NaCl solution to remove any remaining contaminants. A tissue sample was then preserved in Bouin’s solution for 48 h, and over the next 5d (twice daily), they were then rinsed with 70% ethanol. Fixed tissue was cleaned, and dehydrated in a progressive higher concentration of ethanol up to 100% before washing with xylene. The dried tissue was then covered with paraffin wax and was left overnight at 4 °C to solidify into a block. Using a rotatory microtome (YSI062 Yorco Precision Rotary Microtome, India), sections of 5 μm were cut and stained with hematoxylin and eosin stain. Further, the dyed tissue was mounted with DPX and placed on a level surface to ensure adequate drying. The observations were made using an oil immersion Nikon ECLIPSE Ci microscope with a 10/x40 magnification36.
Ultra-structural analysis
1% osmium tetroxide solution (prepared in 0.2 M phosphate buffer, pH 7.4) was used to fix 20 nm thick tissue (2 h, 4 °C) for SEM. Before fixation, tissue was placed in 2.5% glutaraldehyde for 24 h at 4 °C. Following fixation, tissue was dehydrated with progressively increasing concentration of acetone up to 100%. The critical point dryer (Emitech K 850 of Quorum Technology of UK) was used to dry the tissue and was mounted on metal stubs coated with gold. Tissue was observed under SEM (JSM 6490). Massar et al.56 was followed for analysis.
Data analysis
The data for every group (triplicate) represented as mean ± standard error mean (S.E.M.) were studied using one-way ANOVA with Tukey’s post hoc test at a significant level (p < 0.05). One-way ANOVA was also used for transcriptional analysis. A two-way ANOVA for exploring interactions between factors (concentration and exposure period) was applied to ROS, oxidative stress markers, and hormones. ANOVA was done by using IBM 232 SPSS Statistics for Windows (Version 27.0). A regression and correlation analysis at 60d was executed to establish the relationship between oxidative stress and target genes by using MS Excel tools. XLSTAT 2023.1.1.1398 was used to perform PCA. Biplots were created using the mean values of ROS, markers of oxidative stress, and target genes, which were measured in the blood and ovary tissue subjected to three sub-lethal doses of 96 h-LC50 of PD (SL) for 30 and 60d. Through the use of PCA, the primary correlations between the variables that account for the overall variability of the data under study were identified. Graphpad prism (version 10.2.0) was used to prepare graphs.
Data availability
This publication (as well as its Supplementary Information files) contains all of the data created or examined during the present study.
Abbreviations
- AKT:
-
AK transforming
- APHA:
-
American Public Health Association
- ASC:
-
Apoptosis-associated speck-like protein
- CaCO3 :
-
Calcium carbonate
- Cas1:
-
Caspase 1
- CAT:
-
Catalase
- cDNA:
-
Complementary deoxyribonucleic acid
- CDNB:
-
2,4-Dinitrochlorobenzene
- DMSO:
-
Dimethylsulfoxide
- DPX:
-
Dibutylphthalate polystyrene xylene
- DTNB:
-
5,5′-Dithiobis(2-nitrobenzoic acid)
- DTT:
-
Dithiothreitol
- EDTA:
-
Ethylenediaminetetraacetic acid
- ELF1α:
-
Elongation factor 1 alpha
- FOXO3a:
-
Forkhead box O3A
- GPx:
-
Glutathione peroxidase
- GR:
-
Glutathione reductase
- GSH:
-
Glutathione
- GSI:
-
Gonadosomatic index
- GSSG:
-
Glutathione disulphide
- GST:
-
Glutathione-S-transferase
- H2DCF-DA:
-
2’, 7’-Dichlorodihydrofluoresceindiacetate
- H2O2 :
-
Hydrogen peroxide
- IL-18:
-
Interleukin-18
- Il-1β:
-
Interleukin-1β
- MS-222:
-
Tricainemethanesulfonate
- mTOR:
-
Mammalian target of rapamycin
- NADH:
-
Nicotinamide adenine dinucleotide hydrogen
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate hydrogen
- NBT:
-
Nitroblue tetrazolium
- NLRP3:
-
NLR family, pyrin ___domain containing 3
- OECD:
-
Organization for Economic Co-operation and Development
- PBS:
-
Phosphate buffer saline
- PD:
-
Paraquat dichloride
- PI3K:
-
Phosphoinositide 3-kinase
- PMS:
-
Phenazinemetasulphate
- PMSF:
-
Pheny methyl sulfonyl fluoride
- PTENb:
-
Phosphatase and tensin homolog B
- RNA:
-
Ribonucleic acid
- ROS:
-
Reactive oxygen species
- S6K1:
-
Ribosomal protein S6 kinase b, polypeptide 1b
- SOD:
-
Superoxide dismutase
- TCA:
-
Trichloroacetic acid
- TNFα:
-
Tumour necrosis factor-alpha
References
Central Insecticides Board & Registration Committee. Herbicides (Directorate of Plant Protection, Quarantine & Storage, Ministry of Agriculture & Farmers Welfare, 2024).
Chang, S. et al. The early impact of paraquat ban on suicide in Taiwan. Clin. Toxicol. 60, 131–135 (2022).
Chen, J. et al. Effect of paraquat on cytotoxicity involved in oxidative stress and inflammatory reaction: a review of mechanisms and ecological implications. Ecotoxicol. Environ. Saf. 224, 112711 (2021).
Sun, I. O. et al. Predicting the probability of survival in acute paraquat poisoning. Kidney Res. Clin. Pract. 35, 102–106 (2016).
Aribisala, O. A. et al. Genotoxic, biochemical and histological biomarkers of subacute concentrations of paraquat and glyphosate in Nile Tilapia. Environ. Anal. Health Toxicol. 37, 1 (2022).
Das, P. et al. Studies on toxicological effect of the herbicide paraquat dichloride on the air breathing Singhi Catfish, Heteropneustes fossilis (Bloch). Proc. Zool. Soc. 73, 406–417 (2020).
Li, H. et al. Paraquat exposure delays stem/progenitor leydig cell regeneration in the adult rat testis. Chemosphere 231, 60–71 (2019).
Soni, R. et al. Paraquat induced impaired reproductive function and modulation of retinal and extra-retinal photoreceptors in Japanese quail (Coturnixcoturnix Japonica). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 224, 108568 (2019).
Ogunwole, G. et al. Paraquat toxicity on selected biomarkers in Clarias gariepinus handbook of ecomaterials view project seasonal occurence of phthalates and ibuprofen in River Owena and River Ogbese, Ondo State. Nigeria. View project paraquat toxicity on selected biomarkers in Clarias gariepinus. IOSR J. Environ. Sci. 12, 66–75 (2018).
Wang, S. et al. The role of antioxidant enzymes in the ovaries. Oxid. Med. Cell. Longev. 2017, 1 (2017).
Shi, X. et al. Paraquat induces apoptosis, programmed necrosis, and immune dysfunction in CIK cells via the PTEN/PI3K/AKT axis. Fish. Shellfish Immunol. 130, 309–316 (2022).
Shiau, J. P. et al. Impacts of oxidative stress and PI3K/AKT/mTOR on metabolism and the future direction of investigating fucoidan-modulated metabolism. Antioxidants 11, 911 (2022).
De Felici, M. & Klinger, F. G. Pi3k/pten/aktsignaling pathways in germ cell development and their involvement in germ cell tumors and ovarian dysfunctions. Int. J. Mol. Sci. 22, 9838 (2021).
Ding, C. et al. Deoxynivalenol induces carp neutrophil apoptosis and necroptosis via CYP450s/ROS/PI3K/AKT pathway. Aquaculture 545, 1 (2021).
Jiang, Q. et al. Dietary isoleucine improved flesh quality, muscle antioxidant capacity, and muscle growth associated with AKT/TOR/S6K1 and AKT/FOXO3a signaling in hybrid bagrid catfish (Pelteobagrus vachelli♀ × Leiocassis longirostris♂). J. Anim. Sci. Biotechnol. 12, 1 (2021).
Wang, L. et al. Chlorpyrifos induces the apoptosis and necroptosis of L8824 cells through the ROS/PTEN/PI3K/AKT axis. J. Hazard. Mater. 398, 122905 (2020).
Ma, Z. et al. Rosmarinic acid exerts an anticancer effect on osteosarcoma cells by inhibiting DJ-1 via regulation of the PTEN-PI3K-Akt signaling pathway. Phytomedicine 68, 153186 (2020).
Zhang, J. et al. Cadmium-induced oxidative stress promotes apoptosis and necrosis through the regulation of the miR-216a-PI3K/AKT axis in common carp lymphocytes and antagonized by selenium. Chemosphere 258, 127341 (2020).
Zheng, S. et al. Avermectin inhibits neutrophil extracellular traps release by activating PTEN demethylation to negatively regulate the PI3K-ERK pathway and reducing respiratory burst in carp. J. Hazard. Mater. 389, 121885 (2020).
Cui, F. Q. et al. Effects of BSF on podocyte apoptosis via regulating the ROS-mediated PI3K/AKT pathway in DN. J. Diabetes Res. 2019, 1–10 (2019).
Feng, Y. et al. ROS play an important role in ATPR inducing differentiation and inhibiting proliferation of leukemia cells by regulating the PTEN/PI3K/AKT signaling pathway. Biol. Res. 52, 26 (2019).
Zhang, B. F. et al. The role of AKT and FOXO3 in preventing ovarian toxicity induced by cyclophosphamide. PLoS ONE 13, e0201136 (2018).
Amin, F. et al. Systemic inflammation and oxidative stress induced by inhaled paraquat in rat improved by carvacrol, possible role of PPARγ receptors. BioFactors 47, 778–787 (2021).
Cai, J. et al. NLRP3 inflammasome mediated pyroptosis is involved in cadmium exposure-induced neuroinflammation through the IL-1β/IkB-α-NF-κB-NLRP3 feedback loop in swine. Toxicology 453, 152720 (2021).
SreeHarsha, N. Embelin impact on paraquat-induced lung injury through suppressing oxidative stress, inflammatory cascade, and MAPK/NF-κBsignaling pathway. J. Biochem. Mol. Toxicol. 34, e22456 (2020).
Liu, H. et al. High-dose acute exposure of paraquat induces injuries of swim bladder, gastrointestinal tract and liver via neutrophil-mediated ROS in zebrafish and their relevance for human health risk assessment. Chemosphere 205, 662–673 (2018).
Qiang, J. et al. Optimal combination of temperature and photoperiod for sex steroid hormone secretion and egg development of Oreochromis niloticus as determined by response surface methodology. J. Therm. Biol. 97, 102889 (2021).
Li, M. et al. Fluoride impairs ovary development by affecting oogenesis and inducing oxidative stress and apoptosis in female zebrafish (Danio rerio). Chemosphere 256, 127105 (2020).
Norhan, N. A. S. et al. Paraquat-induced histopathological changes on the gills, kidney and liver tissues of Anabas testudineus (Bloch 1792). J. Sustain. Sci. Manag. 17, 169–178 (2022).
Sule, R. O. et al. A common feature of pesticides: Oxidative stress—The role of oxidative stress in pesticide-induced toxicity. Oxid. Med. Cell. Longev. 2022, 5563759 (2022).
Snezhkina, A. V. et al. ROS generation and antioxidant defense systems in normal and malignant cells. Oxid. Med. Cell. Long. 2019, 1–17 (2020).
Schieber, M. & Chandel, N. S. ROS function in redox signaling and oxidative stress. Curr. Biol. 24, R453–R462 (2014).
Jomova, K. et al. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: chronic diseases and aging. Arch. Toxicol. 97, 2499–2574 (2023).
Ismail, R. F. et al. Impacts of microplastics on reproductive performance of male tilapia (Oreochromis niloticus) pre-fed on Amphora coffeaeformis. Environ. Sci. Pollut. Res. 28, 68732–68744 (2021).
Kumar, M. et al. Altered transcriptional levels of autophagy-related genes, induced by oxidative stress in fish Channa punctatus exposed to chromium. Fish. Physiol. Biochem. 48, 1299–1313 (2022).
Kumar, M. et al. Copper-induced genotoxicity, oxidative stress, and alteration in transcriptional level of autophagy-associated genes in snakehead fish Channa punctatus. Biol. Trace Elem. Res. 201, 2022–2035 (2023).
Kuder, R. S. & Philip, G. H. Antioxidant enzymatic activities and lipid peroxidation in liver and ovary of zebrafish (Danio rerio) exposed to deltamethrin. Chem. Ecol. 33, 739–749 (2017).
Kumar, S. et al. Toxic effects of lead exposure on hypothalamo–pituitary–ovarian axis in striped dwarf catfish, Mystus vittatus (Bloch, 1794). Proc. Zool. Soc. 77, 199–213 (2024).
Magnuson, B. et al. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. Biochem. J. 441, 1–21 (2012).
Chen, L. et al. Hydrogen peroxide inhibits mTOR signaling by activation of AMPKα leading to apoptosis of neuronal cells. Lab. Investig. 90, 762–773 (2010).
Zhou, Q. et al. Rotenone induction of hydrogen peroxide inhibits mTOR-mediated S6K1 and 4E-BP1/eIF4E pathways, leading to neuronal apoptosis. Toxicol. Sci. 143, 81–96 (2015).
Fu, H. et al. Effects of glyphosate-based herbicide-contaminated diets on reproductive organ toxicity and hypothalamic-pituitary-ovarian axis hormones in weaned piglets. Environ. Pollut. 272, 115596 (2021).
Baird, R. et al. Standard Methods for the Examination of Water and Wastewater (American Public Health Association, 2017).
OECD. Test No. 203. Fish, Acute Toxicity Testing, Section 2: Effects on Biotic Systems. Guidel. Test. Chem. 10 (2019).
OECD. Test No. 229. Fish Short-Term Reproduction Assay Guidel. Test. Chem. 189–200 (2018).
Lowry, O. et al. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275 (1951).
Kakkar, P. M. et al. A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys. Val. 21, 130–132 (1984).
Hadwan, M. H. et al. An improved method for measuring catalase activity in biological samples. Biol. Methods Protoc. 9, 1 (2024).
Aebi, H. Catalase in vitro. Methods Enzymol. 105, 121–126 (1984).
Ogunwole, G. A. et al. Antioxidant markers in gills, liver and muscle tissue of the African Sharptooth Catfish (Clarias gariepinus) exposed to subchronic levels of Ibuprofen and Dibutyl Phthalate. Sci. Afr. 12, e00816 (2021).
Ellman, G. L. Tissue Su ~ Yd ~ l groups. Arch. Biochem. Biophys. 82, 1 (1959).
Flohé, L. & Günzler, W. Assays of glutathione peroxidase. Methods Enzymol. 105, 114–121 (1984).
Carlberg, I. & Mannervik, B. Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J. Biol. Chem. 250, 5475–5480 (1975).
Mannervik, B. The isoenzymes of glutathione transferase. Adv. Enzymol. Relat. Areas Mol. Biol. 57, 357–341 (1985).
Vandesompele, J. et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3, 1 (2002).
Massar, B. et al. Micro structure analysis of the ovaries of common carp, Cyprinus carpio L. inhabiting a polluted reservoir, Umiam in Meghalaya, India. Microsc. Microanal. 20, 1404–1410 (2014).
Acknowledgements
The authors express their gratitude to the Head of the Department of Zoology, University of Lucknow for granting access to laboratory facilities. The authors also extend their sincere thanks to the University Sophisticated Instrumentation Centre, Babasaheb Bhimrao Ambedkar University, Lucknow, for providing access to the scanning electron microscope.
Author information
Authors and Affiliations
Contributions
Ms. Anamika Jain: Designing the experiment, raw data collection, and writing the first draft of the manuscript. Ms. Shefalee Singh: Experimental setup and Transcriptional analysis. Ms. Seema Yadav & Ms. Aastha Dubey: Procurement, Sampling, and maintenance of fish specimens in the wet lab. Dr. Yashika Awasthi: Statistical analysis of the data. Dr. Vidyanand Tiwari: Analysis of biochemical indices. Dr. Vivek Kumar: Analysis and interpretation of data and management of the references. Dr. Indrani Dubey: Histopathological investigation and analysis. Dr. Sunil P. Trivedi: Supervision, Validation of Methodologies. Dr. Manoj Kumar: Conceptualization, resources, review, and editing of the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The experiment was permitted by the Institutional Animal Ethics Committee (IAEC), University of Lucknow, with Approval No. 07/I/2023/IAEC/LU. Relevant guidelines and regulations were followed throughout the experiment.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jain, A., Singh, S., Yadav, S. et al. Oxidative toxicity mediated oophoritis alters ovarian growth in Channa punctatus under prolonged exposure to herbicide, paraquat dichloride. Sci Rep 15, 1304 (2025). https://doi.org/10.1038/s41598-025-85555-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-85555-5