Abstract
This study aimed to determine whether intermuscular adipose tissue (IMAT) or intramuscular adipose tissue content (IMAC) has a greater effect on skeletal muscle density (SMD) and to explore the underlying mechanisms. We recruited 292 inpatients without musculoskeletal system disease, all of whom underwent abdominal CT. Muscle parameters, including skeletal muscle area (SMA), skeletal muscle index (SMI), SMD, IMAC, and IMAT, as well as fat parameters—subcutaneous fat area (SFA) and subcutaneous fat density (SFD) in the abdominal wall—were measured by two musculoskeletal radiologists using ImageJ software at the third lumbar vertebra (L3) level. One-way ANOVA with LSD (chi-square test for group comparisons where p > 0.05) or Dunnett’s T3 test (p < 0.05) was employed to compare muscle parameters between genders and across age groups. The relationship between SMD and muscle measurements was analyzed using Spearman’s correlation coefficient. Multiple regression analysis identified and compared factors influencing SMD. SMD was highly correlated with IMAT and IMAC (p < 0.05), moderately correlated with gender, age, and SFA (p < 0.05). Multiple linear regression analysis indicated that IMAC, IMAT, and age significantly affected SMD (p < 0.05), with the order of influence being IMAT (β = -0.616), IMAC (β = -0.429), and age (β = -0.098). SFA and gender did not significantly affect SMD (p > 0.05). The findings revealed that age, IMAT, and IMAC influence SMD, with IMAT exerting the most significant impact.
Similar content being viewed by others
Introduction
Sarcopenia, first introduced by Rosenberg in 19841, refers to the age-related decline in skeletal muscle mass and quality. In 2016, sarcopenia was classified under the International Classification of Diseases (ICD-10-CM) with the code M62.842. Numerous studies have confirmed its association with aging3,4, atherosclerosis, diabetes, malignancies, and cognitive disorders5, often leading to increased morbidity, mortality, and healthcare costs.
Early research primarily focused on the link between muscle performance decline and muscle mass loss6,7,8. However, substantial reductions in skeletal muscle function with age can occur with minimal muscle mass loss9,10,11,12,13, leading to a growing interest in muscle quality. Skeletal muscle density (SMD) has been widely used in muscle quality assessment, providing a quantitative measure of the density within the muscle’s region of interest (ROI), which can be directly obtained from computed tomography (CT). Numerous studies have shown a strong correlation between low muscle density and adverse health outcomes, yet the factors contributing to muscle density loss remain unclear.
Skeletal muscle fat infiltration, known as myosteatosis, defined as the accumulation of intramuscular and intermuscular fat within skeletal muscle, serves as an indicator of poor muscle quality14. A decrease in computed tomography (CT) attenuation by 1 Hounsfield unit (HU) corresponds to an increase in lipid concentration of 1 g/100 mL15. The primary cause of altered muscle density is skeletal muscle fat infiltration, assessed by CT parameters such as intermuscular adipose tissue (IMAT) and intramuscular adipose tissue content (IMAC). IMAT, defined as adipose tissue accumulation between muscles and muscle fiber bundles, is directly associated with declines in muscle mass, flexibility, and quality. This measure, quantified by CT attenuation ranging from − 190 HU to -30 HU16,17, has been widely used to assess muscle fat infiltration, with Amini et al.18 noting that 191 of the 388 studies reviewed used IMAT as an evaluation metric.
IMAC, introduced by Kitajima in 201019, represents extra-myocyte adipose tissue within muscle fiber bundles. Calculated as the ratio of CT attenuation in the multifidus muscle to CT attenuation in abdominal wall subcutaneous fat, IMAC serves as a standardized SMD indicator, eliminating variations from CT equipment, scan parameters, and individual differences among patients. Most studies on IMAC have focused on disease prognosis, with high IMAC scores often indicating poor outcomes20. However, fat infiltration distribution within skeletal muscle at the third lumbar vertebra (L3) level is uneven. Some individuals exhibit abdominal wall muscle atrophy, while others show multifidus muscle atrophy. Kitajima’s study evaluated only the multifidus muscle, and the IMAC of a single muscle may not adequately reflect extra-myocyte fat within muscle fiber bundles or provide an accurate measure of overall muscle fat content.
It is hypothesized that increases in IMAT and IMAC contribute to reduced SMD. However, it remains unclear which factor has a greater impact on SMD. Considering this background, our study aims to conduct a cross-sectional analysis to identify factors influencing SMD in the abdominal wall and paravertebral muscle groups (SM) at the L3 level in individuals over 50 years and in postmenopausal women, using opportunistic abdominal CT scans of inpatients.
Results
Baseline characteristics
A total of 499 inpatients were recruited for this study. Of these, 112 patients were excluded due to age mismatch, 84 were excluded due to poor health status or the presence of diseases significantly affecting muscle quality, and 11 were excluded because of poor image quality. Figure 1 illustrates the recruitment process. Ultimately, 292 inpatients were enrolled, consisting of 146 males (mean age 62.25 ± 9.99 years) and 146 females (mean age 69.16 ± 9.95 years), with an overall mean age of 65.71 ± 10.54 years.
No significant differences in age or body mass index (BMI) were observed between genders within each group (Tables 1 and 2). Muscle parameters for males and females are shown in Table 3. The inter-observer reliability, measured by the intraclass correlation coefficient (ICC), exceeded 0.75, indicating excellent repeatability (Table 4).
Correlation analysis and influencing factors of SMD
Table 5 presents the correlation analysis results between SMD and various variables, including age, gender, BMI, and other muscle parameters at the L3 level. The analysis showed a strong correlation between SMD and both IMAT and IMAC (p < 0.05), a moderate correlation with gender, age, SMA, skeletal muscle index (SMI), and subcutaneous fat area (SFA) (p < 0.05), and insignificant correlation with BMI (p > 0.05).
Table 6 details the impact of SMI, IMAC, IMAT, SFA, gender, and age on SMD. Multiple linear regression analysis identified IMAC, IMAT, and age as significant factors influencing SMD (p < 0.05). The order of influence was IMAT (β = -0.616), IMAC (β = -0.429), and age (β = -0.098). Specifically, as IMAT increased by 1 unit, SMD decreased by 0.968 HU; as IMAC increased by 1 unit, SMD decreased by 17.524 HU; and as age increased by 1 year, SMD decreased by 0.097 HU. SMI, SFA and gender did not significantly influence SMD (p > 0.05).
Discussion
Our analysis of 292 inpatients using abdominal opportunistic CT demonstrated that IMAT had a greater effect on SMD than IMAC and age. Previous studies may not have fully investigated the relative impacts of IMAT and IMAC on SMD.
Most research has focused on evaluating healthy individuals21,22. However, a significant proportion of participants in our study were elderly with underlying medical conditions. Jong Hyuk Lee et al. assessed 2,720 patients undergoing annual physical examinations, finding that 12% had a history of cancer, 27% had hypertension, 14% diabetes, 11% cardiovascular disease, and 5.4% chronic liver or kidney disease23. Relying solely on direct assessments of muscle quality in such populations can lead to inaccuracies. We selected inpatients for their clear and comprehensive medical histories, facilitating the exclusion of conditions affecting muscles, reducing experimental error, and enhancing reliability.
Measurement of muscle quality is also influenced by CT equipment, tube voltage, slice thickness, and iodine contrast use. Lamba et al. recruited 48 patients over 18 years who underwent unenhanced CT scans on both GE and Siemens 64-MDCT scanners within 12 months, observing that Hounsfield unit measurements for unenhanced abdominal soft tissues varied between the two manufacturers11. Lortie et al. retrospectively analyzed the effects of iodine contrast and tube voltage on muscle mass and quality evaluation, finding that iodine contrast significantly increased muscle density and area, while lower tube voltage resulted in higher muscle density and lower muscle area, with the impact on muscle density being greater than on muscle area10. Fuchs et al. noted an 11.64% decrease in muscle density (p < 0.0001) and a 1.11% increase in muscle area (p < 0.0001) when measured on 5 mm slice thickness images compared to 2 mm slices24. Therefore, controlling these conditions is essential to ensure measurement stability and reproducibility, minimizing errors. In this study, we used the same CT scanner with standardized parameters: tube voltage at 120 kV, scan slice thickness at 10 mm, reconstructed slice thickness at 0.625 mm, reconstruction window width of 350 HU, and reconstruction window level at 40 HU.
For CT-based muscle quality assessment, inter-observer, intra-observer, and inter-software variability also play roles. Barbalho et al.25 showed that muscle parameters measured by Slice-O-Matic and OsiriX software exhibited excellent consistency (ICC ≥ 0.954, p < 0.001). Our findings demonstrated excellent inter-observer consistency (ICC > 0.75), consistent with other studies26.
Our study demonstrated a strong negative correlation between IMAC and IMAT with SMD, identifying both as influential factors for SMD. Multiple linear regression analysis revealed standardized regression coefficients of -0.616 for IMAT and − 0.429 for IMAC, indicating that a one-unit increase in IMAT led to a 0.968 HU decrease in SMD, while a one-unit increase in IMAC resulted in a 17.524 HU decrease in SMD. These findings underscore the significantly greater impact of IMAT on SMD compared to IMAC. Brennan et al. demonstrated a significant association (p < 0.01) between lower muscle density in the elderly and higher intracellular lipid concentrations; however, SMD showed a weaker correlation with intracellular lipid concentrations, being more influenced by extra-myocyte lipids and independent of age, gender, race, and obesity27. IMAT represents lipid storage in adipose cells beneath the deep muscle fascia, encompassing visible lipids stored within intermuscular fibers and adipocytes28. Therefore, we infer that muscle density is predominantly influenced by the visible fat component located between muscles and fascicles, with minimal impact from intrafascicular and intramuscular cell fat. This aligns with Brennan’s findings27. Further research is needed to determine whether muscle function and metabolism relate to the ___location of fat infiltration within muscle.
Additionally, our study demonstrated a moderate association between age and SMD, with no significant influence from gender. Aging significantly contributed to muscle quality decline in both males and females. Multiple linear regression analysis revealed a standardized regression coefficient of -0.098 for age, indicating that SMD decreased by 0.097 HU annually. Previous studies consistently reported that IMAT increases with age. Our results also align with Tetsuro Hida29, who observed that aging is associated with reduced muscle area and increased fat deposition in muscle, even after controlling for gender and ethnicity. This suggests that the effect of age on muscle quality is universal, irrespective of spinal disease presence, muscle type, gender, or ethnicity.
Our study excluded patients with conditions that could severely affect muscle quality, such as subcutaneous oedema and hemorrhage, the evaluation of the effect of IMAT and IMAC on muscle density was limited in this pathological state. As in many previous large studies, we used a fixed threshold approach to define IMAT. But in a clinical setting, variations in scanning modes and acquisition schemes could potentially affect muscle measurements and limit the power of our analysis30. Further research in this area, conducted through animal testing studies, is recommended to provide more conclusive evidence. Recently, a novel approach to muscle segmentation is beginning to emerge. The method suggested by Mühlberg et al.31 was used to estimate an individualized threshold for the IMAT by using the attenuation of the subcutaneous adipose tissue. This method is currently used for the segmentation of the pectoral and upper femoral thigh muscles31,32, and extending this approach to large-scale multi-center research applications may enable more accurate assessment.
This study has several limitations. First, we evaluated the entire abdominal wall and paraspinal muscles, which show a good linear correlation with total body muscle33. However, delineating the entire muscle group was time-consuming. Future studies should separately analyze the abdominal wall and individual paraspinal muscles to identify the best indicators of changes in muscle mass and quality. Second, due to the difficulty in distinguishing between adipose tissue within muscle bundles and intramuscular lipids, CT attenuation only reflects the overall composition within the muscle. With advances in magnetic resonance spectroscopy (MRS), intramyocellular lipids (IMCL) warrant further investigation in future studies. Finally, our study was a single-center study, and the sample size of this study was relatively small and lack of younger cohort, necessitating further validation of these conclusions through larger-scale studies.
Conclusions
Our findings indicate that IMAT has a greater effect on SMD than IMAC and age, suggesting a distinct causal mechanism for SMD reduction. This insight may encourage a stronger emphasis on preventing IMAT increase.
Materials and methods
Study Design and participants
This retrospective study recruited 499 inpatients from Tianjin Hospital between July 2019 and July 2022. Clinical information and abdominal CT scans were routinely collected for each participant. Inclusion criteria were: (1) males aged ≥ 50 years and females in a postmenopausal state, and (2) abdominal CT images with optimal clarity, showing no density impact on SM, subcutaneous fat tissue in abdominal wall (SAT), or intra-abdominal fat at the L3 level, regardless of underlying causes such as edema, hemorrhage, or artifacts34,35. Exclusion criteria included conditions severely affecting muscle quality, such as musculoskeletal, neuromuscular, malignant, and chronic wasting diseases (e.g., severe diabetes, chronic obstructive pulmonary disease, chronic liver disease). The study followed the Helsinki Declaration and was approved by the Ethics Committee of Tianjin Hospital (2024YLS061). Due to its retrospective nature, Tianjin Hospital waived the requirement for informed consent.
Computed tomography acquisition
Full abdominal spiral CT imaging was performed on all participants using a 128-slice GE Revolution ES CT scanner (GE Medical Systems, LLC*). Scans covered the region from the upper edge of the T12 vertebra to the lower edge of the L5 vertebra. Scan parameters were set as follows: tube voltage, 120 kV4,36; tube current, 230 mA; table height, 85 cm; pitch, 0.948:1; and slice thickness, 10 mm.
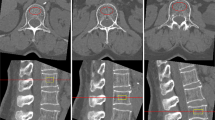
Cross-sectional abdominal images were reconstructed at the L3 level on a GE AW4.7 workstation, aligned parallel to the upper endplate of the L3 vertebra, showing the longest transverse process slice (Fig. 2). Reconstruction parameters were: standard reconstruction algorithm, 0.625 mm reconstruction thickness, window width of 350 HU, window level of 40 HU, and a display field of view (DFOV) of 45 cm.
Muscle quality assessments
Image J 1.53e (Wayne Rasband and contributors, National Institutes of Health, USA) was used for analysis. Muscle segmentation was manually performed using the “Polygon Selections” tool to outline muscle contours at the L3 level. The segmented SM included the psoas, paraspinal (erector spinae and quadratus lumborum), and abdominal wall (external and internal abdominal obliques, transversus abdominis, and rectus abdominis) muscles. SMA (cm2) and SMD (HU) values within the regions of interest were measured using the “Analyze-Measure” function, and muscle area was normalized for height in meters squared (m²) to obtain lumbar SMI (cm²/m²). Within these regions, a threshold of -190 HU to -30 HU was applied to distinguish muscle tissue from fat, allowing IMAT (%) measurement. The same method was used to outline SAT to obtain the cross-sectional area (SFA) and density (SFD) (Fig. 3).
IMAC was calculated using the following formula:
IMAC = SMD / SFD.
All measurements were independently performed by two musculoskeletal radiologists with 10 and 20 years of experience, and mean values were recorded. Figure 4 shows a visual representation of the measurement process.
All the abbreviations of muscle indicators measured at the L3 level are displayed as following:
SM: Abdominal wall and paravertebral muscle groups |
SMD: Skeletal muscle density |
SMA: Skeletal muscle area |
SAT: Subcutaneous fat tissue of abdominal wall |
SFA: Subcutaneous fat area of abdominal wall |
SFD: Subcutaneous fat density of abdominal wall |
IMAC: Intramuscular adipose tissue content |
IMAT: Intermuscular adipose tissue |
Statistical analysis
All statistical analyses were conducted using IBM SPSS Statistics Version 21 (USA). Continuous variables are expressed as mean ± standard deviation (\(\:\stackrel{-}{x}\)±s), with all continuous variables following a normal distribution. The significance level was set at α = 0.05.
The intraclass correlation coefficient (ICC) was used to assess interobserver reliability, with ICC > 0.75 indicating good agreement. An independent samples t-test compared age and BMI between men and women within the same age group. One-way ANOVA with LSD (chi-square test for group comparisons, p > 0.05) or Dunnett’s T3 (p < 0.05) was used to compare muscle parameters by gender and age group. The relationship between SMD and muscle measurements was analyzed using Spearman’s correlation coefficient. Multiple linear regression analysis identified and compared factors influencing SMD.
Data availability
The datasets used during the current study available from the corresponding author on reasonable request.
References
Rosenberg, I. H. Sarcopenia: origins and clinical relevance[J]. J. Nutr. 127 (5 Suppl), 990S–991S (1997).
Anker, S. D., Morley, J. E. & Von Haehling, S. Welcome to the ICD-10 code for sarcopenia[J]. J. Cachexia Sarcopenia Muscle. 7 (5), 512–514 (2016).
Von Haehling, S., Morley, J. E. & Anker, S. D. From muscle wasting to Sarcopenia and Myopenia: update 2012[J]. J. Cachexia Sarcopenia Muscle. 3 (4), 213–217 (2012).
Faulkner, J. A. et al. Age-related changes in the structure and function of skeletal muscles[J]. Clin. Exp. Pharmacol. Physiol. 34 (11), 1091–1096 (2007).
Wang, L. et al. Muscle density discriminates hip fracture better than computed tomography X-ray absorptiometry hip areal bone mineral density[J]. J. Cachexia Sarcopenia Muscle. 11 (6), 1799–1812 (2020).
Janssen, I. Evolution of Sarcopenia research[J]. Appl. Physiol. Nutr. Metab. 35 (5), 707–712 (2010).
Lexell, J., Taylor, C. C. & Sjöström, M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men[J]. J. Neurol. Sci. 84 (2–3), 275–294 (1988).
Lorbergs, A. L. et al. A longitudinal study of Trunk Muscle Properties and Severity of thoracic kyphosis in women and men: the Framingham Study[J]. J. Gerontol. A. 74 (3), 420–427 (2019).
Cheng, X. et al. Opportunistic screening using low-dose CT and the prevalence of osteoporosis in China: a Nationwide, Multicenter Study[J]. J. Bone Mineral. Research: Official J. Am. Soc. Bone Mineral. Res. 36 (3), 427–435 (2021).
Lortie, J. et al. The effect of computed tomography parameters on Sarcopenia and myosteatosis assessment: a scoping review[J]. J. Cachexia Sarcopenia Muscle. 13 (6), 2807–2819 (2022).
Lamba, R. et al. CT hounsfield numbers of soft tissues on unenhanced abdominal CT scans: variability between two different manufacturers’ MDCT scanners[J]. AJR Am. J. Roentgenol. 203 (5), 1013–1020 (2014).
Birnbaum, B. A. et al. Multidetector row CT attenuation measurements: assessment of intra- and interscanner variability with an anthropomorphic body CT phantom[J]. Radiology 242 (1), 109–119 (2007).
Kim, D. W. et al. Reliability of skeletal muscle area measurement on CT with different parameters: a Phantom Study[J]. Korean J. Radiol. 22 (4), 624–633 (2021).
Lee, K. et al. Recent issues on body composition imaging for Sarcopenia Evaluation[J]. Korean J. Radiol. 20 (2), 205–217 (2019).
Goodpaster, B. H., Kelley, D. E., Thaete, F. L., He, J. & Ross, R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J. Appl. Physiol. (1985). 89 (1), 104–110 (2000).
Marcus, R. L. et al. Intramuscular adipose tissue, sarcopenia, and mobility function in older individuals[J]. Journal of Aging Research, 2012: 629637. (2012).
Hamaguchi, Y. et al. Preoperative Visceral Adiposity and Muscularity Predict Poor outcomes after Hepatectomy for Hepatocellular Carcinoma[J]. Liver Cancer, 8(2). (2019).
Jiang, Z., Marriott, K. & Maly, M. R. Impact of Inter- and intramuscular Fat on muscle Architecture and Capacity[J]. Crit. Rev. Biomed. Eng. 47 (6), 515–533 (2019).
Kitajima, Y. et al. Age-related fat deposition in multifidus muscle could be a marker for nonalcoholic fatty liver disease[J]. J. Gastroenterol. 45 (2), 218–224 (2010).
Wang, W. et al. Age-related trends in CT quantitative muscle density and its relationship with bone mineral density[J]. Chin. J. Med. Imaging. 19 (12), 903–908 (2011).
Watanabe, Y. et al. Echo intensity obtained from ultrasonography images reflecting muscle strength in elderly men[J]. Clin. Interv. Aging. 8, 993–998 (2013).
Cadore, E. L. et al. Echo intensity is associated with skeletal muscle power and cardiovascular performance in elderly men[J]. Exp. Gerontol. 47 (6), 473–478 (2012).
Lee, J. H. et al. High visceral fat attenuation and long-term mortality in a health check-up population[J]. J. Cachexia Sarcopenia Muscle. 14 (3), 1495–1507 (2023).
Fuchs, G. et al. Quantifying the effect of slice thickness, intravenous contrast and tube current on muscle segmentation: implications for body composition analysis[J]. Eur. Radiol. 28 (6), 2455–2463 (2018).
Barbalho, E. R. et al. Agreement between software programmes of body composition analyses on abdominal computed tomography scans of obese adults[J]. Archives Endocrinol. Metabolism. 64 (1), 24–29 (2020).
Zuo, Y-Q. et al. Utility of multidetector computed tomography quantitative measurements in identifying Sarcopenia: a propensity score matched study[J]. Skeletal Radiol. 51 (6), 1303–1312 (2022).
Brennan, N. A. et al. Contribution of Intramyocellular Lipids to decreased computed tomography muscle density with Age[J]. Front. Physiol. 12, 632–642 (2021).
Goodpaster, B. H., Thaete, F. L. & Kelley, D. E. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus[J]. Am. J. Clin. Nutr. 71 (4), 885–892 (2000).
Hida, T. et al. Effect of race, age, and gender on lumbar muscle volume and fat infiltration in the degenerative spine[J]. J. Orthop. Science: Official J. Japanese Orthop. Association. 26 (1), 69–74 (2021).
Boutin, R. D., Lenchik, L. & Value-Added Opportunistic, C. T. Insights into osteoporosis and Sarcopenia[J]. AJR Am. J. Roentgenol. 215 (3), 582–594 (2020).
Mühlberg, A. et al. Three-dimensional distribution of muscle and adipose tissue of the thigh at CT: Association with Acute Hip Fracture[J]. Radiology 290 (2), 426–434 (2019).
Ibad, H. A. et al. CT-derived pectoralis composition and incident pneumonia hospitalization using fully automated deep-learning algorithm: multi-ethnic study of atherosclerosis[J]. Eur. Radiol. 34 (6), 4163–4175 (2023).
Schweitzer, L. et al. What is the best reference site for a single MRI slice to assess whole-body skeletal muscle and adipose tissue volumes in healthy adults?[J]. Am. J. Clin. Nutr. 102 (1), 58–65 (2015).
Baggerman, M. R. et al. Edema in critically ill patients leads to overestimation of skeletal muscle mass measurements using computed tomography scans[J]. Nutrition (Burbank, Los Angeles County, Calif.), 89: 111238. (2021).
Wang, C. et al. Quantitative CT analysis of lumbar major muscle changes in postmenopausal osteo-porotic fractures[J]. Chin. J. Bone Joint. 5 (8), 577–581 (2016).
Xia, W. et al. The impact of computed tomography-assessed Sarcopenia on outcomes for trauma patients - a systematic review and meta-analysis[J]. Injury 50 (9), 1565–1576 (2019).
Author information
Authors and Affiliations
Contributions
Conceptualization, X.G. and N.C.; methodology, X.G. and N.C.; software, X.D., N.W., R.L. and F.F.; formal analysis, X.G., N.C. and Z.H.; investigation, X.G.; resources, X.G.; data curation, X.G.; writing—original draft preparation, X.G. and N.C.; writing—review and editing, X.G., N.C., S.R., L.G., L.K., Z.H.; visualization, X.G.; supervision, Z.H.; funding acquisition, Z.H. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Guo, X., Cao, N., Deng, X. et al. Intermuscular adipose tissue affected muscle density more than intramuscular adipose tissue content with opportunistic screening at abdominal CT. Sci Rep 15, 8172 (2025). https://doi.org/10.1038/s41598-025-85946-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-85946-8
This article is cited by
-
BMI-stratified cutoff values for spinal sarcopenia in Chinese adults based on CT measures: a multicentre study
Journal of Orthopaedic Surgery and Research (2025)