Abstract
Silent magnetic resonance angiography (S-MRA) is primarily utilized to assess the blood flow in aneurysms and parent vessels of treated intracranial aneurysms. This study aimed to compare the diagnostic value of S-MRA and three-dimensional time of flight (3D-TOF) MRA for unruptured intracranial aneurysms. We included patients diagnosed with unruptured intracranial aneurysms using digital subtraction angiography (DSA) who subsequently underwent S-MRA and 3D-TOF MRA. Two independent neuroimaging and neurointerventional doctors evaluated the DSA images and measured aneurysm dimensions. Using DSA results as the gold standard, we determined the sensitivity and specificity of S-MRA and 3D-TOF MRA, as well as their accuracy in measuring aneurysm size and identifying aneurysms with daughter sacs. We detected a total of 41 intracranial aneurysms (in 37 patients) on both S-MRA and 3D-TOF MRA, with both techniques achieving a sensitivity and specificity of 100%. For aneurysm height, the intraclass correlation coefficient (ICC) was 0.977 (P < 0.001) between S-MRA and DSA, and 0.908 (P < 0.001) between 3D-TOF MRA and DSA. For neck width, the ICC was 0.663 (P < 0.001) between S-MRA and DSA, and 0.563 (P < 0.001) between 3D-TOF MRA and DSA. In terms of daughter aneurysm detection, 3D-TOF MRA Sensitivity 40%; specificity 92%: positive predictive value 100%; S-MRA sensitivity 60%; specificity 89%; positive predictive value 42%. In conclusion, S-MRA and 3D-TOF MRA did not significantly differ in aneurysm detection ability. For the detection of aneurysm with dauthger sacs indicators, the sensitivity is also higher.
Similar content being viewed by others
Introduction
Intracranial aneurysms (IAs) are pathological expansions of the main intracranial arteries and their branches as shown in postmortem studies. Large intracranial arteries are located in the subarachnoid space, their outer membrane is sparse, and the proportion of elastic fibers is low; therefore, they are more likely to form aneurysms than the extracranial arteries1,2. The incidence of IA accounts for 1–2% of the total population, and the prevalence in adults is 1–5%3–5. Stroke prevalence and mortality rates in China are very high6. Subarachnoid hemorrhage (SAH) accounts for approximately 5–10% of all strokes and is characterized by a low age of onset, high disability rate and high mortality. 80% of all spontaneous SAH cases are caused by IA rupture7. For unruptured IAs, reasonable intervention strategies must be developed based on their ___location, size, morphology, and other factors to prevent SAH8.
With progress in cerebrovascular imaging technology, the detection rate of unruptured IAs has significantly increased4.DSA is considered the gold standard vascular imaging technique for IA diagnostics9.However, owing to its invasive nature, this approach is considered unsuitable for IA detection and postoperative follow-up in routine medical practice9. CTA and 3D- TOF MRA are the most commonly used vascular imaging techniques for IAs10.
Compared to MRA, CTA has a shorter scan time and relatively fewer contraindications and is more widely used. However, due to the introduction of contrast agents, CTA is likely to cause allergic reactions and added risk of radiation exposure. 3D-TOF MRA is widely used in the diagnosis and follow-up of IAs due to its lack of radiation exposure and no contrast enhancement. However, this method is susceptible to saturation effects and provides weak displays of adjacent artifacts and slow-flow vessels. S-MRA is a vascular imaging technique that combines an arterial spin labeling sequence and an ultrashort echo time sequence, which is non-invasive, without radiation exposure, and cannot be affected by the saturation effect11,12,13. S-MRA has a high imaging value after IA stent follow-up9,14.
However, the diagnostic value of S-MRA for unruptured IAs has not yet been confirmed. Therefore, the aim of this study was to elucidate the strengths and weaknesses of S-MRA and 3D-TOF MRA. We evaluated various indicators using DSA as the gold standard15.
Results
This study included 37 patients (14 males and 23 females) aged 46–79 years with a median age of 62 years.
Aneurysm detection and measurement of aneurysm body length diameter and width of aneurysm neck
In 37 patients, 41 IAs were identified in DSA, including 28 in the anterior circulation and 13 in the posterior circulation, and the specific distribution is presented in Table 1. The consistent K values for the judgment of S-MRA and 3D-TOF MRA were 0.79 and 0.73 (k < 0.2, poor; 0.21 < k < 0.40, fair; 0.41 < k<0.60, moderate; 0.61 < k<0.80, good ; 0.81 < k<0.90, very good; k > 0.90, excellent); 41 aneurysms were detected in both methods, and the lesion ___location was consistent with the DSA detection results, and the K value of DSA detection by both methods was 1.
In terms of the measurement aspects, the median measurement value and interquartile spacing of aneurysm body length and neck width were 5.27 (3.61, 8.64) mm and 3.96 (2.71, 5.76) mm; S-MRA median and interquartile spacing of aneurysm body diameter was 5.60 (4.35, 8.40) mm; S-MRA within-group correlation coefficient compared to DSA ICC was 0.977 (P < 0.001); the median and interquartile spacing of the neck width was 3.35 (2.70, 5.40) mm, S-MRA within-group correlation coefficient versus DSA ICC was 0.663 (P < 0.001). 3D-TOF MRA, median and interquartile spacing was 6.20 (4.25,11.75) mm, 3D-TOF MRA ICC versus DSA ICC was 0.908 (P < 0.001); the median and interquartile spacing for neck width was 3.40 (2.40,5.25) mm; and 3D-TOF MRA intra-group correlation versus DSA ICC was 0.563 (P < 0.001). According to the Fig. 1, although TOF MRA detects the presence of the aneurysm but the edge is not clear, which is easy to course measurement error.
(a-b) View of DSA showing a large aneurysm in the basilar artery as the hollow arrow shows (⇨). (c, e, g) View of S-MRA showing the whole fumor boc completehy and the aneurysm blood vessel as shown by double arrows (»). (d, f, h) View of 3D-TOF MRA showing the influence of the vortex in the large aneurysm as indicated by the single arrow (→).
Aneurysms with or without daughter sacs
The consistent K values for the judgment of S-MRA and 3D-TOF MRA were 0.82 and 0.64(k < 0.2, poor; 0.21 < k < 0.40, fair; 0.41 < k<0.60, medium; 0.61 < k<0.80, good; 0.81 < k<0.90, very good; k > 0.90, excellent). This indicated good agreement between the two readers.
According to the DSA results, aneurysms, with daughter sacs, were observed in 5 of the 41 aneurysms. 3D-TOF MRA Sensitivity 40%; specificity 92%: positive predictive value 100%; S-MRA sensitivity 60%; specificity 89%; positive predictive value 42%.The specific distribution is presented in Table 2 and Table 3. S-MRA can show both the lesion and the morphology of the aneurysm in Fig. 2.
(a, c) Coronal view of S-MRA showing the position of the left internal carotid artery aneurysm as shown by double arrows (»); the white arrow points to the bulge and aneurysm on the surface of the lesion. (b, d) 3D-TOF MRA showing the contour of the aneurysm, but the surface morphology of the aneurysm is not clearas indicated by the single arrow (→).
Discussion
In recent years, most studies on S-MRA have focused on the evaluation of stent blood flow after IA clipping, few studies have examined the role of S-MRA in the preoperative evaluation of IAs16. In this study, using the diagnosis of IAs as the standard, we first evaluated the ability of both methods to detect aneurysms at various locations within the skull. In this study, we found that both methods could detect lesions more efficiently in 41 aneurysms. This is due to the fact that both S-MRA and 3D-TOF MRA can show the overall intracranial large and small vessels, and the resolution of normal vessels is similar in two-dimensional images, which makes no difference in aneurysm detection.
Then, by comparing the measurements of S-MRA and 3D-TOF MRA in IA height and neck width, we found that in the measurement of IA height, the ICC values of S-MRA and 3D-TOF MRA were greater than 0.9, indicating that both measures were reproducible; S-MRA (larger ICC value) was closer to the gold standard. In the measurement of IA neck width, the ICC values of both methods are between 0.4 and 0.75, indicating that both are reproducible, and the measurement data of S-MRA (larger ICC value) is closer to that of DSA than 3D-TOF MRA. This may be because S-MRA is arterial spin labeling-based visualization of arterial blood flow with the use of RF pulse9,11,17,18. The most important feature is the ultrashort echo time, where ultrashort echo time reduces sensitivity artifacts associated with metal devices to minimize the phase dispersion of labeled blood flow signals in voxel space, thus reducing magnetic susceptibility and displaying short T2 tissues such as musculoskeletal system, lung parenchyma, or carotid plaques4,12,18,19. However, 3D-TOF MRA is susceptible to characteristic artifacts and saturation effect images, which are limited to fine or slow blood vessels; therefore, some aneurysms are prone to signals, resulting in measurement error; however, S-MRA can well avoid this, especially in lesions in the sinus of the internal carotid artery, and the measured values of aneurysm size and neck width are closer to the DSA results18.
In principle, 3D-TOF MRA is a gradient echo sequence with flow compensation, which depends on the RF excitation between the in-plane stationary proton and the proton flowing into the cross-section20. Stationary proton saturation in the imaging section reduces the signal intensity; therefore, decomposition of the vessels parallel to the scanning section is difficult, and the loss of the signal can also be caused by the vortex in the aneurysm13,21. However, S-MRA is not sensitive to the saturation effect and is not affected by the direction of blood vessels, which can offset the influence of eddy currents in the aneurysm and completely show the size and morphology of the aneurysm. Moreover, owing to different hemodynamic parameters, the aneurysm display may differ between the two models22,23.
Finally, by comparing the results of the two methods, we found that in terms of the aneurysm detection ability, S-MRA did not significantly differ from 3D-TOF MRA.For the detection of aneurysm with, dauthger sacs23, the S-MRA positive rate was higher and more sensitive .However, false positives were evident, indicating a lower specificity for the detection of aneurysms. This may be due to the low spatial resolution of S-MRA and the influence of edge artifacts on the judgment of aneurysm morphology24/25,.S-MRA is superior in the accuracy of anatomical parameters and sensitivity of aneurysm with dauthger sacs detection, so S-MRA is more desirable for aneurysms requiring follow-up, especially aneurysms with morphological changes.
This study has some limitations. First, since IA is a common cause of SAH, the sample size of 41 aneurysms would be considered slightly insufficient. Therefore, more cases should be included in subsequent studies. Second, some studies have shown that the hemodynamic factors of IAs are significantly correlated with the aneurysm ___location, but we did not analyze the display of aneurysm on S-MRA in different distribution locations in detail26. Third, the advantage of S-MRA is more obvious when examining children because of the low noise during scanning21,27; however, this study did not include children, thus this advantage remains unclear.
In summary, Both S-MRA and 3D-TOF MRA can be used as non-invasive examination methods to screen for and follow-up IA in clinical practice. However, S-MRA is more actual and accurate for the measurement of IA anatomical indicators.For the detection of aneurysm with dauthger sacs indicators, the sensitivity is also higher. Therefore, SMRA also has a high imaging value.
Materials and methods
Ethical permission and informed consent
This study was reviewed and approved by the Institution review board of PLA general hospital (Ethical No. S2020-097-02) and was performed guided by the latest version of the Declaration of Helsinki. The principal investigator obtained informed written and verbal consent from all the participants, after clearly explaining the study procedures.
Clinical data collection
Clinical data were prospectively collected from patients with intracranial unruptured IAs diagnosed by DSA in the Neurointerventional Unit of PLA General Hospital from November 2017 to September 2020.
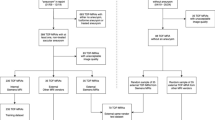
Enrollment criterion was unruptured IAs confirmed using DSA. Exclusion criteria were as follows: (1) IAs that underwent interventional embolization or surgical clipping; (2) unable to obtain DSA images, unclear DSA and MR image quality, or no 3D reconstruction of DSA images; (3) contraindications for MRI and MRI; and (4) patients who explicitly refused to join the study or did not sign informed consent (Fig. 3).
Image examination
A 3T MRI system (GE Healthcare, Milwaukee, Wisconsin, USA) and a 24-channel head coil were employed for 3D-TOF MRA and S-MRA scans, which were obtained in the same scan. The parameters are shown in Table 4.
The DSA parameters were as follows: imaging modality, IA-DSA; exposure time: 6s, frame rate, 4 fps; delay mode, injection delay; high-pressure syringe parameters, injection rate: 6 ms/l; injection volume, 9 ml; and pressure limit, 300 kpl.
Imaging analysis
DSA imaging evaluation by an experienced interventional physician was used as a reference standard, and a blinded analysis was performed by two senior imaging diagnostic physicians who have accumulated more than 5 years of experience in MR diagnosis. The 3D-TOF MRA and S-MRA images were independently analyzed at a PASS workstation, and the source images were acquired using 3D reconstructed image volume reproduction technology by source image (VR, Volume rendering technique and MIP). Both imaging methods were recorded on video readings, with a two-week interval. The consistency between the two diagnostic physicians was tested to determine whether there was an aneurysm, aneurysm site, or aneurysm shape (whether the aneurysm with dauthger sacs was included). The length of the body (the maximum diameter of the body) and width of the neck (the inner diameter of the junction between the parent vessel and the aneurysm) were independently measured, and the average width of the length, diameter, and neck was used.
Statistical analysis
The same MRA examination method of different observers to the consistency of aneurysm judgment, using Kappa test, the observer of IA results are divided into three categories, respectively are (clear aneurysm diagnosis), (clear no aneurysm diagnosis) and suspected aneurysm diagnosis (abnormal vascular morphology, but unable to determine whether the diagnosis of aneurysm, need further judgment by DSA) three aspects. The Kappa value was used to detect the consistency of aneurysm detection and daughter aneurysm detection between the two observers (k < 0.2, poor; 0.21 < k < 0.40, fair; 0.41 < k<0.60, moderate; 0.61 < k<0.80, good ; 0.81 < k<0.90, very good; k > 0.90, excellent).
The Statistical Package for the Social Sciences(SPSS)26.0 data processing software was used for statistical data processing, and the intra-group correlation coefficient (ICC) was used to compare the measurement data. In general, the ICC values ranged between 0 and 1. For diagnostic testing, we considered the test to be less reproducible if the ICC value was less than 0.4. The diagnostic test was considered more reproducible when the ICC value was greater than 0.75.
Data availability
The datasets generated and/or analysed during the current study are not publicly available because the data are part of an ongoing analysis but are available from the corresponding author on reasonable request.
Abbreviations
- S-MRA:
-
Silent Magnetic Resonance Angiography
- DSA:
-
Digital Subtraction Angiography
- 3D-TOF MRA:
-
3 Dimensional Time of Flight Angiography
- SAH:
-
Subarachnoid hemorrhage
- IA:
-
intracranial aneurysms
- ICC:
-
Intraclass Correlation Coefficient
References
Lv, B. et al. Epidemiological trends of subarachnoid hemorrhage at global, regional, and national level: a trend analysis study from 1990 to 2021. Military Med. Res. 11, 46. https://doi.org/10.1186/s40779-024-00551-6 (2024).
Wardlaw, J. M. & White, P. M. The detection and management of unruptured intracranial aneurysms. Brain: J. Neurol. 123 (Pt 2), 205–221. https://doi.org/10.1093/brain/123.2.205 (2000).
Claassen, J. & Park, S. Spontaneous subarachnoid haemorrhage. Lancet (London England). 400, 846–862. https://doi.org/10.1016/s0140-6736(22)00938-2 (2022).
Ajiboye, N., Chalouhi, N., Starke, R. M., Zanaty, M. & Bell, R. Unruptured Cerebral Aneurysms: Evaluation and Management. TheScientificWorldJournal 954954, (2015). https://doi.org/10.1155/2015/954954 (2015).
Jakubowski, J. & Kendall, B. Coincidental aneurysms with tumours of pituitary origin. J. Neurol. Neurosurg. Psychiatry. 41, 972–979. https://doi.org/10.1136/jnnp.41.11.972 (1978).
Lv, B. et al. Mortality from cerebrovascular diseases in China: exploration of recent and future trends. Chin. Med. J. 137, 588–595. https://doi.org/10.1097/cm9.0000000000002760 (2024).
Steiner, T. et al. European Stroke Organization guidelines for the management of intracranial aneurysms and subarachnoid haemorrhage. Cerebrovasc. Dis. 35, 93–112. https://doi.org/10.1159/000346087 (2013).
Sosnov, A. O. et al. [Surgical treatment of «Mirror» aneurysms of the internal carotid artery]. Zh. Vopr. Neirokhir. Im. N. N. Burdenko. 80, 44–50. https://doi.org/10.17116/neiro201680544-50 (2016).
Tanoue, S. et al. Follow-up non-contrast MRA after treatment of intracranial aneurysms using microcoils with prominent metallic artifact: a comparative study of TOF-MRA and Silent MRA. Japanese J. Radiol. 38, 853–859. https://doi.org/10.1007/s11604-020-00981-x (2020).
Piotin, M. et al. CT angiography, MR Angiography and rotational digital subtraction angiography for volumetric assessment of intracranial aneurysms. An experimental study. Neuroradiology 45, 404–409. https://doi.org/10.1007/s00234-002-0922-8 (2003).
Irie, R. et al. Assessing blood Flow in an intracranial stent: a feasibility study of MR Angiography using a Silent scan after stent-assisted Coil embolization for anterior circulation aneurysms. AJNR Am. J. Neuroradiol. 36, 967–970. https://doi.org/10.3174/ajnr.A4199 (2015).
Tomura, N., Kokubun, M., Horiuchi, K. & Watanabe, Z. Comparison of TOF-MRA and silent scan-MRA in depicting cerebral arteries in patients with Moyamoya disease. Acta Radiol. (Stockholm Sweden: 1987). 60, 1321–1328. https://doi.org/10.1177/0284185118824782 (2019).
Ryu, K. H. et al. Usefulness of noncontrast-enhanced silent magnetic resonance angiography (MRA) for treated intracranial aneurysm follow-up in comparison with Time-of-flight MRA. Neurosurgery 87, 220–228. https://doi.org/10.1093/neuros/nyz421 (2020).
van Osch, M. J. et al. Advances in arterial spin labelling MRI methods for measuring perfusion and collateral flow. J. Cereb. Blood flow. Metabolism: Official J. Int. Soc. Cereb. Blood Flow. Metabolism. 38, 1461–1480. https://doi.org/10.1177/0271678x17713434 (2018).
Liu, H. J. et al. Intracranial Mirror Aneurysm: Epidemiology, rupture risk, New Imaging, controversies, and treatment strategies. World Neurosurg. 127, 165–175. https://doi.org/10.1016/j.wneu.2019.03.275 (2019).
Oishi, H. et al. Usefulness of Silent MR Angiography for Intracranial Aneurysms Treated with a Flow-Diverter device. AJNR Am. J. Neuroradiol. 40, 808–814. https://doi.org/10.3174/ajnr.A6047 (2019).
Takano, N. et al. Non-contrast-enhanced Silent scan MR Angiography of Intracranial Anterior circulation aneurysms treated with a Low-Profile visualized Intraluminal Support device. AJNR Am. J. Neuroradiol. 38, 1610–1616. https://doi.org/10.3174/ajnr.A5223 (2017).
Kim, Y. N. et al. Usefulness of Silent MRA for evaluation of aneurysm after stent-assisted Coil Embolization. Korean J. Radiol. 23, 246–255. https://doi.org/10.3348/kjr.2021.0332 (2022).
Arai, N. et al. Silent MRA: arterial spin labeling magnetic resonant angiography with ultra-short time echo assessing cerebral arteriovenous malformation. Neuroradiology 62, 455–461. https://doi.org/10.1007/s00234-019-02345-3 (2020).
Takano, N. et al. Usefulness of Non-contrast-enhanced MR Angiography using a Silent scan for Follow-Up after Y-Configuration stent-assisted Coil Embolization for basilar tip aneurysms. AJNR Am. J. Neuroradiol. 38, 577–581. https://doi.org/10.3174/ajnr.A5033 (2017).
Holdsworth, S. J., Macpherson, S. J., Yeom, K. W., Wintermark, M. & Zaharchuk, G. Clinical evaluation of Silent T1-Weighted MRI and Silent MR Angiography of the brain. AJR Am. J. Roentgenol. 210, 404–411. https://doi.org/10.2214/ajr.17.18247 (2018).
Ren, Y. et al. Reproducibility of image-based computational models of intracranial aneurysm: a comparison between 3D rotational angiography, CT angiography and MR angiography. Biomed. Eng. Online. 15 https://doi.org/10.1186/s12938-016-0163-4 (2016).
Bjorkman, J. F. & Antti, J. E. L. JuhanaTahtinen, OlliBackes, DaanHuttunen, TerhiHarju, JaakkoHuttunen, JukkaKurki, Mitja I.Fraunberg, Mikael von Und ZuKoivisto, TimoManninen, HannuJaaskelainen, E. Irregular Shape Identifies Ruptured Intracranial Aneurysm in Subarachnoid Hemorrhage Patients With Multiple Aneurysms. Stroke: A Journal of Cerebral Circulation 48.7 (2017).
Hwang, Z. A. et al. Intensity of arterial structure acquired by Silent MRA estimates cerebral blood flow. Insights into Imaging. 12 https://doi.org/10.1186/s13244-021-01132-0 (2021).
Lu, Y. et al. Non-contrast enhanced silent MR Angiography to evaluate hemodynamics and morphology of unruptured intracranial aneurysms: a comparative computational fluid dynamics study. J. Neurointerventional Surg. 15, 753–759. https://doi.org/10.1136/jnis-2022-018901 (2023).
Can, A. & Du, R. Association of Hemodynamic Factors with Intracranial Aneurysm Formation and rupture: systematic review and Meta-analysis. Neurosurgery 78, 510–520. https://doi.org/10.1227/neu.0000000000001083 (2016).
Zhang, J. et al. Evaluation of chronic carotid artery occlusion by non-contrast 3D-MERGE MR vessel wall imaging: comparison with 3D-TOF-MRA, contrast-enhanced MRA, and DSA. Eur. Radiol. 30, 5805–5814. https://doi.org/10.1007/s00330-020-06989-1 (2020).
Author information
Authors and Affiliations
Contributions
Bin Lv, Tingyang Zhang and Ning Wang: Conceptualization, Methodology, Data curation, Writing - original draft preparation. Liu Lu, Mingyu Li, Mingguang Sun and Xiao Zang: Visualization, Investigation, Resources, Formal analysis. Xinfeng Liu, Rongju Zhang, Xiangyu Cao and Zhihua Du: Supervision, Project administration. Jun Wang, Jinhao Lyu and Qi Duan: Resources, Data curation, Investigation. Fangfang Guo and Xin Lou: Supervision, Project administration, Resources. Chenglin Tian: Project administration , Supervision, Writing - review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lv, B., Zhang, T., Wang, N. et al. Silent magnetic resonance angiography diagnostic value of intracranial unruptured aneurysms. Sci Rep 15, 4549 (2025). https://doi.org/10.1038/s41598-025-87646-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-87646-9