Abstract
The main objective of the present study was to investigate the toxicity of orally administered organophosphorus dimethoate pesticide in Rattus norvegicus on the basis of biochemical alterations in the blood. There was a total of eight groups: two control groups and six treatment groups. Albino rats of the exposed groups were fed simple laboratory chow along with a low dose: five milliliters were given to the T1 group; the medium dose, ten milliliters, was given to the T2 group; and the high dose, twenty milliliters, was given to the T3 group of dimethoate pesticide for thirty, sixty, or ninety days under controlled laboratory conditions. At the end of the experiment, any weight gain, changes in the blood parameters, or biochemical changes were determined. The results revealed increases in the total glucose, aspartate aminotransferase, and alanine aminotransferase levels in the exposed groups of both genders of albino rats. There were decreases in the levels of urea, uric acid and total bilirubin in the exposed groups of both genders of albino rats. These effects did not vary between the sexes. This study provides a foundation for further research on the long-term effects of dimethoate and similar pesticides, promoting the development of safer alternatives.
Similar content being viewed by others
Introduction
Dimethoate is one of the most important organophosphorus pesticides and is used extensively in agriculture as a systemic and contact pesticide to manage a wide range of pests and insects1. Dimethoate, also known as O, O-dimethyl S-(N-methylcarbamoylmethyl) phosphonodithioate, is a common organophosphorus pesticide that was first licensed in 1962 by American Cyanamid, a US-based company2,3. It is widely used in nonagricultural applications, including pest management, as well as for crops such as fruits, vegetables, and cereals3.
The aim of this research was to investigate the dimethoate toxicity when it is taken orally, with a particular focus on changes in blood biochemistry in Rattus norvegicus. Because of their physiological and metabolic resemblance to humans, albino rats are approved model organisms for toxicity research4. Through the analysis of biochemical markers such as glucose, alanine aminotransferase, aspartate aminotransferase, urea, uric acid, and bilirubin levels, we aimed to obtain thorough knowledge of the sublethal effects of dimethoate at different exposure times. The liver is the main organ involved in the metabolism of xenobiotics, making it a primary target organ for chemicals and medications5.
Mice exposed to tolerable doses of organophosphates over an extended period of time may have minor but considerable alterations in their biochemistry and behavior6. In a similar vein, one study reported changes in metabolic profiles and liver enzyme activity in mice exposed to low doses of dimethoate, highlighting the possibility of long-term health effects even at dosages below the threshold for acute toxicity7. Histopathological modifications in tissues and organs, as well as changes in biochemical parameters, are usually utilized to assess the toxicity of organophosphorus insecticides in humans and animals8. The kidney is one of the organs that organophosphorus chemicals target in experimental animals. Dimethoate induces hyperglycemia and causes various toxic effects on the rat pancreas following acute, sub chronic and chronic exposure10.
Objectives: The main objective of this study was to investigate how dimethoate affects the biochemical parameters of albino rats of both genders using serum analysis. Because serum analysis offers important insights into physiological processes, disease states, and the impact of environmental variables on health, it is widely used in clinical practice, research, and public health11. Acetylcholinesterase, an enzyme essential for the control of neurotransmitters, is inhibited by dimethoate as its primary mode of action. Current studies highlight the biochemical alterations associated with long-term dimethoate intake. Understanding the biochemical response to dimethoate is crucial for assessing its potential health risks. By focusing on biochemical changes, this research aims to clarify the biochemical impact of dimethoate and contributes to the development of safer agricultural alternatives.
Results
Blood parameters
The results of the biochemical analysis of albino rats of both genders after 30, 60, and 90 days of exposure to dimethoate pesticide are shown in the tables.
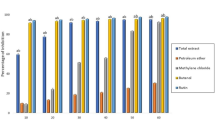
Compared with the control group, Table 1. Effects of different dosages of dimethoate pesticide (5 ml, 10 ml, and 20 ml) on a number of biochemical markers in male and female albino rats over the course of a 30-day period. The statistical significance of each parameter is shown by the p-value, which is represented as the mean ± SEM (standard error of the mean). The effects of dimethoate on biochemical parameters are shown in Fig. 1. Compared with those of control albino rats, urea P ≤ 0.03 (♀) P ≤ 0.00 (♂), uric acid P ≤ 0.00 (♀) P ≤ 0.00 (♂), and total bilirubin P ≤ 0.00 (♀) P ≤ 0.00 (♂) were significantly lower in male and female albino rats treated with dimethoate. In general, there was also a significant increase in total glucose (P ≤ 0.05 (♀) P ≤ 0.01 (♂)), aspartate aminotransferase (AST) P ≤ 0.00 (♀) P ≤ 0.00 (♂), and alanine aminotransferase (ALT) P ≤ 0.001 (♀) P ≤ 0.00 (♂). The level of cholesterol significantly increased in females (P ≤ 0.00) but decreased in males (P ≤ 0.05) (Table. 1).
Compared with the control group, the table. Figure 2 shows the effects of different dosages of dimethoate pesticide (5 ml, 10 ml, and 20 ml) on a number of biochemical markers in male and female albino rats over the course of a 60-day period. The statistical significance of each parameter is shown by the p-value, which is represented as the mean ± SEM (standard error of the mean). The effects of dimethoate on biochemical parameters are shown in Fig. 2. Compared with those of control albino rats, urea P ≤ 0.008 (♀) P ≤ 0.00 (♂), uric acid P ≤ 0.01 (♀) P ≤ 0.06 (♂), and cholesterol level P ≤ 0.02 (♀) P ≤ 0.002 (♂) were significantly lower in male and female albino rats treated with dimethoate. In general, there was also a significant increase in the total glucose P ≤ 0.01 (♀) P ≤ 0.02 (♂), aspartate aminotransferase (AST) P ≤ 0.003 (♀) P ≤ 0.00 (♂), alanine aminotransferase (ALT) P ≤ 0.00 (♀) P ≤ 0.002 (♂), total bilirubin P ≤ 0.01 (♂), and a nonsignificant decrease in the total bilirubin level P ≤ 0.14 (♀).
Compared with the control group, Table 3. Effects of different dosages of dimethoate pesticide (5 ml, 10 ml, and 20 ml) on a number of biochemical markers in male and female albino rats over the course of a 60-day period. The statistical significance of each parameter is shown by the p-value, which is represented as the mean ± SEM (standard error of the mean). The effects of dimethoate on biochemical parameters are shown in Fig. 3. Compared with those of control albino rats, the urea P ≤ 0.02 (♀) P ≤ 0.00 (♂), uric acid P ≤ 0.01 (♀) P ≤ 0.001 (♂), and cholesterol level P ≤ 0.005 (♀) P ≤ 0.00 (♂) of male and female albino rats treated with dimethoate were significantly lower. In general, there was also a significant increase in total glucose, P ≤ 0.04 (♀), P ≤ 0.00 (♂), and alanine aminotransferase (ALT), P ≤ 0.001 (♀), P ≤ 0.00 (♂). The level of aspartate aminotransferase (AST) significantly increased in females with P ≤ 0.00 (♀) and non-significantly increased in males with P ≤ 0.19 (♂). The level of total bilirubin did not significantly decrease in male animals (P ≤ 0.00) (♂), whereas total bilirubin significantly increased in female animals (P ≤ 0.00) (♀).
Discussion
The present study evaluated the biochemical effects of dimethoate pesticide on albino rats over 30, 60, and 90 days. Significant changes in glucose, urea, uric acid, bilirubin, cholesterol, ALT, and AST levels were observed in both male and female rats.
Glucose
The glucose levels in the treatment groups were significantly greater than those in the control groups, according to the findings. Increased blood sugar levels suggest potential issues with carbohydrate metabolism, which can result in diabetes and hyperglycemia. Rats exposed to organophosphate pesticides presented increased blood glucose levels, according to previous studies12. According to research by13, exposure to organophosphate pesticides can disrupt the body’s natural metabolism of glucose, which can increase blood glucose levels. Fish exposed to organophosphates presented elevated glucose levels, as shown by12, suggesting a potential mechanism of metabolic disruption shared by many species14. also reported similar results, indicating that dimethoate exposure increased blood glucose levels in mice and may be associated with insulin resistance. Fifteen studies reported that rats exposed to pesticides, such as dimethoate, over an extended period of time developed significant hyperglycemia.
There are several possible explanations for the increase in blood glucose levels observed in albino rats treated with the pesticide dimethoate.
Insulin signaling disruption
Insulin sensitivity may decrease due to pesticides such as dimethoate and other organophosphate compounds that interfere with insulin signaling pathways. Blood glucose levels can increase as a result of poor cell absorption of glucose caused by insulin resistance12.
Oxidative stress
Dimethoate and other pesticides can cause oxidative stress, which harms the β-cells in the pancreas that produce insulin. This damage may result in reduced insulin secretion and hyperglycemia.
Stimulation of the adrenal gland
The release of stress hormones such as cortisol and adrenaline may be triggered by organophosphate insecticides. These hormones increase blood glucose levels by promoting gluconeogenesis and glycogenolysis11.
Glucose metabolic enzyme interference
Dimethoate exposure can disrupt the activity of enzymes involved in glucose metabolism, such as hexokinase and glucose-6-phosphatase. This disruption can impair glucose utilization and storage, resulting in elevated blood glucose13.
Inflammatory response
Chronic pesticide exposure can cause an inflammatory response, which has been connected to elevated blood glucose and insulin resistance. Insulin signaling and glucose metabolism may be impaired by inflammatory cytokines15.
Cholesterol
Although they varied, cholesterol levels generally decreased, especially after prolonged treatment. This result suggests alterations in lipid metabolism, which is supported by16findings that rats exposed to pesticides had different cholesterol levels17. reported that rat exposed to organophosphate pesticides presented significantly lower cholesterol levels, which they associated with a decrease in lipid synthesis and liver function. According to18, long-term exposure to pesticides in agriculture lowered blood cholesterol levels, suggesting possible disturbances in lipid homeostasis. According to previous studies19, exposure to certain pesticides significantly reduces cholesterol levels, indicating negative effects on the metabolic pathways involved in lipid metabolism.
Twenty-three mice exposed to pesticides presented altered cholesterol metabolism, which led to decreased cholesterol levels and related metabolic disorders. According to21, pesticides have a major influence on lipid profiles, lowering cholesterol and affecting the general health of the metabolic system.
Dysfunction of the liver
According to18, exposure to pesticides can damage the ability of the liver to manufacture and control the production of cholesterol.
Lipid metabolism alteration: Accordingto16, pesticides can interfere with metabolic pathways that are involved in the production and breakdown of lipids, resulting in lower cholesterol levels.
Cholesterol-synthesizing enzyme inhibition
According to19, some pesticides may block important enzymes in the cholesterol biosynthesis pathway, which decreases the amount of cholesterol synthesized.
Increased peroxidation of lipids
According to20, pesticides can cause oxidative stress, which can result in increased lipid peroxidation. This can change lipid profiles and lower cholesterol levels.
Impaired absorption of lipids
Pesticide exposure can cause damage to intestinal cells, which can interfere with the body’s ability to absorb dietary lipids and decrease cholesterol levels18.
Urea and uric acid
In the treatment groups, there was a significant reduction in both the urea and uric acid levels. Since the kidneys normally eliminate these compounds, this decline might be a sign of renal impairment. Reductions in urea and uric acid levels after pesticide exposure have also been reported in other investigations22, with nephrotoxicity being associated with these alterations. According to previous studies23, rats exposed to organophosphate insecticides presented noticeably decreased levels of urea and uric acid, which may indicate renal damage. Similar declines in renal function indicators were noted by24in animals exposed to several agricultural pesticides, underscoring the nephrotoxic consequences. Significant decreases in urea levels were observed15 in rats exposed to several pesticides, suggesting a possible risk for kidney injury.
25highlighted the possible nephrotoxic effects of pesticide exposure by showing that long-term exposure to dimethoate resulted in lower levels of urea and uric acid.
Impaired kidney
Since the kidneys are generally responsible for filtering and excreting these compounds, decreased levels of urea and uric acid frequently signify renal failure. Reduced excretion may result from damage to renal tissues8.
Altered metabolism
Pesticide exposure can disrupt metabolic pathways, affecting protein metabolism and the production of nitrogenous waste, leading to lower urea levels26.
Damage to cells
The nephrotoxic effects of pesticides can harm kidney cells, making it more difficult for organs to process and eliminate waste27.
Enzyme activity inhibition
Pesticides have the potential to block urea cycle-related enzymes, which decreases the amount of ammonia converted to urea and, in turn, lowers urea levels28.
Increased reabsorption
Kidney damage may lead to altered reabsorption processes, causing reduced excretion of urea and uric acid back into circulation29.
Bilirubin
Since bilirubin is a byproduct of hemoglobin breakdown and is eliminated by the liver, a significant decrease in bilirubin levels increases the possibility of liver dysfunction. A lower bilirubin level may be a sign of liver damage, which is consistent with research by21, who reported that pesticide exposure had a similar effect on the liver. Rats exposed to organophosphate pesticides had lower bilirubin levels, which may indicate hepatotoxicity and compromised liver function, according to32research. In mice exposed to agricultural pesticides31, showed substantial decreases in total bilirubin levels, indicating liver damage and impaired bilirubin metabolism. Prolonged exposure to pesticides has been shown to lower bilirubin levels, which is associated with changes in hepatic enzyme activity and liver dysfunction.
Similar results were published by25, who reported that liver function was significantly affected by exposure to pesticides, such as dimethoate, which led to decreased bilirubin production.
Dysfunction of the liver
Since the liver is in charge of metabolizing and excreting bilirubin, decreased bilirubin levels are frequently indicative of liver damage. This function may be diminished by damage to liver cells23.
Modification in the breakdown of hemoglobin
Exposure to pesticides may interfere with the normal metabolism of hemoglobin, which will reduce the amount of bilirubin that is produced as a result of hemoglobin breakdown30.
Liver enzyme inhibition: Accordingto31, pesticides can reduce the activity of liver enzymes that are involved in bilirubin metabolism and conjugation. This lowers bilirubin levels.
Compromised liver function
Liver injury caused by toxins can affect the ability of the liver to synthesize and secrete bile, which can lead to reduced bilirubin levels21.
Increase in hepatic uptake
Liver dysfunction may alter the uptake and processing of bilirubin, leading to decreased levels in circulation32.
ALT and AST
These findings revealed that the aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels in the treatment groups were significantly greater than those in the control groups. The ALT and AST levels significantly increased after the mice were exposed to dimethoate and ethanol, indicating damage to the liver. Research has also revealed alterations in the liver and kidney’s oxidative state, suggesting that oxidative stress is a possible contributing factor. According to previous studies33, rats given diets containing fumigated wheat grain byproducts presented increased liver enzyme levels. This study emphasized the function of oxidative stress in liver damage as well as the hepatotoxic effects of phosphorus toxins34. investigated the hepatotoxic effects of carbofuran on albino rats.
In albino rats exposed to dimethoate, this study revealed substantial increases in ALT and AST levels, suggesting hepatotoxicity. Histological findings of liver injury confirmed the biochemical alterations, indicating a clear link between exposure to carbofuran and elevated liver enzymes. Exposure to chlorpyrifos increased the ALT and AST levels, which are indicators of liver injury. This study also investigated the preventive function of isoflavones, which decrease liver enzyme levels and reduce oxidative stress, emphasizing the function of antioxidants in preventing pesticide-induced liver damage.
When albino rats were treated with carbendazim35, researchers reported increased levels of ALT and AST, which suggests damage to the liver. Investigations into the hepatoprotective properties of aloe vera revealed a significant decrease in liver enzyme levels, indicating a defense mechanism against the hepatotoxicity caused by pesticides. According to a previous study36, rabbits treated with cypermethrin presented substantial increases in ALT and AST levels, indicating liver injury. The hepatotoxic effects of CYP were highlighted by histological results that supported the observed biochemical changes and demonstrated inflammation and damage to liver tissue.
Toxic chemicals, including pesticides, can cause damage to liver cells, which in turn causes the blood to produce AST and ALT. The hepatotoxic effects of dimethoate were highlighted by9 who reported significant elevations in ALT and AST levels in mice exposed to the pesticide. The increase in ALT and AST levels can be attributed to several factors, which are as follows.
Hepatotoxicity
Exposure to toxic substances, such as pesticides, can lead to liver cell damage, resulting in the release of ALT and AST into the bloodstream9. reported significant increases in ALT and AST levels in mice exposed to dimethoate, highlighting the hepatotoxic effects of this pesticide.
Oxidative stress
Reactive oxygen species produced by pesticides can cause oxidative stress, which damages liver cells. ALT and AST levels increase as a result of this injury. Rats fed diets containing fumigated wheat grain byproducts presented biochemical alterations37, including increased liver enzymes associated with oxidative stress. Pesticide exposure over an extended period of time can cause the liver to become inflamed, which damages liver cells and increases enzyme levels.
Inflammation
Chronic exposure to pesticides can trigger inflammatory responses in the liver, causing liver cell damage and elevated enzyme levels. The inflammatory cytokines produced during this response can further exacerbate liver injury.
Conclusion
In conclusion, this study demonstrated that dimethoate pesticide in albino rats led to significant biochemical alterations, including increased alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels and decreased cholesterol, urea, uric acid, and total bilirubin levels. These changes were the same for both male and female rats. These findings suggest that dimethoate has significant health risks and highlight the need for safer pesticide alternatives.
Materials and methods
Ethical consideration
Rats’ overall health was maintained. Seventy-two (72) rats between the ages of six and seven weeks and weights between 160 and 180 g were used in this investigation. The floor of each metal cage containing the animals was splintered soft wood. The environment was kept at 23–25 °C with an air humidity of 50 ± 10% and a 12-hour light/dark cycle. In order to protect the research animals’ health and prevent infections, the bedding was routinely changed out with fresh wood splinters. Animals were housed in the Primate facility of the Faculty of Chemical and Biological Sciences, The Islamia University of Bahawalpur, Pakistan, in compliance with the National Institute of Health, United States guidelines for the care and use of laboratory animals. They were provided with standard rodent food and water ad libitum. All research work in the submitted paper was conducted in an ethical and responsible manner and was in full compliance with all relevant codes of experimentation and legislation. This study was approved by the ethical committee of The Islamia University of Bahawalpur. The meeting was held on 25-05-2023 and approved on 13/11/2023. approval No 1112/AS&R adhered with strict caution to diminish animal distress. This study was reported in accordance with ARRIVE guidelines.
Study Design
The present experiment was carried out at the Zoology Department of The Islamia University of Bahawalpur. I bought 72 healthy albino rats from the Cholistan Institute of Desert Studies, The Islamia University of Bahawalpur. each weighed between 160 and 180 g (See supplementary Fig. S1 online). There were nine rats in each group. Eight groups—four female and four male albino rats—were included. Two control groups and two further subdivided treatment groups were present. Six groups were given various dosages of dimethoate pesticide orally, while the remaining two groups served as the control group (Supplementary Table S1 online).
Toxicity test and preparation of dosages for exposure
The pesticide was manufactured in various concentrations after it was procured in pure form from a reputable pesticide market (Bayer pharmaceutical company). In accordance with the average body weight, three concentrations were created. Before use, all the rats were checked for health issues and given time to adjust to the laboratory setting, such as a 12-hour light/dark cycle. The study comprised two control groups and six treatment groups (T1F, T1M, T2F, T2M, T3F, and T3M) that were exposed to varying doses of dimethoate pesticides. T1 received a low dose of 5 ml, T2 received a medium dose of 10 ml, and T3 received a high dose of 20 ml (See supplementary Fig. S2 online).
Designing the experiment and acclimatization of albino rats
In the laboratory study, the animals were housed in individual cages with a minimum height of 5 inches (12.7 cm) and a floor area per mouse ranging from 6 to 15 inches (38.7 to 96.7 cm) squared, as determined by body weight. They were provided with normal laboratory chow or water ad libitum. For a period of ninety days, the animals in the treatment groups were fed dimethoate once a week and laboratory feed every day, whereas the animals in the control group were fed regular laboratory chow every day (See supplementary Fig. S3 online). Throughout the trial, all of the animals were weighed once a week.
Euthanasia- anesthesia
In accordance with the protocol of (Refer to IACUC Policy No. 9906), euthanasia with CO2 guidelines was used for euthanasia of albino rats. The IAS euthanasia facilities conform to current AVMA guidelines to displace 20% of the mouse container air per minute. To use CO2 in the laboratory, a flow meter was attached to the system to confirm that the air displacement rate was 20% of the volume of the container per minute11 (See supplementary file online).
Sample collection
During the 90-day study, a total of 24 albino rat blood samples were drawn every 30 days via 0.5 ml insulin syringes with the ventral side of the heart needles being 5/16 inch (8 mm) in accordance with the recommended protocols for rat euthanasia, and blood was collected. Blood was taken after three rats from each of the eight groups were dissected. Every albino rat had a maximum blood sample obtained from it (See supplementary Fig. S4 online) Biochemical parameters were examined in the blood. Blood samples were centrifuged for 10–15 min at 3500 rpm to separate the serum, after which they were stored at −20 °C until they were analyzed via a biochemical test (See supplementary file online).
Performance of biochemical tests
The following biochemical characteristics were examined: glucose, urea, uric acid, bilirubin, cholesterol, ALT, and AST levels. A Biosystem BTS-350, a semiautomated biochemical analyzer from Spain, was used to test the serum from each sample (See supplementary Fig. S5 online). Centronic GmbH (Waterberg/Germany) kits were used for biochemical analysis (See supplementary file online).
Statistical analysis
The results of the biochemical analysis are presented as the means and standard deviations. Comparisons between the control and treated groups were performed via one-way analysis of variance. Statistical values of P ≤ 0.05 were considered statistically significant. In IBM SPSS statistics version 20, the obtained findings were subjected to one-way ANOVA.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Sharma, Y. et al. Effects of acute dimethoate administration on antioxidant status of liver and brain of experimental rats. Toxicology 206, 49–57. https://doi.org/10.1016/j.tox.2004.06.062 (2005).
Dissanayake, K. et al. Impaired neuromuscular function by conjoint actions of organophosphorus insecticide metabolites omethoate and cyclohexanol with implications for treatment of respiratory failure. Clin. Toxicol. 59 (12), 1239–1258. https://doi.org/10.1080/15563650.2021.1916519 (2021).
Van, S., Yue, M. & Tjeerdema, R. Environmental fate and toxicology of dimethoate. Reviews of Environmental Contamination and Toxicology. 237: 53–70. (2016). https://doi.org/10.1007/978-3-319-23573-8_3
Palanivelu, M., Anitha, J. & Geetha, S. Biochemical alterations in blood following oral administration of dimethoate in albino rats (Rattus norvegicus). J. Toxicol. Environ. Health Sci. 15 (2), 65–72. https://doi.org/10.5897/JTEHS2023.0507 (2023).
Travlos, G. S. et al. Frequency and relationship of clinical chemistry and liver and kidney histopathology findings in 13-week toxicity studies in rats. Toxicology 107, 17–29. https://doi.org/10.1016/0300-483X(95)03197-N (1996).
Li, Q. et al. Chronic exposure to low doses of organophosphates leads to subtle yet significant biochemical and behavioral changes in rodents. Environmental Toxicology and Pharmacology. 88, 103742. (2021). https://doi.org/10.1016/j.etap.2021.103742
Rajeshwari, Y., Rao, J. & Reddy, P. Alterations in liver enzyme activities and metabolic profiles in animals exposed to low levels of dimethoate. Toxicol. Rep. 7, 1056–1064. https://doi.org/10.1016/j.toxrep.2020.08.009 (2020).
Ahmed, N., Ghannam, H. & Tayel, S. Biochemical and histopathological responses of Oreochromis niloticus and Cyprinus carpio to sub-lethal exposure of ictacrune pesticide. Egypt. J. Histol. 43 (3), 918–930. https://doi.org/10.21608/ejh.2020.21602.1224 (2020).
Sivapiriya, V., Jayanthisakthisekaran, J. & Venkatraman, S. Effects of dimethoate (O, O-dimethyl S-methyl carbamoylmethylphosphorodithioate) and ethanol on antioxidant status of liver and kidney of experimental mice. Pestic. Biochem. Physiol. 85 (1), 115–121. https://doi.org/10.1016/j.pestbp.2005.12.001 (2006).
Hagar, H. H. & Fahmy, A. H. A biochemical, histochemical, and ultrastructural evaluation of the effect of dimethoate intoxication on rat pancreas. Toxicol. Lett. 133, 161–170. https://doi.org/10.1016/S0378-4274(02)00128-5 (2002).
Etten, V. Recommended methods of Anesthesia, Analgesia, and Euthanasia for Laboratory Animal species. Instant Anim. Stud. 460 (718), 839–7100 (2002). https://www.spandidos-publications.com/var/RecommendedMethodsAnesthesiaAnalgesiaEuthanasia.pdf
Joshi, A. & Rajini, P. Organophosphorus insecticides and glucose homeostasis. Insecticides. Pest Engineering. Rijeka: InTech. 63–84. Organophosphorus-Insecticides-and-Glucose-Homeostasis.pdf (2012).
Zhang, J., Song, W., Sun, Y., Cheng, B. & Shan, A. Changes in glucose metabolism and mRNA expression of IRS-2 in rats exposed to phoxim and the protective effects of vitamin E. Toxicol. Res. 7 (2), 201–210. https://doi.org/10.1039/c7tx00243b (2018).
Lasram, M. et al. A review on the molecular mechanisms involved in insulin resistance induced by organophosphorus pesticides. Toxicology 322, 1–13. https://doi.org/10.1016/j.tox.2014.04.009 (2014).
Kori, R., Singh, M., Jain, A. & Yadav, R. Neurochemical and behavioral dysfunctions in pesticide exposed farm workers: a clinical outcome. Indian Journal of Clinical Biochemistry. 33, 372–381. (2018). https://link.springer.com/article/10.1007/s12291-018-0791-5
Akbel, E. et al. The subchronic exposure to malathion, an organophosphate pesticide, causes lipid peroxidation, oxidative stress, and tissue damage in rats: the protective role of resveratrol. Toxicol. Res. 7 (3), 503–512. https://doi.org/10.1039/c8tx00030a (2018).
Ojha, A., Yaduvanshi, S. K. & Srivastava, N. Effect of combined exposure of commonly used organophosphate pesticides on lipid peroxidation and antioxidant enzymes in rat tissues. Pestic. Biochem. Physiol. 99(2), 148–156. https://doi.org/10.1016/j.pestbp.2010.11.011(2011).
Prakasam, A. & Sethupathy, S. Vitamin E supplementation on biochemical changes observed in agricultural field workers exposed to different classes of pesticides. Indian J. Clin. Biochem. 16, 185–189 (2001). https://link.springer.com/article/10.1007/BF02864858
Pothu, U., Thammisetty, A. & Nelakuditi, L. Evaluation of cholinesterase and lipid profile levels in chronic pesticide exposed persons. J. Family Med. Prim. care. 8 (6), 2073–2078. https://doi.org/10.4103/jfmpc.jfmpc_239_19 (2019).
Banerjee, B., Seth, V. & Ahmed, R. Pesticide-induced oxidative stress: perspective and trends. Rev. Environ. Health. 16 (1), 1–40. https://doi.org/10.1515/REVEH.2001.16.1.1 (2001).
Cataudella, E. et al. Pesticides exposure and the management of acute hepatic injury. Acta Med. Mediterranea. 28 (3), 245–252 (2012). https://www.iris.unict.it/bitstream/20.500.11769/42536/1/Pesticides%20exposur.pdf
Prudente, I. et al. Evidence of risks of renal function reduction due to occupational exposure to agrochemicals: a systematic review. Environ. Toxicol. Pharmacol. 63, 21–28. https://doi.org/10.1016/j.etap.2018.08.006 (2018).
Jacobson, M. et al. Organophosphate pesticides and progression of chronic kidney disease among children: a prospective cohort study. Environ. Int. 155, 106597. https://doi.org/10.1016/j.envint.2021.106597 (2021).
Petejova, N., Martinek, A., Zadrazil, J. & Teplan, V. Acute toxic kidney injury. Ren. Fail. 41 (1), 576–594. https://doi.org/10.1080/0886022X.2019.1628780 (2019).
Yang, L. et al. Toxicity of mercury: molecular evidence. Chemosphere 245, 125586. https://doi.org/10.1016/j.chemosphere.2019.125586 (2020).
Klaassen, C., Watkins, J. & Casarett Doull’s. Essentials of Toxicology 3rd edn (McGraw-Hill Education, 2019).
Moulis, J. Pesticides and the kidney. In (ed Gupta, R. C.) Handbook of Toxicology of Chemical Warfare Agents (613–626). Academic. (2012).
Othmene, Y. et al. Oxidative stress, DNA damage and apoptosis induced by tebuconazole in the kidney of male Wistar rat. Chemico-Biol. Interact. 330, 109114. https://doi.org/10.1016/j.cbi.2020.109114 (2020).
Sobolev, V., Sokolova, M., Jenkins, R. & Goncharov, N. Molecular mechanisms of acute organophosphate nephrotoxicity. Int. J. Mol. Sci. 23 (16), 8855. https://doi.org/10.3390/ijms23168855 (2022).
Patel, S. & Singh, P. Hepatotoxicity of organophosphate pesticides: evidence from bilirubin levels. J. Biochem. Mol. Toxicol. 32 (6), e22150 (2018).
Manfo, F. et al. Evaluation of the effects of agro pesticides use on liver and kidney function in farmers from Buea, Cameroon. J. Toxicol. 1, 2305764. https://doi.org/10.1155/2020/2305764 (2020).
VoPham, T. et al. Pesticide exposure and liver cancer: a review. Cancer Causes Control. 28, 177–190. https://doi.org/10.1007/s10552-017-0854-6 (2017).
Yadav, D., Bhattacharyya, R. & Banerjee, D. Acute aluminum phosphide poisoning: the menace of phosphine exposure. Clin. Chim. Acta. 520, 34–42. https://doi.org/10.1016/j.cca.2021.05.026 (2021).
Kaliwal, B. & Ksheerasagar, R. Histological and biochemical changes in the liver of albino mice on exposure to insecticide, carbosulfan. Casp. J. Environ. Sci. 4 (1), 67–76 (2006). https://cjes.guilan.ac.ir/article_965_3d17341e41716fb1d57e4eb530cfd209.pdf
Alghamdi, S. A. Effect of Nigella sativa and Foeniculum vulgare seeds extracts on male mice exposed to carbendazim. Saudi J. Biol. Sci. 27 (10), 2521–2530. https://doi.org/10.1016/j.sjbs.2020.04.016 (2020).
Abdus Sallam, M., Zubair, M., Tehseen Gul, S., Ullah, Q. & Idrees, M. Evaluating the protective effects of vitamin E and selenium on hematology and liver, lung and uterus histopathology of rabbits with cypermethrin toxicity. Toxin Reviews. 39 (3), 236–241. https://doi.org/10.1080/15569543.2018.1518335 (2020).
Massoud, A. et al. Biochemical and histopathological effects of repeated low oral doses of malathion, metalaxyl and cymoxanil on different tissues of rats. Pakistan J. Zool. 55 (1), 11–21. https://doi.org/10.17582/journal.pjz/20210518100521 (2022).
Acknowledgements
The authors gratefully acknowledge Department of Zoology for the support and laboratory facilities for conduction of experiments.
Author information
Authors and Affiliations
Contributions
Nida Saleem: Writing—original draft, methodology, conceptualization, investigation, data curation, data analysis, and formal analysis. Mushtaq Hussain Lashari: Supervision, Validation. Hafiz Ishfaq Ahmad: Supervision, Validation, Proofreading.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Saleem, N., Lashari, M.H. & Ahmad, H.I. Orally administered organophosphorus dimethoate mediated biochemical alterations in male and female experimental Rattus norvegicus (albino rats). Sci Rep 15, 4371 (2025). https://doi.org/10.1038/s41598-025-88023-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-88023-2