Abstract
Forsythia suspensa leaf fermented tea (FSLFT) is made from tender buds of Forsythia suspensa collected in spring. The main active components of FSLFT include forsythiaside, forsythia ester glycoside, rutin, and forsythia flavonoids, which have antibacterial, antioxidant, liver-protective, and immune-regulatory effects. Oxidative stress can trigger excessive apoptosis in intestinal epithelial cells, leading to dysfunction of the small intestinal mucosa and impaired intestinal absorption. This study focused on Kunming mice as research subjects and used hydrogen peroxide as an inducer to investigate the antioxidant and anti-inflammatory effects of FSLFT in vivo, as well as its regulatory effects on the intestinal microbiota of mice. The aim of this study was to establish a theoretical foundation for the functional study of Forsythia suspensa leaves and provide specific recommendations for their growth and application. The results showed that H2O2 treatment led to an increase in oxidative levels in mice. FSLFT has been shown to have antioxidant effects via the Redox Factor-1(Ref-1)/ hypoxia-inducible factor-1 alpha (HIF-1α) pathway, reduce inflammation caused by hydrogen peroxide through the Toll-like receptor 4 (TLR4)/nuclear factor kappa-B (NF-κB) signaling pathway, and protect mouse colons from oxidative stress by repairing gut microbiota imbalance and increasing microbial diversity and abundance. These findings establish a theoretical basis for studying the functional properties of FSLFT.
Similar content being viewed by others
Introduction
Forsythia suspensa (Thunb). Vahl, a traditional Chinese medicine, has the following effects: pyrexia, inflammation, gonorrhea, carbuncle, and erysipelas and so on 1. Modern pharmacology has also shown that Forsythia suspensa has important anti-inflammatory, antioxidant, anti-cancer, and antiviral properties2. According to new research, Forsythia suspensa leaves contain more of the useful chemicals phillyrin and phillygenin than fruit. These chemicals help to protect the liver from damage caused by alcohol and CCl43,4.
Tea infusions prepared from Forsythia suspensa leaves have been commonly consumed as medicinal beverages in Chinese folk for centuries5. FSLFT is made from the tender buds of Forsythia suspensa collected in spring, and undergoes steps such as withering, fixing, rolling, fermenting, and baking. Research has found that the main active components o FSLFT include forsythoside A, flavonoids, forsythin, and rutin6. These active components have antibacterial, antioxidant, liver-protective, and immune-regulatory effects7,8,9.
Physiologically generated Reactive oxygen species (ROS) also serve as second messengers in multiple signal transduction pathways stimulated by cytokines and growth factors, and participate in cellular signaling10. The imbalance between ROS production and the abilities of biological systems to detoxify and repair secondary damage is called oxidative stress11. Research has shown that oxidative stress can trigger excessive apoptosis of intestinal epithelial cells, leading to dysfunction of the small intestinal mucosa and impaired intestinal absorption12, which impacts animal digestion and absorption of nutrients13. ROS can activate NF-κB to initiate or boost inflammatory responses, which causes different cytokines to be made14. Ref-1 is a multifunctional 35.6 kDa protein that responds to DNA damage by oxidative stress15. Ref-1 plays a crucial role in initiating redox processes and interacts with many transcription factors that are essential for cell development, differentiation, and stress responses. Additionally, Ref-1's collaboration with HIF-1 is significant in controlling the expression of genes that are dependent on low oxygen levels (hypoxia)16. Oxidative stress can affect the balance of gut microbiota17. For instance, it has been shown that oxidative stress may favor the growth of certain pathogenic or proinflammatory bacteria while reducing the populations of beneficial or commensal bacteria18,19. A balanced gut microbiota is essential for maintaining a healthy gut environment. When the gut microbial balance is disrupted, deregulated bacteria can produce more ROS and impair gut barrier function20, which allows harmful substances and antigens to pass through the gut lining, further triggering oxidative stress21. Therefore, the gut microbiota can exhibit a reciprocal response to oxidative stress. The gut microbiota has an impact on oxidative stress through metabolite synthesis, regulation of antioxidant enzymes, and maintenance of gut homeostasis22,23, the modulation of antioxidant enzyme production and activity by gut bacteria enables them to regulate oxidative stress within the host; for example, specific strains of bacteria, such as Bifidobacterium longum CCFM752, Lactobacillus plantarum CCFM10, and L. plantarum CCFM1149 can induce the production of crucial antioxidant defense enzymes such as glutathione peroxidase24, catalase25 and superoxide dismutase(SOD)26.
Oxidative stress is a key pathophysiological mechanism in intestinal diseases such as Crohn’s disease (CD) and ulcerative colitis. There have been many studies on the antioxidant and anti-inflammatory functions and related mechanisms (signaling pathways) of Forsythia suspensa leaf (tea) extracts, but there have been no reports on the effects of Forsythia suspensa leaf tea extract (Forsythia suspensa extract) on the Ref-1/HIF-1α signaling pathway in vivo and in vitro. This study specifically examined Kunming mice as the objects of inquiry, utilizing hydrogen peroxide as an inducer. This study examined the antioxidant and anti-inflammatory properties of Forsythia suspensa leaf fermented tea extract by analyzing its effect on the Ref-1/HIF-1α and NF-κB signaling pathways. Furthermore, we investigated the impact of Forsythia suspensa leaf fermented tea extract on the gut microbiota. To establish a theoretical foundation for the functional study of FLFT and provide guidance for the development and utilization of Forsythia suspensa leaves.
Results
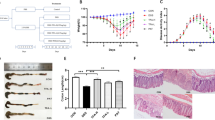
The effect of forsythia suspensa leaf fermented tea extract (FSLFTE) on the body weight of mice under oxidative stress.
Figure 1A indicates that the body weight of the H2O2 group mice was significantly lower than that of the control group, whereas the body weight of the H2O2-tea group mice was significantly higher than that of the H2O2 group. Figure 1B shows that the body weight of mice in the H2O2 and H2O2-tea groups was significantly lower than that of the control group, whereas the body weight of mice in the H2O2-tea group was significantly higher than that in the H2O2 group. These results indicate that H2O2-induced oxidative stress led to weight loss in mice and that the FSLFTE could correct the weight loss induced by H2O2 in mice.
The effect of FSLFTE on plasma indicators in oxidative stress mice
These images (Fig. 2A and B) demonstrate that the amounts of T-SOD and T-AOC in the plasma of mice in the H2O2 and H2O2-tea groups were significantly lower than those in the control group. However, the levels of T-SOD and T-AOC in the plasma of mice in the H2O2-tea group were significantly higher than those in the H2O2 group. These images (Fig. 2C and D) show that the amounts of LPO and MDA in the plasma of mice in the H2O2 group were higher than those in the control group. The amounts of LPO and MDA in the plasma of mice in the H2O2-tea group were higher than those in the control group but much lower than those in the H2O2 group. These results indicated that H2O2 led to an increase of oxidative levels in mice, and FLFTE could improve the antioxidant levels in oxidative stress mice.
The effect of FLFTE on plasma indicators in mice with oxidative stress. (A) T-SOD activity, (B) T-AOC concentration, (C) LPO concentration, (D) MDA concentration, (E) TNF-α concentration, (F) IL-6 concentration, (G) LPS activity, (H) IL-10 concentration. The data are shown as the means ± SEM. n = 10 per group.*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Figure 2E, F, and G show that the levels of TNF-α, IL-6, and lipopolysaccharides (LPS) in the H2O2 and H2O2-tea groups were significantly higher than those in the control group, and the levels of TNF-α, IL-6, and LPS in the blood of mice in the H2O2-tea group were significantly lower than those in the H2O2 group. Figure 2H shows that the amounts of IL-10 in the blood of mice in the H2O2 and H2O2-tea groups were significantly lower than those in the control group, and the levels of IL-10 in the blood of mice in the H2O2-tea group were significantly higher than those in the H2O2 group. These findings indicated that H2O2 treatment caused an increase in inflammation response in mice, while FSLFTE attenuated inflammatory reaction in mice under oxidative stress by reducing the levels of inflammatory cytokines such as IL-6 and TNF-alpha, and increasing the levels of anti-inflammatory cytokines such as IL-10.
The effect of FSLFTE on intestinal oxidative stress indicators in oxidative stress mice
The levels of LPO and MDA in the colons of mice in the H2O2 group were significantly higher than those in the control group (Fig. 3A and B). In the H2O2-tea group, the levels of LPO and MDA in mouse colons were significantly higher than those in the control group, but significantly lower than those in the H2O2 group. As shown in Fig. 3C and D, the amounts of T-SOD and T-AOC in mouse colons in the H2O2 and H2O2-tea groups were significantly lower than those in the control group. In contrast, the amounts of T-SOD and T-AOC in mouse colons in the H2O2-tea group were significantly higher. At the same time, we discovered that the expressions of Ref-1 and HIF-1 α in mice’s colons in the H2O2 group were significantly higher than that in the control and H2O2-tea groups in Fig. 3E and F. These findings suggested that H2O2 treatment increased the level of oxidation in mouse colons, whereas FSLFTE lowered the level of oxidation in mice under oxidative stress. This could be influenced by the Ref-1/HIF-1α signaling pathway.
The effect of FLFTE on oxidative stress indicators in the intestines of mice. (A) LPO concentration(n = 10 per group), (B) MDA concentration(n = 10 per group), (C) T-SOD activity(n = 10 per group), (D) T-AOC concentration (n = 10 per group), (E) HIF-α mRNA expression level (n = 3 per group), (F) Ref-1 mRNA expression level (n = 3 per group). The results are shown as the means ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
The effect of FSLFTE on intestinal inflammatory in oxidative stress mice
The colons of mice in the H2O2 group exhibited significantly higher levels of TNF-α, IL-6, and LPS compared to the control group or the H2O2-tea group (Fig. 4A, B, and C). The concentration of IL-10 in mouse colons in the H2O2 group was notably reduced compared to both the control group and the H2O2-tea group (Fig. 4D). The H2O2 group exhibited a significantly higher abundance of TLR4, NF-κB (p65), TNF-α, and IL-6 genes compared to the control and H2O2-tea groups, as shown in Fig. 4E, F, G, and H. The findings indicated that H2O2 exacerbated colon inflammation in mice, but FSLFTE reduced inflammation in mice experiencing oxidative stress. This could potentially be associated with the TLR4/NF-κB signaling pathway.
The effect of FLFTE on inflammatory markers in mice intestines. (A) TNF-α concentration(n = 10 per group), (B) IL-6 concentration(n = 10 per group), (C) LPS concentration(n = 10 per group), (D) IL-10 concentration(n = 10 per group), (E) TLR4 mRNA expression level(n = 3 per group), (F) NF-κB (p65) mRNA expression level(n = 3 per group), (G) TNF-α mRNA expression level(n = 3 per group), (H) IL-6 mRNA expression level(n = 3 per group). The results are shown as the means ± SEM, ns P > 0.05, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
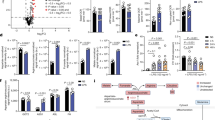
The effect of FLFTE on the diversity of intestinal flora in oxidative stress mice
As shown in Fig. 5A and B, the ACE and Chao1 indices of the control and H2O2-tea groups were significantly or extremely significantly higher than those of the H2O2 group. The Simpson and Shannon indices of the control, H2O2, and H2O2-tea groups were not significantly different, indicating that the species uniformity of intestinal flora in mice was not significantly different among the three groups. Figure 5C and D show that the.
The effect of FSLFTE on the diversity of gut microbiota in mice under oxidative stress. (A) ACE index (n = 5 per group), (B) Chao 1 index(n = 5 per group), (C) Shannon index(n = 5 per group ), (D) Simpson index(n = 5 per group), (E) PCoA (n = 5 per group), (F) PLS-DA(n = 5 per group), (G) Ansiom analysis(n = 5 per group), (H) Permanova analysis(n = 5 per group). The results are shown as the means ± SEM, ns P > 0.05, *P < 0.05, **P < 0.01.
Simpson index and Shannon index of the control, H2O2 group, and H2O2-tea group were not significantly different, which means that the species uniformity of intestinal flora in mice was not significantly different between the three groups. Figure 5E shows that the control and H2O2-tea groups’ PD_whole_tree were significantly higher than the H2O2 groups. These results showed that the α-diversity of intestinal flora in the control and H2O2-tea groups was significantly higher than that in the H2O2 group, mainly due to the difference in the number of flora.
We performed unsupervised principal coordinates analysis (PCoA) on all samples, as shown in Fig. 5F. The first and second explanatory factors separated the microbial communities in the control, H2O2, and H2O2-tea groups into distinct quadrants. We conducted further supervised partial least squares (PLS-DA) analysis on all samples, as illustrated in Fig. 5G. We found that the flora of the control, H2O2, and H2O2-tea groups remained well separated, indicating obvious differences in the species composition of these three groups. Anosim (analysis of similarities, Fig. 5H) showed that the difference between groups was greater than that within groups. Permanova (permutational multivariate analysis of variance, Fig. 5I) showed that different groups had significant differences in the interpretation of samples, which was consistent with anosim results. These results demonstrated that the intestinal microbiota of the three groups of mice was different, with significant differences between and within the groups. suggesting that using QIIME software for beta diversity analysis can reveal the degree of similarity in species diversity.
The effect of FSLFTE on the species distribution of intestinal flora in oxidative stress mice
Figure 6A shows the distribution of the H2O2, Control, and H2O2-tea groups throughout the top 10 phyla. More specifically, 38.20%, 32.01%, and 17 of these groups were composed of Bacteroidetes, Firmicutes, and Proteobacteria. Figure 6B illustrates that the H2O2-tea group contained significantly more Firmicutes than the H2O2 group. Fusobacteria abundance in the H2O2 group was significantly higher than that in the control and H2O2-tea groups (Fig. 6C). Figure 6D demonstrates the presence of more Acidobacteria in the H2O2-tea group compared to the H2O2 group. As shown in Fig. 6E, the ratio of Firmicutes to Bacteroidetes (F/B) was higher in the H2O2-tea group compared to both the control and H2O2 groups. These findings suggest that H2O2 disrupts the dominant bacterial phyla in the mouse colon by increasing the abundance of Fusobacteria. Meanwhile, the administration of FSLFT significantly increased the abundance of Firmicutes.
The effect of FLFTE on the gut microbiota phylum structure of mice under oxidative stress. (A) The phylum abundance, (B) the Firmicutes relative abundance, (C) the Fusobacteria relative abundance, (D) the Acidobacteria relative abundance, (E) the ratio of Firmicutes to Bacteroidetes. The results are shown as the means ± SEM, ns P > 0.05, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Figure 7A shows the distribution of the genera in the three groups (control, H2O2, and H2O2-tea) within the top 10 genera. The abundances of Prevotella_9 and Bacteroides in the H2O2-tea group was significantly lower than that in the control group and the H2O2 group(Fig. 7B, C); Fig. 7D demonstrates that the abundance of Escherichia-Shigella in the H2O2-tea group was significantly higher than that in the hydrogen peroxide group; Fig. 7E reveals that the abundance of uncultured bacterium f_lachnospiraceae in both the H2O2 group and the H2O2-tea group was significantly higher than that in the control group; Fig. 7F and G illustrate that the abundances of uncultured bacterium f_muribaculaceae and Lactobacillus in the H2O2-tea group was significantly higher than that in the control group and the H2O2 group; Fig. 7H displays that the abundance of Fusobacterium in the H2O2 group was significantly higher than that in the control group and the H2O2-tea group; According to Fig. 7I, the abundance of Phascolarctobacterium in the H2O2-tea group was significantly lower than that in the H2O2 group. These results demonstrate that H2O2-induced increased the abundance of Fusobacterium and uncultured.
The effect of FLFTE on the gut microbiota genus structure of mice under oxidative stress. (A) The phylum abundance, (B) the Firmicutes relative abundance, (C) the Fusobacteria relative abundance, (D) the Acidobacteria relative abundance, (E) the ratio of Firmicutes to Bacteroidetes. The results are shown as the means ± SEM, ns P > 0.05, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
_bacterium_f_lachnospiraceae in the gut microbiota of mice, whereas FLFTE balanced the gut microbiota of mice by decreasing the abundance of Prevotella_9, Fusobacterium, Phascolarctobacterium, and increasing the abundance of Escherichia-Shigella, Lactobacillus and uncultured_bacterium_f_muribaculaceae.
The effect of FSLFTE on the differences in gut microbiota among mice under oxidative stress
Linear Discriminant Analysis (LDA) can identify biomarkers with statistical differences between different groups. Figure 8A displays the signature phylum of gut microbiota in mice. The Firmicutes phylum served as the signature for the H2O2-tea group, while the Fusobacteria and Epsilonproteobacteria phyla were signatures for the H2O2 group. The Spirochaetes phylum represented the control group’s signature. Figure 8B illustrates the signature genera of gut microbiota in mice. The genera g_R_UCG_005, g_Prevotellaceae_UCG_001, g_uncultured_bacterium_f_Spirochactaceae, and g_Lachnospiraceae_NK4A136_group belonged to the control group. Helicobacter, Phascolarctobacterium, Anaerostipes, and Fusobacterium were the signature genera for the H2O2 group. The genera g_Bacteroides, Lactobacillus, and g_R_UCG_005 were considered as signatures for the H2O2-tea group.
Analysis of correlation
We found that the phylum Fusobacteria was positively correlated with MDA, LPO (Fig. 9A), and TNF-α, LPS, IL-6 (Fig. 9B), while it was negatively correlated with T-SOD, T-AOC (Fig. 9A), and IL-10 (Fig. 9B); Fusobacterium, Anaerobiospirillum, Alloprevotella, Blautia, Megamonas, and Erysipelatoclostridium were inversely correlated with T-SOD, T-AOC (Fig. 9C), and IL-10 (Fig. 9D); Fusobacterium, Anaerobiospirillum, Alloprevotella, Blautia, Megamonas, and Erysipelatoclostridium genera were inversely proportional to T-SOD, T-AOC (Fig. 9C), and IL-10 (Fig. 9D); The Lachnospiraceae_NK4-A136_group was positively correlated with T-SOD, T-AOC.
Analysis of the correlation between gut microbiota and antioxidant performance. (A) The correlation between gut microbiota and antioxidant indicators at phylum level (n = 5 per group), (B) the correlation between gut microbiota and inflammatory indicators at genus level (n = 5 per group), (C) the correlation between gut microbiota and antioxidant indicators at phylum level (n = 5 per group), (D) the correlation between gut microbiota and inflammatory indicators at genus level (n = 5 per group). *P < 0.05, **P < 0.01, ***P < 0.001.
(Fig. 9C), and IL-10; Fusobacterium, Megamonas, Anaerobiospirillum, and Blautia genus were positively correlated with MDA, LPO (Fig. 9C), as well as TNF-α, LPS, and IL-6 (Fig. 9D).
Dicussion
H2O2 can be metabolized to several ROS, including hydroxyl radicals, which are considered the most dangerous compounds to organisms. Excess uneliminated H2O2 and its metabolites can oxidize virtually all types of macromolecules, including carbohydrates, nucleic acids, lipids and proteins27. The increased levels of ROS overwhelm antioxidant defenses and lead to a state of oxidative stress, which may further impair body function and result in clinical deterioration. The present data indicate that H2O2 inhibited SOD, GSH-Px, and T-AOC activities and increased MDA levels, suggesting a significant disruption in the oxidative balance after exposure to H2O228. This study was in agreement with a previous report, which also demonstrated that hydrogen peroxide induces oxidative stress in the mouse body29. FSLFTE reduced the oxidative level in the mouse body, which may be related to its various active ingredients, such as phillygenin, phillyrin, etc30. According to a previous study, the bioactive substances in Forsythia suspensa reduce oxidative stress caused by ROS through the nuclear factor-erythroid 2-related factor 2 (Nrf2) signaling pathway31. Ref-1 is a multifunctional protein with DNA repair activity that exerts cytoprotective effects by post-translational redox modifications of various transcription factors including HIF-132. Hydrogen peroxide (H2O2) activate HIF-1alpha by inducing the expression of Ref-133. The HIF-1alpha subunit is regulated by O2-dependent hydroxylation of proline residues 402 and 564, or both, by prolyl hydroxylase ___domain protein 2 (PHD2), which promotes binding of the von Hippel-Lindau protein (VHL), leading to ubiquitination and proteasomal degradation, and O2-dependent hydroxylation of asparagine residue 803 by factor inhibiting HIF-1 (FIH-1), which blocks the binding of the 300-kilodalton coactivator protein (p300) and CREB binding protein (CBP). Hydroxylation reactions, which utilize O2 and alpha-ketoglutarate as substrates and generate CO2 and succinate as by-products, provide a mechanism by which changes in cellular oxygenation are transduced to the nucleus as changes in HIF-1 activity34. Therefore, PHD2 and FIH-1 are major regulators of HIF-1alpha, which are induced by external H2O2 but suppressed by ROS-scavenging catalase33. The qPCR results showed that FSLFTE lowered the expression of the Ref-1 and HIF-1α genes in the colon. This suggests that the antioxidant effect of FSLFTE might be linked to the Ref-1/HIF-1α gene. Further research is needed to determine the extent to which PHD2, FIH-1, and other genes are expressed.
Oxidative stress simultaneously activates a large number of inflammation-related transcription factors, initiating the inflammatory process and increasing the production of proinflammatory cytokines35. TNF-α and IL-6 are regarded as two key regulatory factors of pro-inflammatory responses and are involved in promoting inflammation and causing tissue damage36. IL-10 is a product of immunoactivity cells, mainly monocytes and lymphocytes, and is regarded as one of the most important anti-inflammatory immune-regulating cytokines37. The balance between pro-inflammatory cytokines and anti-inflammatory cytokines is crucial for host health, IL-10 reportedly inhibited IL-6 production by halting nuclear translocation of NF-κB in microglia38, which could counteract some of the negative effects of TNF-α39. When mice were under oxidative stress, this study found that FLFTE significantly lowered the levels of TNF-α and IL-6 while significantly increasing the levels of IL-10. This indicated that FLFTE exerted anti-inflammatory effects by regulating the balance of pro-inflammatory and anti-inflammatory cytokines, but its molecular mechanisms require further research. In contrast, we found that FLFTE greatly reduced the expression of TLR4, NF-κB (p65), TNF-α, and IL-6 genes in the colon tissues of mice under oxidative stress. This suggests a link between the anti-inflammatory properties of Forsythia suspensa leaf fermented tea extract and TLR4/NF-κB, potentially due to the presence of phillygenin, phillyrin, and other compounds31,40,41. TLR4, a key receptor for commensal recognition in gut innate immunity, is over-expressed in inflamed colonocytes and is the subject of therapeutics (target inhibition) in inflammatory bowel disease (IBD). TLR4-mediated signal transduction events can lead to the activation of NF-κB, followed by the expression of pleiotropic genes involved in immune and inflammatory responses42. NF-κB, a pleiotropic transcription factor, regulates several other genes involved in inflammatory responses and stimulates numerous cellular signaling pathways, which leads to increased production of inflammatory cytokines such as iNOS, COX-2, IL-6, IL-1β and TNF-α and so on43,44.
Research has shown that oxidative stress can disrupt the structure of gut microbiota, reduce its diversity, and subsequently lead to inflammation45,46. In this study, we employed 16S rRNA gene sequencing technology to analyze changes in the gut microbiota in the colons of various groups of mice. Alpha diversity analysis revealed that hydrogen peroxide reduced species richness and evenness in the microbial community of mice, while Beta diversity analysis indicated that hydrogen peroxide altered the species composition among the microbial communities of mice compared to the control group. However, FSLFTE was able to increase the quantity of gut microbiota in oxidative stress mice and altered the gut microbiota structure in these mice, in agreement with previous studies46,47. In our study, the H2O2-tea group had higher levels of phyla Firmicutes and Acidobacteria than the H2O2 group. In contrast, the H2O2-tea group showed lower levels of Fusobacteria. Firmicutes is believed to be involved in maintaining the integrity of the intestinal barrier, which plays a key role in regulating host inflammation48. Fusobacteria are anaerobic gram-negative bacteria whose members are linked to immune suppression and are involved in inflammatory pathways via the recruitment of inflammatory cytokines and increased production of ROS49. The Firmicutes/Bacteroidetes (F/B) ratio is widely accepted to have an important influence in maintaining normal intestinal homeostasis, and decreased F/B ratio is regarded as dysbiosis, whereby the former is usually observed with obesity, and the latter with inflammatory bowel disease50. Decreased F/B ratios are associated with inflammatory bowel disease, cancer, and enhanced oxidative responses51,52,53. This study found that Forsythia suspensa leaf fermented tea extract increased the F/B ratio. At the gene level, compared to the H2O2 group, the H2O2-tea group significantly upregulated the abundance of uncultured_bacterium_f_Muribaculaceae, Lactobacillus, and Escherichia-Shigella, while downregulating Phascolarctobacterium and Prevotella_9. Lactobacillus, a beneficial bacterium, can significantly improve inflammation and oxidative stress damage in the body. Fusobacterium is a proinflammatory bacterium that can impair the intestinal barrier and serve as a potential microbial marker of hepatic inflammation54. In this study, the H2O2-tea group mice’s signature phylum was Firmicutes (Fig. 8A), and their signature genera were Lactobacillus, Bacteroides, Alloprevotella, and uncultured_bacterium_f_Muribaculaceae (Fig. 7). According to the abundance analysis results in Figs. 6, 7 and 8B, the signature phyla of the H2O2 group were Fusobacteria and Epsilonbacteraeota, and the signature genera were Helicobacter, Phascolarctobacterium, Anaerobiospirillum, and Fusobacterium. To further investigate the relationship between the microbial changes induced by the Forsythia suspensa leaf fermented tea extract and its antioxidant and anti-inflammatory effects, we performed a correlation analysis between the abundance of the microbiota at the phylum and genus levels and indicators of oxidative stress and inflammation. Consistent with earlier studies, we discovered an inverse proportionality between MDA and LPO, and the phylum Firmicutes55. In contrast, Fusobacteria was negatively correlated with T-AOC, IL-10, MDA, LPO, TNF-α, and IL-6 and positive correlated with SOD and T-AOC56. Fusobacterium, Anaerobiospirillum, Alloprevotella, Blautia, Megamonas, and Erysipelatoclostridium were inversely proportional to T-SOD and T-AOC and positively proportional to MDA and LPO, whereas Lachnospiraceae_NK4-A136_group was related to T-SOD and T-AOC (Fig. 9C). The Lachnospiraceae-NK4A136-group, a well-known taxon for its ability to produce butyrate, plays a vital role in preserving the integrity of the mouse intestinal barrier. It also possesses notable anti-inflammatory capabilities and is beneficial for intestinal health57. Studies have shown that low-grade inflammation between gut microbiota and metabolic disorders is significantly influenced by LPS. Dysbiosis can lead to increased intestinal permeability, which allows bacteria-induced LPS to enter the plasma and cause low-grade chronic inflammation58.
Conclusion
In summary, Forsythia suspensa leaf fermented tea extracts has been shown to have antioxidant effects via the Ref-1/HIF-1α pathway, reduce inflammation caused by hydrogen peroxide through the TLR4/NF-κB signaling pathway, and protect mouse colons from oxidative stress by repairing gut microbiota imbalance and increasing microbial diversity and abundance. These findings provide experimental support for potential clinical application.
Methods
Preparation of forsythia suspensa leaf fermented tea extract
Forsythia suspensa leaf fermented tea was obtained from the Medicinal Tea Research Centre, Shanxi Agricultural University. The Forsythia suspensa leaf fermented tea was soaked in boiling water for 30 min (tea/water (w/v) = 1:20) and this was repeated three times. We collected the filtrates through filtration, stored it in a 10 cm petri dish, and then treated it by freezing it overnight at -80 °C and freeze-drying it for 24 h in a freeze dryer. Finally, the tea extract was collected separately in a moisture-proof bag and stored in a dryer.
The main instruments and equipment
The equipment used included a multifunctional enzyme labeler from Swiss Dikon, low-speed centrifuge from Eppendorf AG, high-speed refrigerated centrifuge from German Sigma, RT-PCR instrument from Jena Analytical Instruments, UV–Vis spectrophotometer from Japanese Shimadzu, and NanoDrop 2000 (Nanodrop Technologies, USA).
The main reagents
We sourced the MDA, LPO, T-SOD, and T-AOC kits for small intestine tissue from Nanjing Jiancheng Bioengineering Institute and purchased reverse transcription and RT-PCR kits for the colon tissue supernatant from Takara Bio (Beijing) Co., Ltd.
Animal preparation and experimental protocols
Thirty SPF-grade female Kunming mice weighing 20–24 g were purchased from Spibio Biotechnology Co., Ltd. We placed the mice in a mouse cage at room temperature (22 ± 1 °C) with a 12-h dark/light cycle and 60% relative humidity, allowing them to eat freely. Before the formal trial, the mice were allowed to acclimatize for 7 days and then randomly assigned 10 mice each to the control, H2O2, and H2O2-tea groups, based on their body weight. The animal experimental process is shown in Fig. 10. We administered distilled water to the mice in the control and H2O2 groups daily at 7:00 am for 4 W, and administered the mice in the H2O2-tea group Forsythia suspensa leaf fermented tea extracts (500 mg/kg body weight) daily at 7:00 am for 4 W. The H2O2 and H2O2-tea group mice were intraperitoneally injected with 3% H2O2 (10 mg/kg) at 8:00 am on the first day of the fifth week for 5 days. The control group mice received the same volume of physiological saline as the H2O2 and H2O2-tea group for 5 consecutive days. Weekly weight measurements were conducted on the mice. All animal studies were conducted in accordance with the National Institutes of Health Guidelines, with the approval of the Animal Ethical and Welfare Committee of Changzhi Medical College (Approval Document No. DW2024089), in accordance with ARRIVE guidelines. Mice were euthanized by CO2 asphyxiation. Animal carcasses were packaged as required and handed over to the Experimental Animal Center of Changzhi Medical College for freezing and unified harmless treatment.
Sample collection
After the experiment, the mice were subjected to a 12-h fasting period, followed by euthanasia. Prior to this, the mice had been weighed once a week. Eyeballs were removed to collect blood samples. Subsequently, the samples were centrifuged at 3000 rpm/min for 10 min to separate the plasma, which was then stored at -80 °C for future biochemical analysis. Under sterile conditions, mice were carefully dissected to collect colonic feces for microbiota assessment. The colonic tissue was rapidly extracted and immediately frozen in liquid nitrogen at -80 °C for subsequent biochemical analysis and RT-PCR.
Biochemistry profile
The plasma and colon tissue T-SOD, MDA, T-AOC, LPO, IL-6, TNF-α, LPS, and IL-10 levels were measured according to the manufacturer’s instructions of the reagent kit (Jiancheng Biotech Co., Nanjing, China).
RT-PCR
TRIzol reagent was used to isolate total RNA from colon samples, which were then treated with DNase I according to the manufacturer’s instructions (Wuhan Servicebio Technology Co., Ltd.). The concentration of each RNA sample was measured. The Prime Script RT reagent kit was used to eliminate genomic DNA contamination before reverse transcription. cDNA was synthesized using Prime Script Enzyme Mix 1, RT Primer Mix, and 5 × Primer Script Buffer We performed Reverse transcription at 37 °C for 15 min and 85 °C for 3 s (Takara Bio (Beijing) Co., Ltd). Gene-specific primer sequences were shown in Table 1, designed using NCBI and synthesized by Sangon Biotech Co.Ltd. Real-time PCR was performed according to the manufacturer’s guidelines (Takara Bio (Beijing) Co., Ltd). We calculated the relative expression between the control and treatment groups using the 2-∆∆Ct method, where Ct = Ct (target) – Ct (GAPDH). We selected as a housekeeping gene to normalize the transcript levels of the target gene59 (Table 2).
16S rRNA gene analysis
We conducted 16S rRNA gene analysis in the same manner as previously described60. The readings from each sample were combined using FLASH v1.2.7. To produce high-quality tag sequences, we filtered the spliced raw tags and removed chimeras using Trimmomatic v0.33 and UCHIME v4.2 software. We grouped the tag sequences at a 97% similarity level, denoised and divided the high-quality sequences into ASVs, and then applied an ASV filter to all sequences with a threshold of 0.0005%. We annotated the taxonomic information of each representative sequence using the Silva138 Database and the mother algorithm. We classified the species using AVS sequence composition on the BMK Cloud platform (www. biocloud. ent). We obtained sample taxonomic tree maps based on the AVS analysis results at the phylum and genus levels. We analyzed alpha diversity using QIIME and calculated ACE, Chao1, Shannon, Simpson, and PD_whole_tree indices. To determine how different samples in beta diversity analysis differed in terms of species diversity, we used principal coordinate analysis (PCoA), partial least squares discriminant analysis (PLS-DA), and PERMANOVA/ANOSIM. We examined the difference in the number of species in different samples (groups) based on their taxonomic make-up using an LDA threshold of 4.0, and a linear discriminant analysis effect size (LEfSe). We used the Spearman correlation coefficient to examine the relationship between blood parameters and microbiota abundance based on the species composition distribution. We used the Spearman correlation coefficient to examine the relationship between blood parameters and microbiota abundance based on the species composition distribution.
Statistical analysis
One-way analysis of variance (ANOVA) and Tukey’s test were used to compare group averages. Statistical significance was set at P < 0.05. The mean and SEM were used to express all data.
Data availability
Data is provided within the https://doi.org/10.6084/m9.figshare.26386279.
References
Wu, B. et al. Transcriptomic and Lipidomic Analysis of Lipids in Forsythia suspensa. Front Genet 12, 758326. https://doi.org/10.3389/fgene.2021.758326 (2021).
Wang, Z. et al. Phytochemistry, pharmacology, quality control and future research of Forsythia suspensa (Thunb.) Vahl: A review. Journal of Ethnopharmacology 210, 318–339 (2018). https://doi.org/10.1016/j.jep.2017.08.040
Yuan, J.-F., Liu, X.-Q., Yang, J.-X. & Cui, X.-Q. Forsythia suspense leaves, a plant: extraction, purification and antioxidant activity of main active compounds. European food research and technology 238, 527–533 (2014). https://doi.org/10.1007/s00217-014-2179-y
Dong ZhangLu, D. Z. et al. Forsythiae Fructus: a review on its phytochemistry, quality control, pharmacology and pharmacokinetics. (2017). https://doi.org/10.3390/molecules22091466
Jiao, J. et al. Comparison of main bioactive compounds in tea infusions with different seasonal Forsythia suspensa leaves by liquid chromatography–tandem mass spectrometry and evaluation of antioxidant activity. Food Research International 53, 857–863 (2013). https://doi.org/10.3390/molecules22091466
Wang, D.-H., Wang, M.-Y., Shen, W.-H. & Yuan, J.-F. Analysis of chemical compounds and toxicological evaluation of Forsythia suspensa leaves tea. Food Science and Biotechnology 30, 305–314 (2021). https://doi.org/10.1007/s10068-020-00855-y
Zhang, J. et al. Antibacterial activity and mechanism of phillyrin against selected four foodborne pathogens. Food Science and Technology 42, e32922 (2022).https://doi.org/10.1590/fst.32922
Pan, L. et al. Forsythia suspensa extract attenuates breast muscle oxidative injury induced by transport stress in broilers. Poultry Science 97, 1554–1563 (2018). https://doi.org/10.3382/ps/pey012
Fan, B.-y. et al. Protective mechanism of Forsythia suspense leaves extract against drug-induced liver injury. (2022). https://doi.org/10.5555/20220296553
Checa, J. & Aran, J. M. Reactive oxygen species: drivers of physiological and pathological processes. Journal of Inflammation research, 1057–1073 (2020). https://doi.org/10.2147/JIR.S275595
Adwas, A. A., Elsayed, A., Azab, A. E. & Quwaydir, F. A. Oxidative stress and antioxidant mechanisms in human body. J. Appl. Biotechnol. Bioeng 6, 43–47 (2019). https://doi.org/10.15406/jabb.2019.06.00173
Xu, L. et al. Hydrogen peroxide induces oxidative stress and the mitochondrial pathway of apoptosis in RAT intestinal epithelial cells (IEC-6). Molecular Biology 50, 270–277. https://doi.org/10.7868/S0026898416020269 (2016).
Zhang, J.-X. et al. Soybean β-conglycinin induces inflammation and oxidation and causes dysfunction of intestinal digestion and absorption in fish. PloS one 8, e58115. https://doi.org/10.1371/journal.pone.0058115 (2013).
Leszek, J., E Barreto, G., Gasiorowski, K., Koutsouraki, E. & Aliev, G. Inflammatory mechanisms and oxidative stress as key factors responsible for progression of neurodegeneration: role of brain innate immune system. CNS & Neurological Disorders-Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders) 15, 329–336 (2016). https://doi.org/10.2174/1871527315666160202125914
Oliveira, T. T. et al. APE1/Ref-1 Role in Inflammation and Immune Response. Front Immunol 13, 793096. https://doi.org/10.3389/fimmu.2022.793096 (2022).
Loboda, A. et al. HIF-1 attenuates Ref-1 expression in endothelial cells: Reversal by siRNA and inhibition of geranylgeranylation. Vascular Pharmacology 51, 133–139 (2009). https://doi.org/10.1016/j.vph.2009.05.005
Martens, E. C., Neumann, M. & Desai, M. S. Interactions of commensal and pathogenic microorganisms with the intestinal mucosal barrier. Nature Reviews Microbiology 16, 457–470. https://doi.org/10.1038/s41579-018-0036-x (2018).
Morais, L. H., Schreiber Iv, H. L. & Mazmanian, S. K. The gut microbiota–brain axis in behaviour and brain disorders. Nature Reviews Microbiology 19, 241–255 (2021). https://doi.org/10.1038/s41579-020-00460-0
From disease to therapy. Mitra, S., Dash, R., Al Nishan, A., Habiba, S. U. & Moon, I. S. Brain modulation by the gut microbiota. Journal of Advanced Research 53, 153–173. https://doi.org/10.1016/j.jare.2022.12.001 (2023).
Jackson, D. N. & Theiss, A. L. Gut bacteria signaling to mitochondria in intestinal inflammation and cancer. Gut microbes 11, 285–304. https://doi.org/10.1080/19490976.2019.1592421 (2020).
Singh, T. P., Kadyan, S., Devi, H., Park, G. & Nagpal, R. Gut microbiome as a therapeutic target for liver diseases. Life Sciences 322, 121685 (2023). https://doi.org/10.1016/j.lfs.2023.121685.
Parker, A. et al. (2022).
Ballard, J. W. O. & Towarnicki, S. G. Mitochondria, the gut microbiome and ROS. Cellular signalling 75, 109737. https://doi.org/10.1016/j.cellsig.2020.109737 (2020).
Guo, X. et al. Flavonoids from Rhododendron nivale Hook. f delay aging via modulation of gut microbiota and glutathione metabolism. Phytomedicine 104, 154270 (2022). https://doi.org/10.1016/j.phymed.2022.154270
Chen, S. et al. Bifidobacterium adolescentis regulates catalase activity and host metabolism and improves healthspan and lifespan in multiple species. Nature Aging 1, 991–1001. https://doi.org/10.1038/s43587-021-00129-0 (2021).
Wang, N. et al. Effect on intestinal microbiota, bioaccumulation, and oxidative stress of Carassius auratus gibelio under waterborne cadmium exposure. Fish physiology and biochemistry 46, 2299–2309. https://doi.org/10.1007/s10695-020-00870-0 (2020).
Yin, J. et al. Hydrogen peroxide-induced oxidative stress activates NF-κB and Nrf2/Keap1 signals and triggers autophagy in piglets. RSC Advances 5, 15479–15486. https://doi.org/10.1039/C4RA13557A (2015).
Jin, Y. et al. Rhizoma Dioscoreae Nipponicae polysaccharides protect HUVECs from H2O2-induced injury by regulating PPARγ factor and the NADPH oxidase/ROS–NF-κB signal pathway. Toxicology letters 232, 149–158. https://doi.org/10.1016/j.toxlet.2014.10.006 (2015).
Oshikawa, J. et al. Extracellular SOD-derived H2O2 promotes VEGF signaling in caveolae/lipid rafts and post-ischemic angiogenesis in mice. PloS one 5, e10189. https://doi.org/10.1371/journal.pone.0010189 (2010).
Lu, T. et al. Protective effects of Forsythia suspensa extract against oxidative stress induced by diquat in rats. Food and chemical toxicology 48, 764–770. https://doi.org/10.1016/j.fct.2009.12.018 (2010).
Zhao, P. et al. Forsythia suspensa extract attenuates lipopolysaccharide‐induced inflammatory liver injury in rats via promoting antioxidant defense mechanisms. Animal Science Journal 88, 873–881 (2017). https://doi.org/10.1111/asj.12717
Loboda, A. et al. HIF-1 attenuates Ref-1 expression in endothelial cells: reversal by siRNA and inhibition of geranylgeranylation. Vascular pharmacology 51, 133–139. https://doi.org/10.1016/j.vph.2009.05.005 (2009).
Kobayashi, Y., Oguro, A. & Imaoka, S. Feedback of hypoxia-inducible factor-1alpha (HIF-1alpha) transcriptional activity via redox factor-1 (Ref-1) induction by reactive oxygen species (ROS). Free radical research 55, 154–164. https://doi.org/10.1080/10715762.2020.1870685 (2021).
Semenza, G. L. Hypoxia-inducible factor 1 (HIF-1) pathway. Science’s STKE 2007, cm8-cm8 (2007). https://doi.org/10.1126/stke.4072007cm8
McGarry, T., Biniecka, M., Veale, D. J. & Fearon, U. Hypoxia, oxidative stress and inflammation. Free Radical Biology and Medicine 125, 15–24. https://doi.org/10.1016/j.freeradbiomed (2018).
Bian, X. et al. Saccharin induced liver inflammation in mice by altering the gut microbiota and its metabolic functions. Food and Chemical Toxicology 107, 530–539. https://doi.org/10.1016/j.fct.2017.04.045 (2017).
Stenvinkel, P. et al. IL-10, IL-6, and TNF-α: central factors in the altered cytokine network of uremia—the good, the bad, and the ugly. Kidney international 67, 1216–1233. https://doi.org/10.1111/j.1523-1755.2005.00200.x (2005).
Heyen, J. R. R., Ye, S.-M., Finck, B. N. & Johnson, R. W. Interleukin (IL)-10 inhibits IL-6 production in microglia by preventing activation of NF-κB. Molecular Brain Research 77, 138–147. https://doi.org/10.1016/s0169-328x(00)00042-5 (2000).
Kaur, K., Sharma, A. K., Dhingra, S. & Singal, P. K. Interplay of TNF-alpha and IL-10 in regulating oxidative stress in isolated adult cardiac myocytes. J Mol Cell Cardiol 41, 1023–1030. https://doi.org/10.1016/j.yjmcc.2006.08.005 (2006).
Hu, N. et al. Phillygenin inhibits LPS-induced activation and inflammation of LX2 cells by TLR4/MyD88/NF-κB signaling pathway. Journal of ethnopharmacology 248, 112361. https://doi.org/10.1016/j.jep.2019.112361 (2020).
Guo, J. et al. Phillygenin from Forsythia suspensa leaves exhibits analgesic potential and anti‐inflammatory activity in carrageenan‐induced paw edema in mice. Journal of Food Biochemistry 46, e14460 (2022). https://doi.org/10.1111/jfbc.14460
Cui, L., Feng, L., Zhang, Z. H. & Jia, X. B. The anti-inflammation effect of baicalin on experimental colitis through inhibiting TLR4/NF-κB pathway activation. International Immunopharmacology 23, 294–303. https://doi.org/10.1016/j.intimp.2014.09.005 (2014).
Muhammad, T., Ikram, M., Ullah, R., Rehman, S. U. & Kim, M. O. Hesperetin, a citrus flavonoid, attenuates LPS-induced neuroinflammation, apoptosis and memory impairments by modulating TLR4/NF-κB signaling. Nutrients 11, 648 (2019). https://doi.org/10.3390/nu11030648
Zhou, M. et al. Phillygenin inhibited LPS-induced RAW 264.7 cell inflammation by NF-κB pathway. European Journal of Pharmacology 899, 174043 (2021). https://doi.org/10.1016/j.ejphar.2021.174043
Wang, J. et al. Spirulina platensis aqueous extracts ameliorate colonic mucosal damage and modulate gut microbiota disorder in mice with ulcerative colitis by inhibiting inflammation and oxidative stress. Journal of Zhejiang University-SCIENCE B 23, 481–501. https://doi.org/10.1631/jzus.B2100988 (2022).
Li, M. et al. Theaflavins in black tea mitigate aging-associated cognitive dysfunction via the microbiota–gut–brain axis. Journal of Agricultural and Food Chemistry 71, 2356–2369. https://doi.org/10.1021/acs.jafc.2c06679 (2023).
Zhao, D. & Shah, N. P. Synergistic application of black tea extracts and lactic acid bacteria in protecting human colonocytes against oxidative damage. Journal of agricultural and food chemistry 64, 2238–2246. https://doi.org/10.1021/acs.jafc.5b05742 (2016).
Louis, P. & Flint, H. J. Formation of propionate and butyrate by the human colonic microbiota. Environ Microbiol 19, 29–41. https://doi.org/10.1111/1462-2920.13589 (2017).
Gur, C., Mandelboim, O. & Bachrach, G. “Messieurs, c’est les microbes qui auront le dernier mot”: Gentlemen, it is the microbes who have the last word (Louis Pasteur)—Fusobacterium nucleatum protect tumors from killing by immune cells. Oncoimmunology 4, e1038690. https://doi.org/10.1080/2162402X.2015.1038690 (2015).
Stojanov, S., Berlec, A. & Štrukelj, B. The influence of probiotics on the firmicutes/bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms 8, 1715. https://doi.org/10.3390/microorganisms8111715 (2020).
Stojanov, S., Berlec, A. & Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms 8 (2020). https://doi.org/10.3390/microorganisms8111715
Ni, H. et al. Maternal cysteine intake influenced oxidative status and lipid-related gut microbiota and plasma metabolomics in male suckling piglets. Animal Feed Science and Technology 276, 114947 (2021). https://doi.org/10.1016/j.anifeedsci.2021.114947
Wang, M. et al. Olive Fruit Extracts Supplement Improve Antioxidant Capacity via Altering Colonic Microbiota Composition in Mice. Front Nutr 8, 645099. https://doi.org/10.3389/fnut.2021.645099 (2021).
Gu, X., Liao, S., Li, M., Wang, J. & Tan, B. Chloroquine Downregulation of Intestinal Autophagy Changed Intestinal Microbial Community Compositions and Metabolite Profiles in Piglets. Veterinary Sciences 11, 333. https://doi.org/10.3390/vetsci11080333 (2024).
González, S. et al. Microbiota and oxidant-antioxidant balance in systemic lupus erythematosus. Nutrición Hospitalaria 34, 934–941 (2017). https://doi.org/10.20960/nh.546
Zheng, T., Tao, Y., Lu, S., Qiang, J. & Xu, P. Integrated transcriptome and 16S rDNA analyses reveal that transport stress induces oxidative stress and immune and metabolic disorders in the intestine of hybrid yellow catfish (Tachysurus fulvidraco♀× Pseudobagrus vachellii♂). Antioxidants 11, 1737. https://doi.org/10.3390/antiox11091737 (2022).
Zhu, X. et al. A high-fat diet increases the characteristics of gut microbial composition and the intestinal damage associated with non-alcoholic fatty liver disease. International Journal of Molecular Sciences 24, 16733. https://doi.org/10.3390/ijms242316733 (2023).
Wang, W. et al. Lactobacillus paracasei Jlus66 extenuate oxidative stress and inflammation via regulation of intestinal flora in rats with non alcoholic fatty liver disease. Food Science & Nutrition 7, 2636–2646. https://doi.org/10.1002/fsn3.1118 (2019).
Álvarez-Amor, L. et al. Extra virgin olive oil improved body weight and insulin sensitivity in high fat diet-induced obese LDLr−/−.Leiden mice without attenuationof steatohepatitis. Sci. Rep. 11, 8250. https://doi.org/10.1038/s41598-021-87761-3 (2021).
Xie, Y. et al. Supplement of high protein-enriched diet modulates the diversity of gut microbiota in WT or PD-1H-depleted mice. Journal of Microbiology and Biotechnology 31, 207. https://doi.org/10.4014/jmb.2008.08003 (2021).
Acknowledgements
The authors are thankful to the Institute of Economic Crop Research at the University of Shanxi Agriculture and the Garden Pharmacy Tea Practice Center at the Shanxi Agricultural University for their support.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
LL: Conceptualization, Data curation, formal analysis, Investigation, Methodology, Writing of the original draft. YZ: Conceptualization, Methodology, Supervision, Writing-Review and Editing. YD: Formal analysis, Software, Validation, Visualization, Writing, review, and editing. LG: Conceptualization, Investigation, Methodology, Writing—Review, and Editing. RD: Data curation, formal analysis, Software, Writing of the original draft. AC: Conceptualization, Funding acquisition, Investigation, Writing, review, and editing. GD: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing, review, and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, L., zhao, Y., Ding, Y. et al. Forsythia suspensa leaf fermented tea extracts attenuated oxidative stress in mice via the Ref-1/HIF-1α signal pathway and modulation of gut microbiota. Sci Rep 15, 4106 (2025). https://doi.org/10.1038/s41598-025-87182-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-87182-6