Abstract
Drought is widespread worldwide and has a negative impact on the growth and development of plants. As a kind of high-quality feed resource with great potential, nettle is also facing the severe test of drought stress. At present, more and more attention has been paid to the strategy of microbial drought resistance, which is expected to bring a turning point for alleviating the survival pressure of nettles under drought. In this study, nettle plants (Urtica cannabina) were obtained from a temperate desert steppe in the Tianshan Mountains, Xinjiang, China. Polyethylene glycol (PEG) was used to simulate a high/low gradient of drought stress. The results indicate that under mild drought stress, drought damage in nettle is reduced through proline (Pro), soluble protein (SP) and soluble sugar (SS) accumulation and increased superoxide dismutase (SOD), peroxidase (POD) and catalase (CAT) activity. At the same time, the net photosynthetic rate (Pn), transpiration rate (Tr) and stomatal conductance (Gs) of nettle leaves decreased to resist mild drought stress. However, if the drought is too severe or too prolonged, nettle plants wilt considerably. Under drought stress, the community structure of endophytic bacteria in the nettle plants changed, and the relative abundances of Pseudomonas, Halomonas, Nesterenkonia and Aliihoeflea decreased, while that of Romboutsia increased. Halomonas, Romboutsia, Sphingomonas, Bifidobacterium and Pseudomonas are highly correlated with the physiological characteristics and chlorophyll content of nettle, among which Pseudomonas is the key factor of endophytic bacterial in nettle under drought stress. In this study, the changes of physiological characteristics and endophytic bacterial community of Urtica cannabina under different degrees of drought stress provided a preliminary foundation for field experiments under natural drought conditions and the verification of drought-related microorganisms.
Similar content being viewed by others
Introduction
Nettle is a perennial herb1, which is widely distributed in the world and has great feeding2,3, medicinal and ecological value4,5. It can also be used as a renewable source of fiber for oil absorbency6. Additionally, the use of nettle as animal feed could improve animal production and resistance to infection and disease because nettle leaves contain high levels of nutrition and biologically active compounds1,7.
Plant growth is influenced by many factors, among which environmental factors are particularly prominent, and drought stress is the most widespread among environmental factors. Climate change is a key factor for drought stress, which causes several problems that affect plant growth and development8. Researchers observed 21% and 41% reductions in wheat and maize yield attributed to drought stress worldwide9. In addition, drought could cause serious plant growth issues in over 50% of land area by 205010. Because of the sustainability and environmental friendliness of microbial strategy to agriculture, the study of microbial-assisted drought stress resistance strategy has become the focus of attention at present8. Several studies have reported that rhizosphere bacteria, such as promoting rhizobacterial (PGPR) strains, have a strong ability to promote plant growth under drought stress10. However, endophytic bacteria (living inside of roots or tissues of plants) are more efficient in protecting plants from environmental stress due to their ability to live inside plants11.
There are few studies on drought stress of nettles, and all of them focus on the physiological and photosynthetic characteristics of nettles under drought stress. For example, the research on photooxidative stress and photoprotection of nettles by Bismancas et al. shows that severe drought stress will significantly increase the degree of lipid peroxidation of nettles, and the application of NPK fertilizer can reduce this degree12,13. Considering that the fungi and bacteria living on and in plants and in the rhizosphere were both involved in the response of plants to drought stress, the co-occurrence networks of rhizosphere fungi and leaf bacteria were strengthened due to drought14. Clearly, leaf bacteria are also important for the plant response to drought stress. However, most studies have focused on the subsurface parts of plants15,16, and thus far, the effects of aboveground endophytic bacteria on plant growth remain unclear8. To our knowledge, no study has focused on the endophytic bacterial community in nettle leaves. Thus, we hypothesize that the endophytic bacterial community probably plays a role in the resistance of nettle to drought stress. To provide a preliminary basis for identifying microbial strategies for plants under drought stress, we studied the effects of drought stress at different levels on the physiological characteristics and endophytic bacterial communities of nettle plants and investigated the correlations between these two factors.
Materials and methods
Simulation of drought stress in nettle
The simulated drought experiment was conducted in Shawan County, central mountains of Tianshan, Xinjiang, China (E 84°58′–86°24′; N 43°26′–45°20′) were randomly selected (July 06. 2021), and the local area is a warm grassland and has a temperate continental arid and semiarid climate. The winter is long and cold, while the summer is short and hot. The temperature difference between day and night is large, with an average annual temperature of 17.7 ℃. The frost-free period is 165–172 d throughout the year, and the annual precipitation is 545 mm. 90 naturally growing nettle plants of the same age in the local area, which are free of diseases and pests and have an intact and uniform appearance and growth, were selected as the experimental materials. 30 nettles with distilled water as the control group, 30 nettles with 15% polyethylene glycol (PEG, Sigma, molecular weight 6000) water solution (3:17, PEG: water, v/v) as the low level of drought stress treatment (TL), and 30 nettles with 30% PEG water solution (3:7, PEG: water, v/v) as the high level of drought stress treatment (TH). Each nettle plant treated with TL and TH was irrigated with 2 L of 15% and 30% PEG6000, respectively, divided into 4 watering sessions with an interval of 2 h (the previous irrigation was fully absorbed). After the photosynthetic parameters of each treatment group were measured at 0, 1, 4, and 8 d, complete nettle leaf samples were randomly collected from each treatment group and control group and stored in liquid nitrogen containers until further analysis. On the same day, the relative water content and chlorophyll content of the leaves were measured, and the remaining samples were stored in a -80℃ ultralow temperature refrigerator. After all the samples were collected, other physical and chemical properties were analyzed.
Characteristics of nettle leaf during drought stress
A JK-100 soil moisture detector (Zhonglutong, China) was used to measure the soil moisture content (SW) of the nettle root soil at a depth of 40 cm, and the relative water content (RWC) of nettle leaves was determined by the drying and weighing method as described by Bowers, 201617.
Relative water content of leaves: RWC (%) = (leaf fresh weight-leaf dry weight)/(saturated fresh weight-leaf fresh weight)×100%. Chlorophyll content was determined by grinding and filtering with 95% ethanol18. Chlorophyll content (mg/g)= (chlorophyll concentration × extract volume × dilution multiple)/sample fresh weight.
The acidic ninhydrin method was used to determine the proline (Pro) content, the soluble protein (SP) content was determined using Coomassie Brilliant Blue staining, the soluble sugar (SS) content was determined using the anthrone colorimetric method, the malondialdehyde (MDA) content was determined using the thiobarbituric acid method, and the peroxidase (POD), superoxide dismutase (SOD), and catalase (CAT) contents in the leaves of the nettle were measured according to the methods of Zhai19. Make 5 technical copies of each sample.
The chlorophyll content was measured via the 95% ethanol grinding filtration method under dark conditions, and the photosynthetic parameters were measured by a hand-held CI-340 photosynthetic measuring instrument (CID, Inc.). The field measurement was carried out when the light intensity was stable at 1500 p·µmol/m−2·s−1 on sunny days. The light intensity was measured for 2 h from 10:00 to 12:00, 5 leaves were measured for each plant, and each leaf was measured 3 times. The instrument automatically records parameters such as net photosynthetic rate (Pn), transpiration rate (Tr), intercellular CO2 concentration (Ci), and stomatal conductance (Gs) and calculates the water use efficiency (WUEi) of leaves17.
Isolation of endophytic bacteria from nettle leaves
According to Siddique20, 70% ethanol/water solution (v/v) was used for surface sterilization of nettle leaves for 1 min, and then the leaves were chopped into 1 cm pieces, which were processed under sterile conditions at an aseptic work table. All samples were stored at −80 °C for further analysis.
The DNA of bacterial community extract and sequence analysis
The collected nettle leaves were surface sterilized (soaked in 75% alcohol for 1 min and rinsed with sterile water). The total DNA of each sample was extracted with a commercial DNA Kit (FastDNA® Spin Kit for Soil, MP Biomedicals, New York, NY, USA). According to21, the V4-V5 region of bacterial 16 S rDNA was amplified by blocking PCR to remove the influence of chloroplasts. The primers used were (515 F: 5’- GTGCAGAGCCGGTAA-3’, 926R: 5’- CGTCAATTCMTTTRAGTTT-3’), and the amplicons were extracted and further analyzed, sequencing by Parseno platform (Personalbio Biopharm Technology Corporation, Shanghai, China). The Quantitative Insights Into Microbial Ecology (QIIME, v1.8.0) pipeline was employed to process the sequencing data, as previously described22. OTU classification was conducted by BLAST searching the representative sequences against the Greengenes Database using the best hit23. The raw sequences of all samples were deposited in the NCBI Sequence Read Archive under accession number: PRJNA911972.
Statistical analysis
The characteristics of the nettle leaves were subjected to a two-way analysis of variance (ANOVA) with a 3 × 4 factorial complete randomized design (TL, TH and control groups × 0, 1, 4 and 8 four drought stress days). The data were analyzed using IBM SPSS 22 Statistics (IBM Corp., Armonk, NY, United States). Significant differences between treatments were determined using Tukey’s test at p < 0.05. Sequence data analyses were mainly performed using QIIME and R packages (v3.2.0) through the GeneCloud platform (Personalbio Biopharm Technology Corporation, Shanghai, China, https://www.genescloud.cn). Briefly, alpha diversity indicxes such as the Chao1 richness estimator, Shannon diversity index, and Simpson index were calculated using the OTU table in QIIME. Beta diversity analysis through UniFrac distance metrics24. The significance of the differentiation of microbiota structure among groups was assessed by permutational multivariate analysis of variance (PERMANOVA) and analysis of similarities (ANOSIM)25,26. LEfSe (linear discriminant analysis effect size) was performed to detect differentially abundant taxa across groups using the default parameters27.
Results
The characteristics of nettle leaves affected by drought stress
Overall, under simulated drought stress, compared with the CK treatment, significant changes were observed in SW, RWC and osmoregulatory substances under TL and TH treatment (Table 1).With increasing duration and severity of drought stress, On the 8th day, the SW of TL treatment decreased by 55.3%, TH treatment decreased by 69.29%, and RWC decreased by 48.87% and 57.46% respectively (p < 0.05), ultimately leading to wilting or even withering of nettle leaves (Fig. 1). The proline content continued to increase, with the maximum increase in TL (91.61%) and TH (155.6%) on the 8th day, and TH treatment remained higher than TL treatment. With the increase of stress time, the SP content of TL treatment showed an upward trend, while TH treatment first increased and then decreased. TL and TH treatments were significantly higher than CK on the 4th day (p < 0.05), but on the 8th day, the SP content of TH treatment decreased to the same level as CK. The SS content of TL treatment showed a trend of first decreasing and then increasing, while TH treatment showed a trend of first decreasing, then increasing and then decreasing. On the 4th day, TH treatment was significantly higher than TL and CK (p < 0.05), and on the 8th day, TL treatment was significantly higher than TH and CK (p < 0.05). SW, RWC, SP and SS showed obvious dose-dependent relationship, but this phenomenon of SP and SS disappeared on the 8th day of TH treatment.
Growth of nettle under drought stress. CK, TL and TH represent the control, low-gradient drought stress and high-gradient drought stress treatments, respectively, and 0 d, 1 d, 4 d, and 8 d represent different days. CK0d: control on day 0; CK1d: control on day 1; CK4d: control on day 4; CK8d: control on day 8; TL0d: lower drought stress on day 0; TL1d: lower drought stress on day 1; TL4d: lower drought stress on day 4; TL8d: lower drought stress on day 8; TH0d: greater drought stress on day 0; TH1d: greater drought stress on day 1; TH4d: greater drought stress on day 4; TH8d: greater drought stress on day 8.
With the increase of stress time, there was no change in CK treatment, but the MDA content in TL treatment significantly increased. Compared with CK, the MDA content increased by 160.82% on the 8th day. Compared with CK, the difference of TH treatment increased by 155.90% on the 4th day, but decreased significantly from the 8th day and was lower than CK (p < 0.05). With the prolongation of drought stress time, the activities of SOD, POD, and CAT in TL treatment significantly increased, while TH treatment showed an initial increase and began to decrease after the 4th day. On the 8th day, the SOD and CAT activities in the TH treated group were significantly lower than those in the CK group (p < 0.05). MDA, SOD, POD and CAT also showed a clear dose-dependent relationship, but this phenomenon disappeared on the 8th day of TH treatment(Table 2).
The results showed (Tables 3 and 4) that under simulated drought stress, the photosynthetic parameters (Pn, Tr and Gs), WUEi and chlorophyll content of the nettle leaves decreased significantly (p < 0.05). On the 8th day, the chlorophyll a content decreased by 42.43% and 70.59% under the TL and TH treatments, respectively, and the chlorophyll b content decreased by 32.37% and 65.77% under the TL and TH treatments, respectively. Compared to TL treatment, TH treatment showed a more severe decrease in chlorophyll content. However, the Ci in the TL treatment first decreased and then increased, while that in the TH treatment increased significantly (p < 0.05). The photosynthetic parameters (Pn, Tr and Gs) of the nettle leaves increased in a dose-dependent manner on the 4th and 8th days, while the WUEi and chlorophyll content increased in a dose-dependent manner on the 8th day.
Bacterial community analysis of nettle leaves
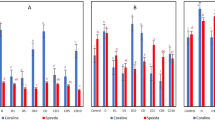
The sequencing results of endophytes from nettle under drought stress showed that 8,057,675 high-quality sequences were obtained, with an average sequence length of 376 bp. The sequencing depth is sufficient to comprehensively reflect the microbial composition of nettle samples under drought stress. Figure 2 shows that the addition of PEG to simulate drought stress had an impact on the richness and diversity of endophytic bacteria in nettle leaves to a certain extent. Compared with those in the CK group, the Chao1 and observed species indices of endophytic bacteria in the nettle leaves increased to varying degrees, except for those in the TH treatment group on the 4th day. These indicators showed maximum values on the 8th day of TL processing. Shannon index of TL and TH treatments was higher than CK, but the difference was not significant.
Phylum-level bacterial community analysis revealed that (Fig. 3A) Proteobacteria (39.14–62.74%) was dominant, followed by Actinobacteria (15.26–33.25%) and Firmicutes (5.58–21.59%), in the nettle. During drought stress simulation, the abundance of Proteobacteria tended to decrease, while that of Actinobacteria and Firmicutes tended to increase. Specifically, compared with CK, Proteobacteria treated with TL and TH decreased by 32.93% and 32.94% on the 1st day of drought stress, by 14.75% and 9.53% on the 4th day, and by 23.54% and 13.18% on the 8th day. Compared with CK, Actinobacteria treated with TL and TH increased by 11.02% and 9.78% on the 1st day, by 3.58% on the 4th day and by 20.92% on the 8th day. Compared with CK, Firmicutes treated with TL and TH increased by 9.63% and 7.09% on the 1st day, 3.98% and 5.31% on the 4th day and 3.5% and 17.78% on the 8th day.
Genus-level bacterial community analysis indicated that (Fig. 3B), the four bacteria with the highest relative abundances on CK0d were Halomonas, Pseudomonas, Nesterenkonia and Romboutsia in nettle, with relative abundances of 10.05%, 4.43%, 3.51% and 3.39%, respectively. On the 1st day of drought stress, Halomonas was dominant in CK, TL and TH treatments (16.41%, 8.15% and 9.37%), followed by Pseudomonas (14.51%), Romboutsia (5.28%) and Romboutsia (4.17%), respectively. On the 4th day of drought stress, the dominant treatments of CK, TL and TH were Pseudomonas (24.05%), Spiroplasma (8.83%) and Pseudomonas (16.05%), respectively, Halomonas (relative abundance: 9.36%, 7.27%, 9.86%, respectively) followed. On the 8th day of drought stress, the dominant treatments of CK, TL and TH were Pseudomonas (22.35%), Bifidobacterium (8.76%), Pseudomonas (6.68%), respectively, followed by Halomonas (6.82%), Pseudomonas (7.27%), Arthrobacter (4.87%), respectively.
During drought stress simulation, compared with the CK1d, the relative abundance of Pseudomonas decreased rapidly after 1 d of TL and TH treatment, decreasing by 67.15% and 90.45%, respectively, followed by decreases in that of Halomonas, Nesterenkonia, Aliihoeflea and Ochrobactrum. However, the relative abundance of Romboutsia increased after 1 d of TL and TH treatment when compared with the control (increased 210.04% and 144.88%, respectively). The relative abundance of Pseudomonas decreased rapidly after 4 d of TL treatment, decreasing 82.12% when compared with the control; however, the relative abundance of Nesterenkonia increased 45.22% when compared with the control after 4 d of TH treatment; the relative abundance of Romboutsia increased in both the TL and TH treatments when compared with the control, which were 175.20% and 268.56%, respectively. The relative abundance of Pseudomonas decreased after 8 d of TL and TH treatment when compared with the control, which were 84.67% and 74.09%, respectively, followed by a decrease in the relative abundance of Halomonas in both the TL and TH treatments. The relative abundances of Romboutsia and Aliihoeflea were increased after 8 d of TH treatment when compared with the control, which were 154.16% and 61.29%, respectively. Additionally, the TL treatment increased the relative abundance of Nocardioides after 8 d of simulation, which increased by 48.53% when compared with the control group. The LEfSe analysis among the control, TL and TH groups on different simulation days is shown in Fig. 4. Considering that the relative abundance of bacteria > 1%, the LAD results showed (LAD > 2, p < 0.05) that the most significant difference in bacteria at the genus level was in Halomonas after 1 d of simulation of drought stress in the control group (p < 0.05). The most significantly different bacteria at the genus level was in Nocardioides after 8 d of drought stress simulation in the TL group (p < 0.05).
Correlation analysis between bacteria and characteristics of nettle leaves
As shown in Fig. 5A–C, spearman was used to analyze the correlation between the characteristic factors and the samples on the 0–8 day of drought stress simulation, with p < 0.05 as the statistical basis. In CK, only Halomonas and Romboutsia were negatively correlated with SP (R = −0.57 and R = −0.52). Romboutsia was positively correlated with CAT (R = 0.58). In TL, Halomonas was positively correlated with SW, RWC and Ch1 respectively (R = 0.62, R = 0.66, R = 0.63), and negatively correlated with Pro, SP, SS and CAT respectively (R = −0.69, R = −0.69, R = −0.66, R = −0.61). Romboutsia was positively correlated with SW, RWC and Chl (R = 0.78, R = 0.84, R = 0.74), and negatively correlated with MDA, SP and POD (R = −0.74, R = −0.73, R = −0.61). Spiroplasma was positively correlated with SS (R = 0.53), and Bifidobacterium was positively correlated with Pro, SS and CAT respectively (R = 0.61, R = 0.68, R = 0.62). In TH, Pseudomonas was positively correlated with SP and Pro (R = 0.66, R = 0.66). Halomonas was positively correlated with Ch1 (R = 0.59) and negatively correlated with pro (R = −0.55). Romboutsia was positively correlated with SOD (R = −0.63) and Nesterenkoniawas positively correlated with MDA, SP, CAT (R = 0.74, R = 0.59, R = 0.59). Redundancy analysis shows that (Fig. 5a–c) Pseudomonas and Halomonas have certain influence on the composition of nettle community, Spiroplasma and Bifidobacterium are associated with shifts in the composition of the nettle community under drought stress. Pseudomonas and Halomonas are significant contributors to the nettle community structure under drought stress.
Heatmaps of the correlation analysis for bacteria and characteristics of nettle leaves. A and a: 1–8 days control, B and b: 1–8 days the low level of drought stress treatment (TL), C and c: 1–8 days the high level of drought stress treatment (TH). ∗p < 0.05. SS: soil moisture content; RWC: relative moisture content of leaves; Ch1: chlorophyll; MDA: malondialdehyde; PRO: proline; SP: soluble protein; SS: soluble carbohydrate; POD: peroxidase; CAT: catalase; SOD: superoxide dismutase. The control and low drought stress (TL) treatments are not statistically significant (p = 0.87 and p = 0.63, respectively), whereas the TH condition is significant (p = 0.033).
Discussion
The characteristics of nettle leaves during simulated drought stress
In general, the SW and RWC decreased with prolonged time and drought development, as described by Xia28. In the present study, long-term (TL 8th day) or severe drought stress (TH 1ht day) significantly changed the shape of nettle leaves, resulting in heavy damage (Fig. 1). Plants resist drought stress by accumulating many osmotic adjustment substances, such as SP, SS and especially Pro29,30. In the present study, the SP content increased in a dose-dependent manner after 4 d of drought stress, but the same effect of PEG on Pro was shown only after drought stress was prolonged to 8 d. Based on the duration of drought stress, SP is more sensitive to drought stress, and Pro is more important for plants to resist drought stress.
The MDA content is often used as a common index in the study of plant resistance physiology to indirectly judge the degree of damage and stress resistance of plant biofilm systems31. In the present study, the content of MDA increased in a dose-dependent manner with PEG treatment after four days of drought stress. On the 8th day of drought stress, severe drought stress results in the excessive accumulation of MDA in plants, indicating that the plants lose their capacity for enzyme synthesis, resulting in reduced POD, SOD and CAT activities19,32. In the present study, the changes in the enzyme activities of SOD, POD and CAT in the two treatments were basically the same, indicating that the three enzymes played a synergistic role under drought stress in plants33. Combined with changes in chlorophyll content, the photosynthesis of nettles was affected after four days of drought stress, which may be related to the accumulation of reactive oxygen species under drought stress leading to damage to chloroplast structure or accelerated chlorophyll decomposition34.
In the present study, Pn, Tr, and Gs decreased, which is a normal regulatory plant response to drought stress35. Ci decreased on the 4th day of TL treatment, at which time Pn decreased mainly due to stomatal restriction. On the 8th day of TH treatment and TL treatment, Ci increased, indicating that photosynthesis was damaged and that the decrease in Pn was dominated by nonstomatal restriction35. In addition, drought stress can reduce WUEi, and the more severe the stress, the more pronounced it becomes36 .
Bacterial community analysis of nettle leaves during drought stress
When nettle is faced with drought stress, it will alleviate the harm caused by drought by increasing the diversity and richness of endophytes37, while the richness and diversity of endophytic bacteria in the TH treatment on the 8th day are lower than those in the TL treatment. This result may be due to severe and prolonged drought stress leading to leaf wilting and yellowing, resulting in a decrease in the diversity of endophytic bacteria in the nettle leaves. In addition, the addition of PEG clearly changed the endophytic bacterial community structure on nettle leaves. In general, simulation of drought stress on nettle decreased Proteobacteria activity but increased Actinobacteria, Firmicutes and Bacteroidetes activity. The abundance of Actinobacteria can increase due to drought stress, which enhances plant resistance to drought stress38.
Overall, at the genus level, simulated drought stress decreased Pseudomonas, Halomonas, Nesterenkonia, Aliihoeflea, and Ochrobactrum activity but increased Romboutsia activity in nettle leaves. The high level of drought stress rapidly decreased Pseudomonas activity within 1 day; however, this rapid effect disappeared as the duration of drought stress increased. The nine isolated strains of Pseudomonas showed the capacity to tolerate drought under conditions via the addition of 15% PEG39. As the present study results show, the TH treatment (30% PEG) probably quickly disturbed Pseudomonas bacteria in nettle leaves. The simulation of drought stress on nettle decreased Halomonas activity in both low and high levels of drought stress. Halomonas comprises halophilic and/or halotolerant gram-negative aerobic bacteria, usually found in saline environments, which can survive at a maximum of 30% salt (%, w/v)40. The results of the present study suggested that Halomonas is probably transferred to the rhizosphere of Plants because it prefers saline environments. The TL treatment was more effective in inhibiting Nesterenkonia, Aliihoeflea, and Ochrobactrum activity than the TH treatment during the simulation of drought stress on nettle. Like Halomonas, Nesterenkonia are also halotolerant and/or halophilic bacteria41. The results of the present study showed that different levels of drought stress affected the relative abundance of Nesterenkonia but consistently affected the relative abundance of Halomonas. Thus, the Nesterenkonia activity response to drought stress conditions is likely more complicated than that of Halomonas. However, simulation of drought stress on nettle increased Romboutsia activity; TH treatment showed more effect when compared with low level after 8 d of simulation. Recently, researchers observed that alkaloid levels significantly affected Romboutsia activity42. Consistently, alkaloids, which facilitate plant photosynthesis and cell osmolarity and even delay leaf senescence during drought stress, have been observed in increased abundance43. The present study suggested that increased Romboutsia activity is probably correlated with the response of some compounds by plants to drought stress. Additionally, Nocardioides activity increased after 8 d of low-level drought stress on nettle. These results indicate that endophytic bacteria can help host plants regulate and increase their resistance to drought stress to a certain extent by adjusting their own community structure44.
Correlation analysis between bacteria and characteristics of nettle leaves during drought stress
Usually, endophytic bacteria promote plant tolerance to drought stress through nitrogen fixation, phosphate solubilization, mineral uptake, and the production of siderophores, 1-aminocyclopropane-1-carboxylate (ACC) deaminase, and various phytohormones8. In the present study, with the extension and deepening of drought stress, Pseudomonas occupies a dominant position in the endophytic community structure of nettle, and is closely related to more characters of nettle. These results probably suggest that Pseudomonas had more effects on the characteristics of nettle with prolonged drought stress. Maize plants inoculated with Pseudomonas aeruginosa Pa2 exhibit a maximum decrease in the activity of antioxidant enzymes such as POD, SOD, and CAT and decreased proline content during drought stress45. However, some strains of Pseudomonas could increase the stress tolerance of maize by accumulating proline under drought stress46. Considering that these two studies used the same methods for inoculation with Pseudomonas for maize, the reason for the opposite was mainly from the different strains of Pseudomonas. The reason why inoculation with Pseudomonas in plants results in increased proline content under drought stress is probably due to the upregulation of the proline biosynthesis pathway, in which a high level of proline could protect cells under drought stress30. Under drought stress, bacteria can accumulate high concentrations of organic solutes within the cytoplasm, including polyols, sugars or amino acids, and derivatives of these classes of molecules to prevent water loss from cells40. In addition, as shown in the present results, the Pseudomonas strain in nettle probably did not have the ability to increase proline under drought stress conditions. The proline content of nettle increased with prolonged drought stress time, which indicates that the proline biosynthesis pathway is probably upregulated during drought stress.
Considering the bacteria, treatments, and characteristics of the nettle, the redundancy analysis is to find out the most influential factors in the whole drought stress simulation process. Because there is no significant difference between CK and TL (p > 0.05), the current results show that Pseudomonas may be the key factor involved in plant drought stress response. Many studies have shown that Pseudomonas can help plants resist drought stress37,47, but its potential mechanism is complex. However, these findings are only based on mutual correlation, and the specific mechanism needs further research, such as functional testing, to confirm these conclusions. In addition, PEG-induced osmotic stress can not replicate all aspects of natural drought conditions, and we will verify the research results in the future under natural drought conditions.
Conclusion
Under the condition of shallow drought or short stress time, the SW, RWC, SP, SS, MDA, SOD, POD and CAT of nettle showed obvious dose-dependent relationship, but with the deepening of drought and the extension of time, this phenomenon of SP, SS, MDA, SOD, POD and CAT disappeared. Drought stress reduced the relative abundance of Pseudomonas and Halomonas in nettle leaves but increased the relative abundance of Romboutsia. Halomonas, Romboutsia, Sphingomonas, Bifidobacterium and Pseudomonas are highly correlated with the physiological characteristics and chlorophyll content of nettle, among which Pseudomonas is found to be the most significant contributor of endophytic bacterial in nettle under drought stress. In this study, the changes of endophytic bacterial community and physiological characteristics of Urtica cannabina under different degrees of drought stress were preliminarily revealed. It provides a preliminary research basis for the subsequent field experiments under natural drought conditions and the inoculation verification of plant drought-resistant microbial strategies.
Data availability
The raw sequences of all samples were deposited in the NCBI Sequence Read Archive under accession number: PRJNA911972.The data used in the study analyses can be made available by the corresponding author upon reasonable request.
References
Kregiel, D., Pawlikowska, E. & Antolak, H. Urtica spp.: Ordinary plants with extraordinary properties. Molecules 23, 1664. https://doi.org/10.3390/molecules23071664 (2018).
Durovic, S. et al. Chemical composition of stinging nettle leaves obtained by different analytical approaches. J. Funct. Foods. 32, 18–26. https://doi.org/10.1016/j.jff.2017.02.019 (2017).
Kianbakht, S., Khalighi-Sigaroodi, F. & Dabaghian, F. H. Improved glycemic control in patients with advanced type 2 diabetes mellitus taking Urtica dioica leaf extract: A randomized double-blind placebo-controlled clinical trial. Clin. Lab. 59, 1071–1076. https://doi.org/10.7754/Clin.Lab.2012.121019 (2013).
Opacic, N. et al. Nettle cultivation practices-from open field to modern hydroponics: A case study of specialized metabolites. Plants-Basel 11, 483. (2022). https://doi.org/10.3390/plants11040483
Shah, M. A., Bosco, S. J. D. & Mir, S. A. Plant extracts as natural antioxidants in meat and meat products. Meat Sci. 98, 21–33. https://doi.org/10.1016/j.meatsci.2014.03.020 (2014).
Viju, S. & Thilagavathi, G. Effect of Alkali treatment of Nettle fibers on oil absorbency. J. Nat. Fibers. 18, 2092–2101. https://doi.org/10.1080/15440478.2020.1723776 (2021).
Disler, M., Lvemeyer, S. & Walkenhorst, M. Ethnoveterinary herbal remedies used by farmers in four north-eastern Swiss cantons (St. Gallen, Thurgau, Appenzell Innerrhoden and Appenzell Ausserrhoden). J. Ethnobiol. Ethnomed. 10, 32. https://doi.org/10.1186/1746-4269-10-32 (2014).
Ullah, A. et al. Drought tolerance improvement in plants: An endophytic bacterial approach. Appl. Microbiol. Biotechnol. 103, 7385–7397. https://doi.org/10.1007/s00253-019-10045-4 (2019).
Daryanto, S., Wang, L. X. & Jacinthe, P. A. Global synthesis of drought effects on maize and wheat production. Plos One. 11, e0156362. https://doi.org/10.1371/journal.pone.0156362 (2016).
Vurukonda, S. S. K. P., Vardharajula, S., Shrivastava, M. & SkZ, A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 184, 13–24. https://doi.org/10.1016/j.micres.2015.12.003 (2016).
Lata, R., Chowdhury, S., Gond, S. K. & White, J. F. Induction of abiotic stress tolerance in plants by endophytic microbes. Lett. Appl. Microbiol. 66, 268–276. https://doi.org/10.1111/lam.12855 (2018).
Bansal, S. et al. Changes in crop physiology under drought stress: A review. J. Pharmacognosy Phytochemistry. 8(4), 1251–1253 (2019).
Simancas, B., Juvany, M., Cotado, A. & Munne-Bosch, S. Sex-related differences in photoinhibition, photo-oxidative stress and photoprotection in stinging nettle (Urtica dioica L.) exposed to drought and nutrient deficiency. J. Photochem. Photobiology B-Biology. 156, 22–28. https://doi.org/10.1016/j.jphotobiol.2016.01.005 (2016).
Gao, C. et al. Co-occurrence networks reveal more complexity than community composition in resistance and resilience of microbial communities. Nat. Commun. 13, 3867. https://doi.org/10.1038/s41467-022-31343-y (2022).
Meenakshi, A. K. et al. Mitigation of drought stress in wheat crop by drought tolerant endophytic bacterial isolates. Vegetos 32(4), 486–493. https://doi.org/10.1007/s42535-019-00060-1 (2019).
Salvi, P. et al. Advancement in the molecular perspective of plant-endophytic interaction to mitigate drought stress in plants. Front. Microbiol. 13, 981355. https://doi.org/10.3389/fmicb.2022.981355 (2022).
Bowers, J. E. Effects of drought on shrub surival and longevity in the northermn Sonoran deser. J. Torrey Bot. Soc. 132(03), 421–431 (2016).
González-Villagra, J. et al. Salicylic acid improves antioxidant Defense System and Photosynthetic Performance in Aristotelia chilensis plants subjected to Moderate Drought stress. Plants 11, 639. https://doi.org/10.3390/plants11050639 (2022).
Zhai, H. et al. A myo-inositol-1-phosphate synthase gene, IbMIPS1, enhances salt and drought tolerance and stem nematode resistance in transgenic sweetpotato. Plant Biotechnol. J. 14(2), 592–602. https://doi.org/10.1111/pbi.12402 (2016).
Siddique, S., Naveed, M., Yaseen, M. & Shahbaz, M. Exploring potential of seed endophytic Bacteria for Enhancing Drought stress resilience in Maize (Zea mays L). Sustainbility 14, 673. https://doi.org/10.3390/su14020673 (2022).
Su, R. N. et al. Effects of ferulic acid esterase-producing Lactobacillus fermentum and cellulase additives on the fermentation quality and microbial community of alfalfa silage. PeerJ 7, e7712. https://doi.org/10.7717/peerj.7712 (2019).
Kumaishi, K. et al. High throughput method of 16S rRNA gene sequencing library preparation for plant root microbial community profiling. Sci. Rep. 12(1), 19289. https://doi.org/10.1038/s41598-022-23943-x (2022).
Samal, K. C. et al. Understanding the BLAST (Basic Local Alignment Search Tool) program and a step-by-step guide for its use in life science research. Bhartiya Krishi Anusandhan Patrika. 36(1), 55–61. https://doi.org/10.18805/BKAP283 (2021).
Lozupone, C. & Knight, R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 71, 8228–8235. https://doi.org/10.1128/AEM.71.12.8228-8235.2005 (2005).
Baho, D. L. et al. Assessing temporal scales and patterns in time series: comparing methods based on redundancy analysis. Ecol. Complex. 22, 162–168. https://doi.org/10.1016/j.ecocom.2015.04.001 (2015).
Cheng, Z. et al. Metagenomic and machine learning-aided identification of biomarkers driving distinctive cd accumulation features in the root-associated microbiome of two rice cultivars. ISME Commun. 3(1), 14 (2023).
Segata, N. et al. Metagenomic biomarker discovery and explanation. Genome Biol. 12, R60 (2011).
Xia, H. et al. Melatonin alleviates Drought stress by a non-enzymatic and enzymatic antioxidative system in Kiwifruit Seedlings. Int. J. Mol. Sci. 21, 852. https://doi.org/10.3390/ijms21030852 (2020).
Shinozaki, K. Molecular responses to dehydration and low temperature: Differences and cross-talk between two stress signaling pathways. Curr. Opin. Plant. Biol.. (3), 217–223. https://doi.org/10.1016/S1369-5266(00)80068-0 (2000).
Ozturk, M. et al. Osmoregulation and its actions during the drought stress in plants. Physiol. Plant. 172(2), 1321–1335. https://doi.org/10.1111/ppl.13297 (2021).
Yang, X. et al. Response mechanism of plants to Drought stress. Horticulturae 7, 50. https://doi.org/10.3390/horticulturae7030050 (2021).
Carraro, E. & Di Iorio, A. Eligible strategies of drought response to improve drought resistance in woody crops: A mini-review. Plant. Biotechnol. Rep. 16(3), 265–282. https://doi.org/10.1007/s11816-021-00733-x (2022).
Zhanassova, K. et al. ROS status and antioxidant enzyme activities in response to combined temperature and drought stresses in barley. Acta Physiol. Plant. 43, 1–12. https://doi.org/10.1007/s11738-021-03281-7 (2021).
Liang, G. et al. Effects of drought stress on photosynthetic and physiological parameters of tomato. J. Am. Soc. Hortic. Sci. 145 (1), 12–17. https://doi.org/10.21273/JASHS04725-19 (2020).
Zahra, N. et al. Plant photosynthetic responses under drought stress: Effects and management. J. Agron. Crop. Sci. 209(5), 651–672 (2023).
Yan, K. et al. Responses of photosynthesis and photosystem II to higher temperature and salt stress in sorghum. J. Agron. Crop. Sci. 198(3), 218–225. https://doi.org/10.1111/j.1439-037X.2011.00498.x (2012).
Kour, D. & Yadav, A. N. Bacterial mitigation of Drought stress in plants: Current perspectives and Future challenges. Curr. Microbiol. 79, 248. https://doi.org/10.1007/s00284-022-02939-w (2022).
Song, Y. & Haney, C. H. Drought dampens microbiome development. Nat. Plants. 7, 994–995. https://doi.org/10.1038/s41477-021-00977-z (2021).
Ali, S. Z., Sandhya, V. & Rao, L. V. Isolation and characterization of drought-tolerant ACC deaminase and exopolysaccharide-producing fluorescent Pseudomonas Sp. Ann. Microbiol. 64, 493–502. https://doi.org/10.1007/s13213-013-0680-3 (2014).
Kim, K. K., Lee, J. S. & Stevens, D. A. Microbiology and epidemiology of Halomonas species. Future Microbiol. 8, 1559–1573. https://doi.org/10.2217/fmb.13.108 (2013).
Wang, S., Sun, L., Rao, M. P. N., Fang, B. Z. & Li, W. J. Comparative genome analysis of a novel alkaliphilic actinobacterial species nesterenkonia haasae. Pol. J. Microbiol. 71, 453–461. https://doi.org/10.33073/pjm-2022-040 (2022).
Ijoma, G. N., Ogola, H. J. O., Rashama, C. & Matambo, T. Metagenomic insights into the impacts of phytochemicals on bacterial and archaeal community structure and biogas production patterns during anaerobic digestion of avocado oil processing waste feedstocks. Biomass Convers. Biorefinery. https://doi.org/10.1007/s13399-022-03516-8 (2022).
Fang, Y. J. & Xiong, L. Z. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. 72, 673–689 (2015).
Verma, H. et al. The potential application of endophytes in management of stress from Drought and Salinity in Crop plants. Microorganisms 9, 1729. https://doi.org/10.3390/microorganisms9081729 (2021).
Naseem, H. & Bano, A. Role of plant growth-promoting rhizobacteria and their exopolysaccharide in drought tolerance of maize. J. Plant Interact. 9, 689–701. https://doi.org/10.1080/17429145.2014.902125 (2014).
Sandhya, V., Ali, S. Z., Grover, M., Reddy, G. & Venkateswarlu, B. Effect of plant growth promoting Pseudomonas spp. on compatible solutes, antioxidant status and plant growth of maize under drought stress. Plant. Growth Regul. 62, 21–30 (2010).
Zhang, Y. et al. Pseudomonas monteilii PN1: A great potential P-nitrophenol degrader with plant growth promoting traits under drought and saline–alkali stresses. Biotechnol. Lett. 41, 801–811 (2019).
Funding
This work was supported by the National Natural Science Foundation of China (NSFC) [grant number 32060399] and the China Agriculture Research System of MOF and MARA [grant number CARS].
Author information
Authors and Affiliations
Contributions
Y. C. : Conceptualization, Methodology, Writing - review & editing. Z. X. : Resources, Investigation. X. L. : Resources. R. H. : Conceptualization, Methodology, Writing - review & editing. F. Z. : Resources, Validation, Supervision, review & editing. C. M. : Resources, Validation. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, Y., Huang, R., Xu, Z. et al. Responses of the physiological characteristics and endophytic bacteria of Urtica cannabina to simulated drought stress. Sci Rep 15, 12080 (2025). https://doi.org/10.1038/s41598-025-87172-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-87172-8