Abstract
Extracorporeal cardiopulmonary resuscitation (ECPR) improves survival for prolonged cardiac arrest (CA) but carries significant risks and costs due to ECMO. Previous predictive models have been complex, incorporating both clinical data and parameters obtained after CPR or ECMO initiation. This study aims to compare a simpler clinical-only model with a model that includes both clinical and pre-ECMO laboratory parameters, to refine patient selection and improve ECPR outcomes. Medical records between January 2012 and January 2019 in our institution were retrospectively reviewed. Patients who met the following criteria were enrolled in the ECPR program: age 18–75 years, CCPR started with CA in < 5 min, CA was assumed to be of heart origin, and refractory CA. Survivors had similar underlying diseases and younger age without statistical significance (57.0 vs. 61.0 years, p = 0.117). Survivors had significantly higher rates of initial shockable rhythm, pulseless ventricular tachycardia and ventricular fibrillation, shorter low-flow time (CPR-to-ECMO time), lower lactate levels, and higher initial pH. Survival to discharge was higher for emergency department CA than for out-of-hospital and in-hospital CA (63.3% vs. 35.3%, p = 0.007). Two models were used for evaluating survival to discharge and good neurological outcomes. Model 1, short version based on clinical factors, (S1, survival score 1; F1, function score 1) included the patient’s characteristics before ECPR, whereas Model 2, full version included clinical factors and laboratory data including lactate and pH levels (S2, survival score 2; F2, function score 2). Both Model 1(S1) and Model 2(S2) showed good predictive ability for survival to discharge with areas under the receiver operating characteristic (AUROCs) of 0.79 and 0.83, respectively. Model 1(F1) and Model 2(F2) revealed prediction power for good neurological outcomes, with AUROCs of 0.80 and 0.79, respectively. The AUROCs of survival score Model 1(S1) and 2(S2) and function score Model 1(F1) and 2(F2) were not significantly different. This study demonstrates that clinical factors alone can effectively predict survival to discharge and favorable neurological outcomes at 6 months. This emphasizes the importance of early prognostic evaluation and supports the use of clinical data as a practical tool for clinicians in decision-making for this difficult situation.
Similar content being viewed by others
Introduction
Extracorporeal cardiopulmonary resuscitation (ECPR) is increasingly used as a rescue or salvage therapy for prolonged cardiac arrest (CA) and is associated with improved survival compared with conventional CPR (CCPR)1,2,3,4. Extracorporeal membrane oxygenation (ECMO) has some serious complications, including massive bleeding; ischemia, particularly lower extremity ischemia; and organ failure, particularly hepatic, renal, and brain injury, not to mention the cost and financial burden to the family5,6,7.
Currently, scoring systems to predict survival and good neurological outcomes are unsuitable to provide decision-making for patients requiring ECPR at emergency department. Most prediction scoring systems included parameters after CPR or ECMO implantation, which are not suitable for the critical situation and emergent condition8. However, the use of ECMO is an invasive supportive system that requires intensive critical care, which will consume resources of critical care and add to the socio-economic burden. Predictive models have the potential to assist in crucial decisions, such as determining who would significantly benefit from its support, furthermore, achieving good neurological outcomes at discharge.
The aim of this study is to compare the performance of a clinical-only model (Model 1, short version) with a combined Clinical-Pre-ECMO Model (Model 2, full version), which includes pre-ECMO laboratory parameters such as lactate and pH levels, for evaluating outcomes in patients undergoing extracorporeal cardiopulmonary resuscitation (ECPR). This study aims to improve clinicians’ decision-making in the emergent management of ECPR patients by providing a practical model for predicting survival and neurological function.
Materials and methods
In this retrospective study, medical records between January 2012 and January 2019 in our institution, which was a referred medical center that can provide a 24/7 ECPR service, were reviewed. The study was approved by the Institutional Review Board of Changhua Christian Hospital (IRB No. 220119). Patients who met the following criteria were enrolled in the ECPR program: (1) aged 18–75 years, (2) CCPR initiated within 5 min of cardiac arrest (no-flow time ≤ 5 min), (3) cardiac arrest was presumed to be of cardiac origin, and (4) refractory cardiac arrest (at least 10 min of CCPR with no return of spontaneous circulation [ROSC]).
The exclusion criteria were as follows: duration of CA to CCPR initiation > 5 min or unknown, history of severe head injuries, active acute bleeding, end-stage cancer, severe sepsis, or serious neurological disabilities (including ischemic stroke, hemorrhagic stroke, dementia, and bedridden status).
The ___location of cardiac arrest was categorized as follows: out-of-hospital cardiac arrest (OHCA) occurred outside the hospital, in-hospital cardiac arrest (IHCA) occurred during admission or within the hospital, and emergency department cardiac arrest (EDCA)9 occurred in the emergency department.
Assessment of clinical outcomes
The cause of cardiac arrests, rhythm at CA, and time between CPR and ECMO were examined retrospectively. Sustained ROSC was defined as spontaneous circulation of > 20 min without CA recurrence. Neurological outcomes were evaluated using the Glasgow–Pittsburgh cerebral performance category (CPC) scale. CPC 1 or 2 represents good neurological function, CPC 3 or 4 represents poor neurological function, and CPC 5 represents brain death. Survival outcome and neurological function were evaluated at the day of discharge and 6 months after discharge. Medical records were reviewed, and telephone interviews were conducted to obtain information on patients who survived the ECPR until hospital discharge.
ECMO system and clinical settings
In our institution, ECPR was inaugurated as an alternative for patients who met the inclusion criteria with prolonged CPR, according to the judgment after a discussion among the emergency physician, cardiologist, and our ECMO team. The ECMO system consisted of a patient outflow cannula, a centrifugal blood pump, oxygenator, and patient inflow cannula. The standard ECMO setting included maintaining a minimum flow of 2.0 L/min through centrifugal blood pumps, and the activated clotting time was maintained at 180–220 s by continuous infusion of heparin. ECPR is implemented through femoral cannulation in the ED, general ward, cardiac catheterization laboratory, or intensive care unit. Patients are transferred to the cardiac and surgical intensive care units for diagnosis and treatment while achieving sustained ROSC after ECPR. If there is no sustained organized electrical rhythm after 90 min of ECPR, the patient is declared dead. In patients suspected of acute coronary syndrome, emergency coronary angiography is performed.
Statistical analyses
Demographic and clinical data for continuous variables are presented as median and interquartile, whereas categorical variables are presented as frequencies and percentages. Continuous variables were compared between groups using the Mann–Whitney U test, whereas the chi-square test or Fisher’s exact test was used for categorical variables. The optimal cutoff value for each indicator was determined using receiver operating characteristic (ROC) analysis. Variables that achieved statistical significance (p < 0.05) in the univariate analysis were then analyzed using logistic regression.
Two models were used to evaluate survival to discharge and good neurological outcomes. Model 1 (S1 and F1) included patient characteristics before ECPR, such as age, cardiac arrest ___location, CPR-to-ECMO time, and initial cardiac rhythm. Model 2 (S2 and F2) also considered laboratory results from the emergency department, including lactate and pH levels. Model 1 (S1) and Model 2 (S2) were used to predict survival to discharge, while Model 1 (F1) and Model 2 (F2) predicted good neurological function.
A scoring system was developed based on logistic regression incorporating multiple variables from the each models. The goodness of fit was verified using the Hosmer–Lemeshow test and internally validated using 1000 bootstrap resamplings. Survival and functional component scores were obtained by dividing the coefficients of each predictor retained in the multivariable logistic regression model by the smallest coefficient in the model, multiplying the result by 5 and rounding to the nearest integer. The prediction accuracy of each scaled score was quantified using ROC-derived area under the ROC curve (AUC) estimates. The Delong test method was used to compare the AUC of ROC between the two prediction models. Finally, the clinical outcomes of each patient were validated by classifying their survival and functional scores into three risk categories. The Kaplan–Meier method and the log-rank test were used to compare the survival rates of patients in these risk groups. All statistical analyses were performed using the IBM SPSS Statistics for Windows version 22.0 (IBM Corp., Armonk, NY) and SAS system version 9.4 (SAS Institute, Cary, NC).
Results
Patient characteristics and outcome of refractory CA with ECPR
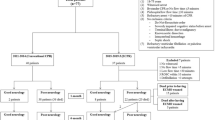
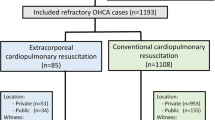
A total of 447 patients received ECMO support at our institution between January 2012 and January 2019, of which 149 patients who met the inclusion and exclusion criteria were enrolled in the study (Fig. 1). The demographic characteristics of the patients are shown in Table 1. Male patients were predominant, which accounted for 73.2%. The median age was 60 years. Depending on the ___location, CAs were classified as IHCA (55.0%), OHCA (24.8%), or EDCA (20.1%). Initially, 86 (57.8%) patients presented with shockable rhythms, namely, pulseless ventricular tachycardia and ventricular fibrillation. Moreover, 63 (42.2%) patients presented with no shockable rhythm, namely, asystole and pulseless electrical activity. The median low-flow time (CRP to CEMO) was 45 (IQR 35.0–55.0) min. The causes of CA were further diagnosed via laboratory examination of a blood sample, computed tomography, or coronary angiography, which included acute myocardial infarction (64.4%), cardiomyopathy (10.1%), myocarditis (10.1%), pulmonary embolism (7.4%), and others (8.1%). Overall, 61 (40.9%) patients survived to discharge, and 45 (30.2%) were alive with good neurological outcomes. Furthermore, 41 (27.5%) patients had good neurological outcomes at the 6-month follow-up after discharge.
Comparison of clinical outcomes between survivors and nonsurvivors
Compared with nonsurvivors (Table 1), survivors had similar underlying diseases and younger age without statistical significance (57.0 [IQR, 48.0–66.0] vs. 61.0 [IQR, 54.0–67.5] years, p = 0.117). Survivors had a significantly higher severity of acute myocardial infarctions (p = 0.024), initial shockable rhythms, pulseless VT and Vf (p < 0.001), shorter low-flow time (CPR-to-ECMO time) (p = 0.001), lower lactate levels (p = 0.002), and higher initial pH (p = 0.039). In the univariate logistic analysis (Table 2), survival to discharge was better for EDCA than for OHCA and IHCA (63.3% vs. 35.3%, p = 0.007).
Factors affecting survival to discharge and good neurological function after ECPR
In the univariate logistic analysis (Table 2), the factors that significantly affected the survival to discharge included age (p = 0.013), EDCA (p = 0.007), CPR-to-ECMO time (p < 0.001), initial shockable cardiac rhythm (p < 0.001), serum lactate concentration (p = 0.005), initial pH (p = 0.004), and CA etiology as myocarditis (p = 0.035).
Considering good neurological function, the significant variables were similar to factors that affected survival, namely, age (p = 0.023), EDCA (p = 0.003), CPR-to-ECMO time (p < 0.001), initial shockable cardiac rhythm (p = 0.001), and serum lactate concentration (p = 0.008), except serum pH level (p = 0.052).
Risk score setting and risk stratification of patients for ECPR
Patient clinical outcomes were stratified before and after ECPR using univariate (Table 2) and multivariate regression (Table 3) analyses. Based on these analyses, two models were developed for comparison: Model 1 (short version, based on clinical factors) and Model 2 (full version, incorporating clinical factors with pre-ECMO parameters). Each model was further refined to predict patient survival to discharge (S1 and S2) and good neurological outcomes (F1 and F2).
In the logistic regression analysis of survival to discharge, Model 1 (S1) multiple analysis showed that three factors (CA ___location, CPR-to-ECMO time, and initial cardiac rhythm) were significant, with survival component scores of 5, 5, and 7 points, respectively. The survival risk of each patient was divided into three risk levels: 0, defined as high; 5–10, defined as medium; and 12–17, defined as low, and the survival outcomes were compared between the three risk groups shown in Fig. 2A.
Patients’ survival and functional scores were classified into three risk categories to validate their clinical outcomes. (A) Model 1(S1) revealed that the low-risk group had good clinical outcomes, with rates of survival to discharge of 65.5% and survival to 6 months after discharge of 63.8%. (B) Model 2(S2) revealed that the low-risk group had the best outcome, with rates of survival to discharge of 84.2% and survival to 6 months after discharge of 78.9%. (C) Model 1(F1) revealed that 49.1% of patients in the low-risk group had good neurological function 6 months after discharge. (D) Model 2(F2) revealed that 52.2% of patients in the low-risk group had good neurological function 6 months after discharge. (E) Kaplan–Meier curves of Model 1(S1) (F) Kaplan–Meier curves of Model 2(S2).
In total, 30 patients were classified as high risk, 61 as medium risk, and 58 as low risk. The low-risk group had good clinical outcomes, with rates of survival to discharge of 65.5% and survival to 6 months after discharge of 63.8%. In the high-risk group, no patients survived to discharge. The Kaplan–Meier curves of Model 1(S1) was showed in Fig. 2E.
Multivariate analysis of Model 2 (S2) identified four significant factors—patient age, CPR-to-ECMO time, initial cardiac rhythm, and initial pH—resulting in survival component scores of 5, 7, 7, and 6 points, respectively.The survival risk was divided into three risk levels: 0–6 defined as high, 7–17 as medium, and 18–25 as low, and the survival outcomes between the three risk groups were compared as shown in Fig. 2B. In total, 31 patients were classified as high risk, 80 as medium risk, and 38 as low risk. The low-risk group had the best outcomes, with rates of survival to discharge of 84.2% and survival to 6 months after discharge of 78.9%. In the high-risk group, no patients survived to discharge.
The Kaplan–Meier curves for Model 2 (S2) are shown in Fig. 2F. Similarly, logistic regression analysis, including multivariate analyses of Model 1 (F1) and Model 2 (F2), was conducted to predict good neurological function. As shown in Fig. 2C, Model 1 (F1) revealed that the rates of good neurological function in the low-risk group were 50.9% at discharge and 49.1% at 6 months after discharge. In Fig. 2D, Model 2(F2) revealed that the rates of good neurological function in the low-risk group were 56.5% at discharge and 52.2% at 6 months after discharge.
Comparative performance of a clinical-only model versus a model with pre-ECMO parameters for survival and neurological outcomes
Two models were used for evaluating survival to discharge and good neurological outcomes. Model 1(S1 and F1) included the patient’s characteristics before ECPR such as age, CA ___location, CPR-to-ECMO time, and initial cardiac rhythm. In Model 2(S2 and F2), laboratory examinations at the ED were considered, including lactate and pH levels. Model 1(S1) and 2(S2) were used to predict survival to discharge, and Model 1(F1) and 2(F2) were used to predict good neurological function. A scoring system was also created according to logistic regression with multiple variables.
Model 1(S1) and 2(S2) showed good ability to predict survival to discharge with AUROCs of 0.79 and 0.83, respectively. Considering good neurological outcomes, the AUROC of Model 1(F1) and 2(F2) were 0.80 and 0.79, respectively. In the comparison of these two models to predict survival to discharge (Fig. 3A), Model 2(S2) showed better predictive power for survival to discharge, but without statistical significance (p = 0.069). However, the ability of Model 1(F1) and 2(F2) to predict good neurological outcomes was not significantly different (p = 0.515) (Fig. 3B). The concordance between the short and long versions was found to be 0.81 for survival to hospital discharge and 0.85 for neurological recovery after hospital discharge (Fig. 4).
Discussion
In this study, the rates of overall survival and good neurological outcomes in patients with refractory CA after ECPR were 40.9% and 30.2%, respectively. Factors that affect survival to discharge and good neurological outcomes were similar, which included younger age, initial shockable rhythms, shorter low-flow time (CPR-to-ECMO time), EDCA, etiology as acute myocardial infarction, lower lactate levels, and higher initial pH levels. Two models were used to predict outcomes. Model 1 involved clinical parameters, and Model 2 included additional parameters using the patient’s blood sample at the ED including serum lactate and pH levels, both of which showed good predictive power for survival and good neurological outcomes.
The prognosis of patients with prolonged CA is dismal when they are refractory to CCPR. The survival to hospital discharge with CA and CCPR was only approximately 7.1%, and the rate of having favorable neurological results of CPC 1 and 2 was only 4.2%10. A meta-analysis showed that the survival-to-discharge rate of patients with IHCA receiving ECPR was 37.9%11. The ARREST study, a phase 2, single-center, randomized controlled trial, showed that the survival rate of patients with OHCA and refractory ventricular fibrillation after early ECPR was 42.9%, which indicated significantly improved survival to discharge and good neurological outcomes compared with CCPR12. Our previous study identified that the survival rate for ECPR for EDCA was higher than that for OHCA and IHCA13. ECPR was associated with higher survival rates and favorable neurological outcomes and was recommended as a class 2a rescue therapy for selected patients but not for routine use; however, CCPR efforts failed to restore spontaneous circulation and can be expeditiously implemented and supported by skilled providers14.
Several factors affected survival after ECPR for CA, namely, age, initial shockable rhythm, duration from CPR initiation to ECMO, and lower lactate and higher pH levels. Several meta-analyses and systematic reviews have shown these important prognostic markers in both IHCA and OHCA11,15,16,17,18. Lactate, as a component of anaerobic metabolism, had been included in the analysis of mortality in CA with CCPR and was found to be a prognostic factor for mortality and neurological outcomes19,20,21,22. Recent prospective studies have revealed the serum lactate as a potential predictor for patients with CA treated with ECPR17,23,24 and lactate clearance as a parameter in the decision to continue ECMO23,25. Except for lactate, arterial pH was also associated with survival outcomes in patients with OHCA receiving ECPR18.
It is difficult to conclude that a single parameter affects ECPR outcomes in patients with CA. Several multivariable mortality prediction models have been used to evaluate the prognosis of ECPR for CA. The modified Survival After Veno-arterial ECMO (SAVE) score, combined lactate and SAVE score, was found to be a useful tool for predicting survival, with AUROCs of 0.84326 in CA and 0.70327 in IHCA receiving ECPR, and demonstrated better predictive power than the SAVE score, which is initially used for refractory cardiogenic shock cases with ECMO support28. Another retrospective study of the American Heart Association developed the Resuscitation Using ECPR During In-Hospital Cardiac Arrest score, which well predicted survival with AUROC of 0.719 and acceptable calibration (Hosmer–Lemeshow goodness of fit, p = 0.079)29. In our analysis, we employed two models for predicting patient outcomes. These models also proved valuable in predicting survival, demonstrating similar AUROCs of 0.738 and 0.770, respectively.
Neurological outcomes were also crucial when deciding ECMO support for patients with CA. Recent studies also evaluated neurological outcomes using the SAVE score and mSAVE score, with AUROCs of 0.629 and 0.693, respectively. Considering body mass index and mSAVE score, the model showed better predictive power, and the AUROC was 0.76027. A study in Japan used a TiPS65 scoring system to predict neurological outcomes for OHCA, with a AUROC of 0.72430. In our analysis, functional Model 1 and 2 demonstrated strong predictive power for favorable neurological outcomes at the 6-month mark, boasting AUROCs of 0.854 and 0.927, respectively. Despite incorporating factors after initiating CPR, such as lactate, in functional Model 2 for evaluation, both models did not exhibit a statistically significant difference. Given that factors such as age, ___location of CPR, and initial cardiac rhythm are non-modifiable in our study, the only adjustable factor influencing patient outcomes is the duration of CPR to ECMO. Hence, timely decisions regarding ECMO implantation to minimize this duration are crucial for optimizing patient outcome.
This study is subject to limitations. It was a single-center, retrospective study with a restricted sample size. In addition, patients with IHCA and OHCA were enrolled, and the quality of CPR was difficult to evaluate. We did not enroll patients from the COVID-19 pandemic period due to the potential confounding effects of the pandemic. The COVID-19 pandemic introduced variables such as the etiology of cardiac arrest being influenced by coronavirus infection, along with potential biases in patient management during that time. This study serves as a pilot to provide valuable insights into the management of patients with cardiac arrest undergoing ECMO, and that further multi-center, large-scale studies are required to validate these findings.
Conclusions
This study is the first to evaluate and compare predictive models based solely on clinical factors with models incorporating pre-ECMO parameters for ECPR patients with refractory cardiac arrest. We found that the clinical-only short model, which relies on clinical factors, accurately predicts survival to discharge and neurological outcomes in patients with refractory cardiac arrest (CA) undergoing ECPR, offering clinicians a practical decision-making tool.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
JK Patel H Meng A Qadeer PB Parikh 2019 Impact of extracorporeal membrane oxygenation on mortality in adults with cardiac arrest Am. J. Cardiol. 124 1857 1861 https://doi.org/10.1016/j.amjcard.2019.09.013
A Inoue T Hifumi T Sakamoto Y Kuroda 2020 Extracorporeal cardiopulmonary resuscitation for out-of-hospital cardiac arrest in adult patients J. Am. Heart Assoc. 9 e015291https://doi.org/10.1161/JAHA.119.015291
YS Chen A Chao HY Yu WJ Ko IH Wu RJ Chen SC Huang FY Lin SS Wang 2003 Analysis and results of prolonged resuscitation in cardiac arrest patients rescued by extracorporeal membrane oxygenation J. Am. Coll. Cardiol. 41 197 203 https://doi.org/10.1016/s0735-1097(02)02716-x
D Abrams G MacLaren R Lorusso S Price D Yannopoulos L Vercaemst J Bělohlávek FS Taccone N Aissaoui K Shekar 2022 Extracorporeal cardiopulmonary resuscitation in adults: Evidence and implications Intensive Care Med. 48 1 15 https://doi.org/10.1007/s00134-021-06514-y
MI Bharmal JM Venturini RFM Chua WW Sharp DG Beiser CE Tabit T Hirai JR Rosenberg J Friant JEA Blair 2019 Cost-utility of extracorporeal cardiopulmonary resuscitation in patients with cardiac arrest Resuscitation 136 126 130 https://doi.org/10.1016/j.resuscitation.2019.01.027
JE Tonna CH Selzman MP Mallin BR Smith ST Youngquist A Koliopoulou F Welt KD Stoddard R Nirula R Barton 2017 Development and implementation of a comprehensive, multidisciplinary emergency department extracorporeal membrane oxygenation program Ann. Emerg. Med. 70 32 40 https://doi.org/10.1016/j.annemergmed.2016.10.001
NJ Johnson M Acker CH Hsu N Desai P Vallabhajosyula S Lazar J Horak J Wald F McCarthy E Rame 2014 Extracorporeal life support as rescue strategy for out-of-hospital and emergency department cardiac arrest Resuscitation 85 1527 1532 https://doi.org/10.1016/j.resuscitation.2014.08.028
LCA Pladet JMM Barten LM Vernooij CVE Kraemer JJH Bunge E Scholten LJ Montenij M Kuijpers DW Donker OL Cremer CL Meuwese 2023 Prognostic models for mortality risk in patients requiring ECMO Intensive Care Med. 49 131 141 https://doi.org/10.1007/s00134-022-06947-z
KS Bilodeau KE Gray DM McMullan 2024 Extracorporeal cardiopulmonary resuscitation outcomes for children with out-of-hospital and emergency department cardiac arrest Am. J. Emerg. Med. 81 35 39 https://doi.org/10.1016/j.ajem.2024.03.035
BJ Bobrow DW Spaite RA Berg U Stolz AB Sanders KB Kern TF Vadeboncoeur LL Clark JV Gallagher JS Stapczynski 2010 Chest compression-only CPR by lay rescuers and survival from out-of-hospital cardiac arrest JAMA 304 1447 1454 https://doi.org/10.1001/jama.2010.1392
S D’Arrigo S Cacciola M Dennis C Jung E Kagawa M Antonelli C Sandroni 2017 Predictors of favourable outcome after in-hospital cardiac arrest treated with extracorporeal cardiopulmonary resuscitation: A systematic review and meta-analysis Resuscitation 121 62 70 https://doi.org/10.1016/j.resuscitation.2017.10.005
D Yannopoulos J Bartos G Raveendran E Walser J Connett TA Murray G Collins L Zhang R Kalra M Kosmopoulos 2020 Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): A phase 2, single centre, open-label, randomised controlled trial Lancet 396 1807 1816 https://doi.org/10.1016/S0140-6736(20)32338-2
FY Siao CW Chiu CC Chiu YJ Chang YC Chen YL Chen YK Hsieh CC Chou HH Yen 2020 Can we predict patient outcome before extracorporeal membrane oxygenation for refractory cardiac arrest? Scand J. Trauma Resusc. Emerg. Med. 28 58 https://doi.org/10.1186/s13049-020-00753-6
SM Perman J Elmer CB Maciel A Uzendu T May BE Mumma JA Bartos AJ Rodriguez MC Kurz AR Panchal 2024 2023 American heart association focused update on adult advanced cardiovascular life support: An update to the american heart association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care Circulation 149 e254 e273 https://doi.org/10.1161/CIR.0000000000001194
JA Bartos K Carlson C Carlson G Raveendran R John TP Aufderheide D Yannopoulos 2018 Surviving refractory out-of-hospital ventricular fibrillation cardiac arrest: Critical care and extracorporeal membrane oxygenation management Resuscitation 132 47 55 https://doi.org/10.1016/j.resuscitation.2018.08.030
J Koen T Nathanaël D Philippe 2020 A systematic review of current ECPR protocols. A step towards standardisation Resusc. Plus. 3 100018https://doi.org/10.1016/j.resplu.2020.100018
G Debaty V Babaz M Durand L Gaide-Chevronnay E Fournel M Blancher H Bouvaist O Chavanon M Maignan P Bouzat 2017 Prognostic factors for extracorporeal cardiopulmonary resuscitation recipients following out-of-hospital refractory cardiac arrest. A systematic review and meta-analysis Resuscitation 112 1 10 https://doi.org/10.1016/j.resuscitation.2016.12.011
Y Zhang CS Li XL Yuan JY Ling Q Zhang Y Liang B Liu LX Zhao 2018 Association of serum biomarkers with outcomes of cardiac arrest patients undergoing ECMO Am. J. Emerg. Med. 36 2020 2028 https://doi.org/10.1016/j.ajem.2018.03.015
JL Vincent D Backer De 2014 Circulatory shock N. Engl. J. Med. 370 583 https://doi.org/10.1056/NEJMc1314999
MW Donnino LW Andersen T Giberson DF Gaieski BS Abella MA Peberdy JC Rittenberger CW Callaway J Ornato J Clore 2014 Initial lactate and lactate change in post-cardiac arrest: A multicenter validation study Crit. Care Med. 42 1804 1811 https://doi.org/10.1097/CCM.0000000000000332
SJ Park SP Kim JB Kim SH Jung SJ Choo CH Chung JW Lee 2014 Blood lactate level during extracorporeal life support as a surrogate marker for survival J. Thorac. Cardiovasc. Surg. 148 714 720 https://doi.org/10.1016/j.jtcvs.2014.02.078
HR Omar JW Handshoe T Tribble M Guglin 2022 Survival on venoarterial extracorporeal membrane oxygenation in cardiogenic shock: Which lactate is most useful? ASAIO J. 68 41 45 https://doi.org/10.1097/MAT.0000000000001413
R Jouffroy L Lamhaut A Guyard P Phillipe T Deluze M Jaffry C Dagron W Bourgoin JP Orsini K An 2014 Base excess and lactate as prognostic indicators for patients treated by extra corporeal life support after out hospital cardiac arrest due to acute coronary syndrome Resuscitation 85 1764 1768 https://doi.org/10.1016/j.resuscitation.2014.10.012
C Jung K Janssen M Kaluza G Fuernau TC Poerner M Fritzenwanger R Pfeifer H Thiele HR Figulla 2016 Outcome predictors in cardiopulmonary resuscitation facilitated by extracorporeal membrane oxygenation Clin. Res. Cardiol. 105 196 205 https://doi.org/10.1007/s00392-015-0906-4
İ Mungan D Kazancı Ş Bektaş D Ademoglu S Turan 2018 Does lactate clearance prognosticates outcomes in ECMO therapy: A retrospective observational study BMC Anesthesiol. 18 152 https://doi.org/10.1186/s12871-018-0618-1
WC Chen KY Huang CW Yao CF Wu SJ Liang CH Li CY Tu HJ Chen 2016 The modified SAVE score: Predicting survival using urgent veno-arterial extracorporeal membrane oxygenation within 24 hours of arrival at the emergency department Crit. Care. 20 336 https://doi.org/10.1186/s13054-016-1520-1
JW Schurr M Noubani LA Santore AP Rabenstein K Dhundale J Fitzgerald J Cahill TV Bilfinger FC Seifert AJ McLarty 2021 Survival and outcomes after cardiac arrest with VA-ECMO rescue therapy Shock 56 939 947 https://doi.org/10.1097/SHK.0000000000001809
M Schmidt A Burrell L Roberts M Bailey J Sheldrake PT Rycus C Hodgson C Scheinkestel DJ Cooper RR Thiagarajan 2015 Predicting survival after ECMO for refractory cardiogenic shock: the survival after veno-arterial-ECMO (SAVE)-score Eur. Heart J. 36 2246 2256 https://doi.org/10.1093/eurheartj/ehv194
JE Tonna CH Selzman S Girotra AP Presson RR Thiagarajan LB Becker C Zhang P Rycus HT Keenan Investigators AHAGWtGR 2022 Resuscitation using ecpr during in-hospital cardiac arrest (RESCUE-IHCA) mortality prediction score and external validation JACC Cardiovasc. Interv. 15 237 247 https://doi.org/10.1016/j.jcin.2021.09.032
Y Okada T Kiguchi T Irisawa T Yamada K Yoshiya C Park T Nishimura T Ishibe Y Yagi M Kishimoto 2020 Development and validation of a clinical score to predict neurological outcomes in patients with out-of-hospital cardiac arrest treated with extracorporeal cardiopulmonary resuscitation JAMA Netw. Open. 3 e2022920https://doi.org/10.1001/jamanetworkopen.2020.22920
Funding
The authors received research fund from Changhua Christian Hospital (111-CCH-IRP-063).
Author information
Authors and Affiliations
Contributions
Conceptualization, Chun-Chieh Chiu, Ying-Chen Chen, Shun-Wen Hsiao, Hsu-Heng Yen and Fu-Yuan Siao; Data curation, Chun-Wen Chiu and Yu-Jun Chang; Formal analysis, Shun-Wen Hsiao and Fu-Yuan Siao; Funding acquisition, and Fu-Yuan Siao; Investigation, Ying-Chen Chen; Methodology, and Fu-Yuan Siao; Software, and Fu-Yuan Siao; Validation, Chun-Chieh Chiu and Ying-Chen Chen; Visualization, Shun-Wen Hsiao; Writing—original draft, Shun-Wen Hsiao and Fu-Yuan Siao; Writing—review and editing, Yu-Jun Chang, Ying-Chen Chen, Yung-Kun Hsieh, Hsu-Heng Yen and Fu-Yuan Siao.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Institutional review board statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Changhua Christian Hospital (CCH IRB No. 220119).
Informed consent
Patient consent was waived due to the retrospective nature of the study and approved by the Institutional Review Board of Changhua Christian Hospital.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chiu, CC., Chang, YJ., Chiu, CW. et al. Comparing clinical only and combined clinical laboratory models for ECPR outcomes in refractory cardiac arrest. Sci Rep 15, 2915 (2025). https://doi.org/10.1038/s41598-025-87200-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-87200-7