Abstract
Atherosclerotic cardiovascular disease (ASCVD) is a leading cause of mortality worldwide. Dysregulation of glucose metabolism and inflammation are key factors in the development of atherosclerosis. The glucose-to-lymphocyte ratio (GLR) is a comprehensive marker for assessing glucose metabolism and inflammation. This study aims to evaluate the association between GLR and all-cause as well as cardiovascular disease (CVD) mortality in patients with ASCVD within the U.S. population. This retrospective cohort study recruited 1,753 ASCVD patients from the 2003–2018 National Health and Nutrition Examination Survey (NHANES) with a median follow-up of 6.25 years. Mortality outcomes were determined by linkage to the National Death Index (NDI) records up to December 31, 2019. Weighted Cox proportional hazard models were used to assess the independent association between GLR and mortality risk. Restricted cubic spline (RCS) curves were used to display the relationship between GLR and all-cause mortality visually, and two-segment Cox proportional hazards models were constructed on either side of the inflection points. Kaplan-Meier survival curves were further used to assess the relationship between GLR and mortality, and further subgroup analyses were performed. Receiver operating characteristic curve (ROC) analysis was conducted to assess the predictive ability of GLR for survival. During a median follow-up of 6.25 years, 624 deaths from various causes were observed, with 254 deaths from CVD. Cox regression analysis revealed a positive association between GLR and both all-cause and CVD mortality. Based on RCS, a J-shaped nonlinear relationship was observed between GLR and all-cause mortality in ASCVD patients, with an inflection point at 3.13. When the GLR < 3.13, it showed a significant negative association with all-cause mortality (HR 0.65, 95% CI 0.47–0.89). When GLR ≥ 3.13 for all-cause mortality, there was a significant positive correlation with all-cause mortality (HR 1.13, 95% CI 1.09–1.17). Subgroup analysis revealed a positive association between GLR and CVD mortality across most subgroups, but the correlation between GLR and CVD mortality was weaker compared to its association with all-cause mortality. In addition, an interaction was detected between GLR and age in relation to all-cause mortality. Moreover, the predictive performance of GLR on all-cause and CVD mortality seemed superior to that of glucose or lymphocytes. Our findings indicate that elevated GLR was closely associated with an increased risk of all-cause mortality and CVD mortality in ASCVD patients. Notably, the relationship between GLR and all-cause mortality exhibited a J-shaped nonlinear pattern, with an inflection point at 3.13.
Similar content being viewed by others
Introduction
Atherosclerosis refers to the formation of fibrofatty lesions in the arterial walls. Cardiovascular disease (CVD) caused by atherosclerosis are collectively termed atherosclerotic cardiovascular disease (ASCVD), which include ischemic stroke, ischemic heart disease, and peripheral arterial atherosclerotic vascular disease1. ASCVD is the most important component of CVD. Recent research indicates that over the past thirty years, the prevalence and related deaths from CVD worldwide have significantly increased, from 271 million to 523 million people2. Additionally, the number of deaths related to CVD increased by 6.5 million2, imposing a huge burden on the global economy and human health3. Consequently, researchers worldwide are dedicated to exploring and addressing its risk factors to improve the prognosis of ASCVD patients.
Dysregulation of glucose metabolism and systemic inflammation are closely related to ASCVD4,5. Diabetes is considered an important independent risk factor for the occurrence and development of ASCVD6. Insulin resistance and hyperglycemia can promote the development of atherosclerosis7. Recent studies have shown that hyperglycemia is associated with higher mortality rates in patients with acute myocardial infarction (AMI)8.
Studies have found that atherosclerosis is essentially vascular wall damage caused by lipid-induced inflammation. Inflammation not only leads to the occurrence of ASCVD but also promotes its progression, eventually resulting in various complications and even death9. There is much research that shows Multiple immune-inflammatory pathways such as NLRP3 inflammasome, Toll-like receptors, proprotein convertase subtilisin/kexin type 9, Notch and Wnt signaling pathways are associated with atherosclerosis prognostic prediction10,11, Therefore, anti-inflammatory treatments targeting inflammatory factors may be an effective strategy to improve the prognosis of ASCVD patients.
Glucose to lymphocyte ratio (GLR), as a novel biomarker, can be quickly obtained through blood glucose tests and routine blood tests, reflecting fasting blood glucose and systemic inflammation levels. In recent years, GLR has been widely used due to its simplicity and cost-effectiveness. Several studies have shown that GLR can serve as a prognostic marker for cancers such as liver cancer12, gastric cancer13, and thyroid cancer14. Additionally, recent studies have shown that GLR also has important value in prognostic evaluation for inflammatory diseases such as pancreatitis15, chronic obstructive pulmonary disease (COPD)16, and acute respiratory distress syndrome (ARDS)17.
However, there is currently limited evidence assessing the relationship between GLR and all-cause or CVD mortality in American ASCVD populations. A study evaluating in-hospital mortality in AMI patients demonstrated that the predictive value of the GLR is superior to using glucose or lymphocytes alone8. Another study found that elevated GLR is related to higher in-hospital mortality in critically ill patients with cerebral hemorrhage18. However, it remains unclear whether GLR is associated with all-cause mortality in non-hospitalized patients previously diagnosed with ASCVD in the U.S. population. Furthermore, these studies did not evaluate the relationship between GLR and CVD mortality.
Therefore, this study utilizes data from the National Health and Nutrition Examination Survey (NHANES) database to evaluate the relationship between GLR and all-cause as well as CVD mortality in U.S. patients with ASCVD.
Materials and methods
The NHANES is an ongoing survey that uses a complex, multistage probability sampling method to select a representative sample of the U.S. population. It aims to assess the health and nutritional status of adults and children. The Centers for Disease Control and Prevention (CDC) is responsible for providing national health statistics. The NHANES study protocol was approved by the National Center for Health Statistics (NCHS) Ethics Review Board, all participants provided written informed consent, and all methods were carried out in accordance with the relevant guidelines and regulations19. The datasets used in this study and their analysis results are available at any time on the CDC’s website (https://www.cdc.gov/nchs/nhanes/index.html)20,21.
Study participants
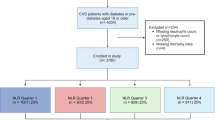
This study analyzed data from the NHANES database from 2003 to 2018. Initially, we recruited 80,312 participants. After excluding participants with missing data on ASCVD (35,533 individuals), those without ASCVD (40,077 individuals), those with missing fasting blood glucose data (2,156 individuals), those with missing lymphocyte count data (471 individuals), those covariate missing data (162 individuals), those unsuitable for follow-up (1 individual), and those with missing subsample weight data (159 individuals), the final analysis included 1,753 participants (Fig. 1).
Calculation method of GLR
The calculation method of GLR is to divide the fasting blood glucose concentration (mmol/L) by the serum lymphocyte count (109 cells/L). Fasting blood glucose concentration was determined by an enzymatic method on the Roche/Hitachi Cobas C 501 chemistry analyzer, using a hexokinase-mediated reaction to assess fasting plasma glucose levels. Lymphocyte count was obtained by performing a complete blood count on blood specimens using the Beckman Coulter automated hematology analyzer, expressed as × 103 cells/µL22.
Mortality assessment
To determine the mortality status of the follow-up population, we used the NHANES Public-use Linked Mortality File, updated through December 31, 2019. The NCHS linked this file to the National Death Index (NDI) using a probabilistic matching algorithm. Additionally, deaths from specific diseases were identified using the International Classification of Diseases, Tenth Revision (ICD-10). Cardiovascular disease deaths included rheumatic heart disease, hypertensive heart disease, ischemic heart disease, acute myocardial infarction, pericardial diseases, acute myocarditis, heart failure, and cerebrovascular diseases. The corresponding disease codes are I00-I09, I11, I13, I20-I51,and I60-I6923,24.
Covariate assessment
Data on various demographic and health-related factors were collected through NHANES household interviews. These comprehensive pieces of information include age, age categorized as “<60” and “>=60”, sex (female/male), race(non-Hispanic white, non-Hispanic black, Mexican American, other), education level categorized as “Less than high school level, “High school or equivalent, “or “Greater than high school level “28, smoking status categorized as never/former/current, and body mass index(BMI) calculated as weight (kg) divided by height (m) squared. Participants were categorized into normal weight (< 25 kg/m2), overweight (25-29.9 kg/m2), and obese (≥ 30 kg/m2) categories29. Laboratory examinations included fasting blood glucose, lymphocyte count, GLR, total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), glycated hemoglobin (HbA1c), creatinine (Cr), and uric acid (UA). The definition of diabetes relied on an affirmative response to any of the following questions: “Have you ever been told by a doctor that you have diabetes?” or “Are you currently taking insulin?” or “Are you currently taking diabetic pills?”30. The definition of hypertension depended on an affirmative response to either of the following questions: “Has a doctor ever told you that you have high blood pressure?” or “Are you currently taking medication for high blood pressure?”
Statistical analysis
The statistical analysis followed guidelines from the Centers for Disease Control and Prevention of the United States. NHANES employed a stratified multistage probability sampling to reduce biases caused by stratification, non-response, and oversampling. Continuous variables were presented as the weighted mean ± standard error, while categorical variables were expressed in terms of frequency and percentage. We divided GLR into four subgroups by quartile, i.e., Q1 (0.05–2.66], Q2 (2.66–3.48], Q3(3.48–4.69], and Q4 (4.69–21.48], with Q1 as the reference group. Analysis of variance (ANOVA) was used to test GLR quartile variations. To assess the hazard ratios (HR) and 95% CIs for the risk of all-cause mortality and CVD mortality associated with GLR, we constructed multivariable Cox proportional hazards regression models. Three models were used to control for confounding factors. Model 1 was unadjusted; Model 229 was adjusted for age, sex, race, education level, body mass index, and smoking status; Model 329was further adjusted for diabetes, hypertension, HbA1c, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, total cholesterol, triglycerides, creatinine, and uric acid. RCS was used to display the relationship between GLR and all-cause mortality visually, and two-segment Cox proportional hazard models were constructed on either side of the inflection points. Kaplan-Meier survival curves were further used to assess the relationship between GLR and mortality. Our results were also subjected to sensitivity analyses stratified according to Model 3 to assess whether the relationship between GLR and mortality was influenced by age, sex, race, education level, body mass index, smoking, diabetes, and hypertension. Subsequently, receiver operating characteristic (ROC) analysis was used to evaluate the predictive ability of GLR, glucose, and lymphocytes for all-cause mortality and CVD mortality. Statistical significance was set at a two-tailed P-value of < 0.05. All analyses were conducted using R version 4.3.3 (http://www.R-project.org, The R Foundation).
Results
Baseline characteristics of study participants
Table 1 Lists the baseline characteristics of study participants stratified by the quartiles GLR. A total of 1,753 participants met the study criteria, with an average age of 64.51 ± 0.40 years. Among them, 55.62% were male and 44.38% were female. Furthermore, among these participants, 74.32% were non-hispanic white, 25.62% had not completed high school or higher education, 44.98% were obese, 23.13% were current smokers, 69.79% had hypertension, and 29.03% had diabetes. The average GLR of the participants was 3.94 ± 0.07, with a GLR range of 0.05–21.48. The GLR range for quartiles 1–4 were 0.05–2.66, 2.66–3.48, 3.48–4.69, and 4.69–21.48, respectively. Statistically significant differences (all P < 0.05) were observed between different quartiles of GLR in terms of age, fasting blood glucose, lymphocyte count, low-density lipoprotein cholesterol, total cholesterol, glycated hemoglobin, creatinine, sex, race, smoking status, and diabetes. Compared with the lowest GLR quartile, individuals in the increased GLR group were more likely to have diabetes, and they had higher levels of fasting blood glucose, creatinine, and glycated hemoglobin. Additionally, they were more likely to be male, non-hispanic white, and non-smokers. There were no statistically significant differences among quartiles in terms of high-density lipoprotein cholesterol, triglycerides, uric acid, body mass index, education level, and hypertension. (all P > 0.05)
Relationship between GLR and all-cause and CVD mortality
Table 2 shows that during a median follow-up of 6.25 years, there were 624 all-cause deaths and 254 cardiovascular-related deaths. To explore the independent association between GLR and the risk of all-cause and CVD mortality, we established three Cox regression models. As a continuous variable, in model 1, GLR was significantly positively associated with the risk of all-cause mortality (HR 1.17, 95% CI 1.13–1.21). This positive association remained in the minimally adjusted model (HR 1.13, 95% CI 1.08–1.17). After full adjustment, GLR was still positively associated with the risk of all-cause mortality (HR 1.10, 95% CI 1.04–1.15). As a quartile variable, in the model3 adjusting for all covariates, compared with participants in Q1, multivariate HRs for all-cause mortality were 0.87 (95% CI 0.64–1.17) for Q2, 1.04 (95% CI 0.74–1.45) for Q3, and 1.17 (95% CI 0.79–1.72) for Q4. Higher GLR quantiles were not associated with an increased risk of all-cause mortality compared to the lowest quantile. Furthermore, we observed a relationship between continuous GLR and CVD mortality, with higher GLR associated with an increased risk of CVD mortality in model 3 after full adjustment for confounders (HR 1.08, 95% CI 1.00-1.16). However, when analyzed as a categorical variable, in the model3 adjusting for all covariates, compared with participants in Q1, multivariate HRs for CVD mortality was 0.82 (95% CI 0.50–1.36) for Q2, 1.09 (95% CI 0.69–1.73) for Q3, and 1.12 (95% CI 0.66–1.90) for Q4. Higher GLR quantiles were not associated with an increased risk of CVD mortality compared to the lowest quantile.
RCS analysis
To further validate the relationship between GLR and the risks of all-cause and CVD mortality, we performed an RCS analysis. The results indicated a J-shaped non-linear relationship between GLR and both all-cause mortality (p for nonlinear = 0.0024) (Fig. 2). Yet, this trend was not observed in CVD mortality. RCS analysis revealed a linear association between GLR and CVD mortality (p for nonlinear = 0.1839) (Fig. 3). Additionally, we further modeled the relationship between GLR and all-cause mortality using a two-segment Cox proportional hazards regression model. The analysis showed that the threshold for all-cause mortality was 3.13 (Table 3). Notably, When GLR < 3.13, we observed that for each unit increase in the GLR index, all-cause mortality decreased by 35% (HR 0.65, 95% CI 0.47–0.89). Moreover, when GLR was ≥ 3.13, each unit increase in GLR was associated with a 13% increase in the risk of all-cause mortality (HR 1.13, 95% CI 1.09–1.17). It is noteworthy that the survival curves showed a significant decrease in survival rates for the higher GLR quantiles compared to the lowest GLR quantile, with significantly increased all-cause and CVD mortality (p < 0.0001) (Figs. 4 and 5).
Subgroup analysis
The analysis was stratified by age, sex, race, education level, body mass index, smoking, diabetes, and hypertension. GLR was further treated as a continuous variable, and the all-cause mortality associated with the GLR among patients with ASCVD, no significant interactions were observed across most subgroups. The exception was a significant interaction detected between GLR and age on all-cause mortality (Table 4).
In subgroup analyses examining CVD mortality associated with the GLR among patients with ASCVD, no significant interactions were observed (Table 5). This suggests that the results are reliable, and that the correlation between GLR and CVD mortality is weaker compared to its association with all-cause mortality.
Predictive performance of GLR, glucose, and lymphocytes on all-cause mortality and CVD mortality
The predictive value of GLR, glucose, and lymphocytes on all-cause mortality and CVD mortality with ASCVD was further assessed. The ROC analysis revealed that the area under the curve (AUC) for GLR in predicting all-cause mortality was 0.58, outperforming glucose 0.41 and lymphocytes 0.53 (Fig. 6). Additionally, the AUC for GLR in predicting CVD mortality was 0.59, which was superior to glucose 0.40 and lymphocytes 0.51 (Fig. 7). The curve of GLR was consistently higher than that of glucose or lymphocytes, and the graphical trend was basically in equilibrium, indicating that the predictive value of GLR may be superior to that of glucose or lymphocytes alone.
Discussion
In this study involving 1,753 American ASCVD patients, we observed a positive association between GLR and both all-cause and CVD mortality. Based on RCS, a J-shaped nonlinear relationship was observed between GLR and all-cause mortality in ASCVD patients, with an inflection point at 3.13. Moreover, the predictive performance of GLR on all-cause and CVD mortality seemed superior to that of glucose or lymphocytes. Overall, our study suggests that GLR is an important indicator of the risk of all-cause and CVD mortality in ASCVD patients, contributing to the advancement of cardiovascular disease prevention measures.
The exact mechanism linking GLR levels in patients with ASCVD to the risk of all-cause mortality and CVD mortality remains unclear. It has been reported that high blood glucose levels lead to prolonged oxidative stress, causing endothelial dysfunction and promoting the development of atherosclerosis31,32,33. Moreover, under hyperglycemic conditions, inflammatory responses intensify, manifested by elevated levels of inflammatory cytokines such as TNF-α, IL-6, and IL-8. These cytokines contribute to smooth muscle cell proliferation and collagen deposition, ultimately triggering atherosclerosis34,35,36. Diabetes also promotes the production of plasminogen activator inhibitor-1, further facilitating thrombus formation37. Numerous studies indicate that diabetes is associated with platelet activation38.
In recent years, large-scale clinical studies such as the CANTOS study39, the COLCOT study40, and the LoDoCo2 study41 have confirmed that anti-inflammatory therapies can significantly reduce the risk of ASCVD mortality. Reduced lymphocyte count is a fundamental characteristic of systemic inflammation, as the release of anti-inflammatory factors can induce immune suppression, leading to extensive lymphocyte apoptosis18. Studies have reported that reduced lymphocyte count is closely associated with adverse outcomes in stroke patients42. Reduced lymphocyte count may also be related to the activation of the renin-angiotensin system and the adrenergic nervous system, where the produced adrenaline and angiotensin further promote lymphocyte apoptosis43. Therefore, targeting lymphocytes with anti-inflammatory therapies provides a novel approach to reducing CVD mortality risk in ASCVD patients.
Research has already reported the predictive value of GLR in malignant tumors and inflammatory diseases. Yılmaz A and colleagues discovered that pre-treatment GLR levels with sorafenib could serve as a novel prognostic biomarker, accurately predicting survival in patients with advanced hepatocellular carcinoma12. Another study indicated that GLR could independently and rapidly predict the mortality of hospitalized patients with acute pancreatitis15. Similarly, in our study, we found that high levels of GLR were associated with an increased risk of both all-cause and CVD mortality in patients with ASCVD. We further evaluated the predictive value of GLR and compared it with the predictive values of glucose and lymphocytes, finding that GLR’s predictive performance might surpass that of the other two indicators. Therefore, our results provide some evidence that GLR could be a potentially good predictive factor for patients with ASCVD.
Although the GLR is measured at a single time point, it may represent the overall health status of a patient at that time. As a biomarker, GLR reflects the relationship between the body’s glucose metabolism and immune function. Elevated glucose levels are often associated with multiple disease states such as diabetes and metabolic syndrome, both of which are linked to increased risk of mortality. High blood glucose can induce oxidative stress and promote the formation of advanced glycation end-products, thus exacerbating vascular inflammation and atherosclerosis44. Additionally, a reduction in lymphocyte count, an indicator of immune status, often signifies a weakened body’s resistance to disease. A decrease in lymphocytes is associated with increased inflammatory activity45, a known factor in the progression of atherosclerotic plaques. In summary, a single-time measurement of GLR can reflect key aspects of disease pathology and has significant prognostic value for the long-term mortality of patients with ASCVD.
According to the results of the subgroup analysis, higher GLR levels were significantly associated with an increased risk of all-cause mortality in ASCVD patients older than 60 years. Both glucose tolerance and adaptive immune function showed significant age-related changes. A study in elderly mice showed that T lymphocyte depletion improves age-related metabolic damage46. In our study, the elevated risk of all-cause mortality from ASCVD in the elderly is linked to poor overall health and increased complications, requiring heightened attention as the population ages.
Our study subjects were determined using a complex multistage probability sampling weighted method, resulting in a representative NHANES cohort that included 1,753 ASCVD patients, providing a relatively large sample size. Secondly, our study evaluated the association between GLR and both all-cause mortality and CVD mortality in ASCVD patients, enriching the research in this field. Thirdly, this study employed stratified analysis and K-M survival curves, utilizing diverse analytical methods and a longer follow-up period, further ensuring the reliability and robustness of the study results.
This study also has some noteworthy limitations. First, our subjects were primarily Americans, which may limit the applicability of the results to different race and living environments, necessitating further validation of our main findings in other countries. Secondly, despite adjustments for potential confounders, it is impossible to rule out all potential confounders completely; for example, due to the design limitations of the NHANES database, we did not include diseases that cause lymphocyte reduction, such as acquired immunodeficiency syndrome (AIDS). Thirdly, GLR was measured as a single baseline value, and the impact of fluctuations in this index on mortality risk during follow-up remains unclear. Fourthly, the definition of ASCVD in NHANES relies on self-reported physician-diagnosed surveys rather than imaging assessments or laboratory biomarkers, potentially introducing retrospective bias and limiting the accuracy of ASCVD diagnosis. Lastly, our study is based on the NHANES database, which is cross-sectional in nature. We hope that the findings of this study will be validated in future longitudinal cohort studies.
Conclusion
Our study results indicate that GLR is a rapid, economical, and valuable biomarker for the risk of all-cause and CVD mortality in American ASCVD patients. Furthermore, we found a non-linear association between GLR and all-cause mortality and a linear relationship with CVD mortality. Therefore, we recommend monitoring GLR in ASCVD patients and consider it as a potentially beneficial method for assessing clinical prognosis and mortality risk in these patients.
Data availability
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/index.htm.
References
Libby, P. et al. Atherosclerosis. Nat. Rev. Dis. Primers 5(1): 56. (2019).
Roth, G. A. et al. Global Burden of Cardiovascular diseases and Risk factors, 1990–2019: Update from the GBD 2019 study. J. Am. Coll. Cardiol. 76(25), 2982–3021 (2020).
Vaduganathan, M., Mensah, G. A., Turco, J. V., Fuster, V. & Roth, G. A. The Global Burden of Cardiovascular diseases and Risk: a compass for Future Health. J. Am. Coll. Cardiol. 80(25), 2361–2371 (2022).
International, H. S. G. Hypoglycaemia, cardiovascular disease, and mortality in diabetes: epidemiology, pathogenesis, and management. Lancet Diabetes Endocrinol. 7(5), 385–396 (2019).
Golia, E. et al. Inflammation and cardiovascular disease: from pathogenesis to therapeutic target. Curr. Atheroscler Rep. 16(9), 435 (2014).
Zhao, D., Liu, J., Wang, M., Zhang, X. & Zhou, M. Epidemiology of cardiovascular disease in China: current features and implications. Nat. Rev. Cardiol. 16(4), 203–212 (2019).
Bornfeldt, K. E. & Tabas, I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell. Metab. 14(5), 575–585 (2011).
Liu, J. & Hu, X. Association between glucose-to-lymphocyte ratio and in-hospital mortality in acute myocardial infarction patients. PLoS One. 18(12), e0295602 (2023).
Bäck, M., Yurdagul, A. Jr, Tabas, I., Öörni, K. & Kovanen, P. T. Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nat. Rev. Cardiol. 16(7), 389–406 (2019).
Ridker, P. M. & Rane, M. Interleukin-6 signaling and anti-interleukin-6 therapeutics in Cardiovascular Disease. Circ. Res. 128(11), 1728–1746 (2021).
Ridker, P. M. From C-Reactive protein to Interleukin-6 to Interleukin-1: moving Upstream to identify novel targets for atheroprotection. Circ. Res. 118(1), 145–156 (2016).
Yılmaz, A. et al. The importance of the glucose-to-lymphocyte ratio in patients with hepatocellular carcinoma treated with sorafenib. Future Oncol. 17(33), 4545–4559 (2021).
Hannarici, Z. et al. The value of pretreatment glucose-to-lymphocyte ratio for predicting survival of metastatic gastric cancer. Future Oncol. 19(4), 315–325 (2023).
Jin, L. et al. Glucose-to-lymphocyte ratio (GLR) as a predictor of Preoperative Central Lymph Node Metastasis in Papillary thyroid Cancer patients with type 2 diabetes Mellitus and Construction of the Nomogram. Front. Endocrinol. (Lausanne). 13, 829009 (2022).
Chen, Y., Tang, S. & Wang, Y. Prognostic value of glucose-to-lymphocyte ratio in critically ill patients with Acute Pancreatitis. Int. J. Gen. Med. 14, 5449–5460 (2021).
Hu, T., Liu, X. & Liu, Y. Usefulness of glucose to lymphocyte ratio to Predict in-hospital mortality in patients with AECOPD admitted to the Intensive Care Unit. COPD 19(1), 158–165 (2022).
Zhang, Y. & Zhang, S. Prognostic value of glucose-to-lymphocyte ratio in critically ill patients with acute respiratory distress syndrome: a retrospective cohort study. J. Clin. Lab. Anal. 36(5), e24397 (2022).
Yang, S. et al. Association between high serum blood glucose lymphocyte ratio and all-cause mortality in non-traumatic cerebral hemorrhage: a retrospective analysis of the MIMIC-IV database. Front. Endocrinol. (Lausanne). 14, 1290176 (2023).
Lumley, T. Analysis of Complex Survey samples. J. Stat. Softw. 9(8), 1–19 (2004).
Johnson, C. L., Dohrmann, S. M., Burt, V. L. & Mohadjer, L. K. National health and nutrition examination survey: sample design, 2011–2014. Vital Health Stat. 2(162): 1–33. (2014).
Ahluwalia, N., Dwyer, J., Terry, A., Moshfegh, A. & Johnson, C. Update on NHANES Dietary Data: Focus on Collection, Release, Analytical considerations, and uses to inform Public Policy. Adv. Nutr. 7(1), 121–134 (2016).
Lan, Y. et al. The role of glucose-to-lymphocyte ratio in patients with Cardiovascular Disease: a Population-based cross-sectional study. J. Biol. Regul. Homeost. Agents. 37(6), 3151–3160 (2023).
Liu, C., Liang, D., Xiao, K. & Xie, L. Association between the triglyceride-glucose index and all-cause and CVD mortality in the young population with diabetes. Cardiovasc. Diabetol. 23(1), 171 (2024).
Wang, X., Mukherjee, B. & Park, S. K. Does information on blood heavy metals improve Cardiovascular Mortality Prediction. J. Am. Heart Assoc. 8(21), e013571 (2019).
Yang, M., Miao, S., Hu, W. & Yan, J. Association between the dietary inflammatory index and all-cause and cardiovascular mortality in patients with atherosclerotic cardiovascular disease. Nutr. Metab. Cardiovasc. Dis. 34(4), 1046–1053 (2024).
Stone, N. J. et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice guidelines. J. Am. Coll. Cardiol. 63(25 Pt B), 2889–2934 (2014).
Liu, C., Lai, W., Zhao, M., Zhang, Y. & Hu, Y. Association between the composite dietary antioxidant index and atherosclerotic Cardiovascular Disease in Postmenopausal women: a cross-sectional study of NHANES Data, 2013–2018. Antioxidants 12(9), 1740 (2023).
Ballou, S. et al. Chronic diarrhea and constipation are more common in depressed individuals. Clin. Gastroenterol. Hepatol. 17(13), 2696–2703 (2019).
Zhang, X. et al. The neutrophil-to-lymphocyte ratio is associated with all-cause and cardiovascular mortality among individuals with hypertension. Cardiovasc. Diabetol. 23(1), 117 (2024).
Li, B. et al. Association of serum uric acid with all-cause and Cardiovascular Mortality in Diabetes. Diabetes Care. 46(2), 425–433 (2023).
Low Wang, C. C., Hess, C. N., Hiatt, W. R. & Goldfine, A. B. Clinical update: Cardiovascular Disease in Diabetes Mellitus: atherosclerotic Cardiovascular Disease and Heart failure in type 2 diabetes Mellitus - mechanisms, Management, and clinical considerations. Circulation 133(24), 2459–2502 (2016).
De Rosa, S. et al. Type 2 diabetes Mellitus and Cardiovascular Disease: genetic and epigenetic links. Front. Endocrinol. (Lausanne). 9, 2 (2018).
Petersen, K. F., Dufour, S., Befroy, D., Garcia, R. & Shulman, G. I. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl. J. Med. 350(7), 664–671 (2004).
Ormazabal, V. et al. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 17(1), 122 (2018).
Shoelson, S. E., Lee, J. & Goldfine, A. B. Inflammation and insulin resistance. J. Clin. Invest. 116(7), 1793–1801 (2006).
Xu, H. et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 112(12), 1821–1830 (2003).
Iwaki, T., Urano, T. & Umemura, K. PAI-1, progress in understanding the clinical problem and its aetiology. Br. J. Haematol. 157(3), 291–298 (2012).
Ross, R. Atherosclerosis–an inflammatory disease. N Engl. J. Med. 340(2), 115–126 (1999).
Ridker, P. M. et al. Antiinflammatory therapy with Canakinumab for atherosclerotic disease. N Engl. J. Med. 377(12), 1119–1131 (2017).
Tardif, J. C. et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl. J. Med. 381(26), 2497–2505 (2019).
Nidorf, S. M. et al. Colchicine in patients with chronic coronary disease. N Engl. J. Med. 383(19), 1838–1847 (2020).
Nishinaka, T. et al. Alterations of lymphocyte count and platelet volume precede cerebrovascular lesions in stroke-prone spontaneously hypertensive rats. Biomarkers 25(3), 305–313 (2020).
Anker, S. D. et al. Hormonal changes and catabolic/anabolic imbalance in chronic heart failure and their importance for cardiac cachexia. Circulation 96(2), 526–534 (1997).
Aronson, D. & Rayfield, E. J. How hyperglycemia promotes atherosclerosis: molecular mechanisms. Cardiovasc. Diabetol. 1, 1 (2002).
Wang, Z., Zhang, W., Chen, L., Lu, X. & Tu, Y. Lymphopenia in sepsis: a narrative review. Crit. Care. 28(1), 315 (2024).
Trott, D. W. et al. T lymphocyte depletion ameliorates age-related metabolic impairments in mice. Geroscience 43(3), 1331–1347 (2021).
Acknowledgements
The authors thank the staff and the participants of the NHANES study for their valuable contributions.
Funding
This work was sponsored by a major Research Project of National Clinical Key Specialties of Hunan Provincial Health Commission(Z2023127), Hunan Provincial Natural Science Foundation Regional Joint Fund(2023JJ50372), Research Project of Hunan Provincial Health Commission (D202303077684), The Stroke Technology Research and Development Center of Chenzhou City AND Xiangnan University College-level Research Project (2022JX109).
Author information
Authors and Affiliations
Contributions
JL and WD were involved in the experiment design. HF, YL, and WZ performed the data analysis, and JL wrote the primary manuscript. HL, XY, and ZH reviewed and revised the manuscript. All the authors have approved the manuscript for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The studies involving human participants were reviewed and approved by the NCHS Research Ethics Review Board (ERB). All participants provided written informed consent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lei, J., Duan, W., Yao, X. et al. Association of glucose to lymphocyte ratio with the risk of death in patients with atherosclerotic cardiovascular disease. Sci Rep 15, 3861 (2025). https://doi.org/10.1038/s41598-025-87260-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-87260-9