Abstract
A nanocomposite composed of rGO and γ-Fe2O3 was prepared using ultrasonication for the adsorption of malachite green (MG) dye. The preferential plane diffractions at 2θ values of 35.54° and 26.45° about γ-Fe2O3 (311) and rGO (002) with 19.85 and 20.92 nm crystallite sizes, respectively confirmed the successful formation of nanocomposite nature of the adsorbent. Moreover, XPS and FTIR results also confirmed the composite formation due to the existence of peaks relevant to the composite’s components. The adsorbent’s surface charge (pHPZC = ~ 7.1) was also estimated using the salt addition technique. To minimize experiments and optimize adsorption parameters for the removal of MG by the nanocomposite at 25 °C, central composite design (CCD) using response surface methodology (RSM) was used. The optimal adsorption parameters obtained from the within-range numerical optimization based on 0.923 Derringer’s desirability function were 200 mg/100 mL dose, 80 ppm dye solution, 7.99 pH and 112.68 min. contact time for ~ 90% MG dye removal and 40.64 mg/g adsorption capacity (qm) by rGO/γ-Fe2O3. However, ~ 98% MG dye removal with 64.26 mg/g (qm) was achieved upon extended-range estimation of adsorption parameters. The adsorption data exhibited the best co-relationship with Freundlich isotherm and pseudo-2nd order kinetic model. According to a thermodynamic analysis, the MG adsorption process on the rGO/γ-Fe2O3 surface is exothermic, spontaneous, and less random. Moreover, the thermal stability, desorption, regeneration and reusability of rGO/γ-Fe2O3 nanocomposite were also explored. Finally, this study shows that RSM can be an excellent technique to optimize the dye adsorption process at industrial scale.
Similar content being viewed by others
Introduction
Safe drinking water is available only to a small proportion of people in the world. The rest of the population is reliant on contaminated water, which is mostly contaminated by sewage and, to a lesser extent, fertilizer, pesticides, and industrial effluents1,2,3. The contaminants present in water can be microbes, pollutants of organic and inorganic nature, and positive and negative charged ions. Organic substances such as phenols, dyes, and pesticides are also regarded as possible water quality risks. All of these compounds are toxic and significantly risk the health of humans and other living things in the ecosystem if they go beyond a certain limit4,5,6. The techniques that are used for pollutant removal from polluted water are precipitation, oxidation, membrane filtration, and adsorption. Adsorption is the most used method, and it has proven an efficient and successful method in the removal of toxins such as metals, phenolic compounds, dyes, and other pollutants7,8,9. Nanomaterials have become increasingly important in the removal of waste materials from contaminated water, particularly dyes and phenolic contents, in recent years10,11.
Many types of adsorbent materials are being used for the elimination of impurities, especially organic toxins. The most used adsorbent for organic pollutants removal is activated carbon. Magnetic nanomaterials are more appropriate for pollutant removal. Magnetic nanomaterials are getting more attention because of their easy separation from the reaction mixture10,11,12,13. The structural unit size of nanomaterials is less than 100 nanometers. The huge surface area, intense chemical reactivity, compact size, and high number of surface atoms of nanomaterials have attracted a lot of interest in the last several decades14. All these qualities work together to enhance the quantity of unsaturated bonds on the surface while reducing surface-molecule interaction. As a result, the atoms on the surface become exceedingly unstable and active, allowing other molecules to easily interact15. The active groups on nano adsorbents’ surfaces form bonds with heavy metals and organic molecules in dyes and wastewater16. All of these phenomena boost nanomaterials’ adsorption ability, making them a viable adsorbent.
Because of their high adsorption capacity at minimal contact time, excellent stability, and ease of separation, magnetic nanoparticles have been the subject of much research over the last five years. They are utilized to remove heavy metals, medicines, organic chemicals, and dyes because they have outstanding physiochemical characteristics and are easy to reproduce12,17. Magnetic nanoscale metal oxides have potential adsorbent properties when compared to other adsorbents such as high surface area and active adsorption pores, as well as a short diffusion distance18,19,20.
In this study, we tried to explore the effect of adsorption parameters using response surface modeling (RSM) by interpreting 3D response surface plots for the elimination of MG dye by rGO/γ-Fe2O3 nanocomposite for the first time. Reduced graphene oxide was chosen because of its high surface area and availability of oxygen containing functional groups to support the dye adsorption20,21. Moreover, γ-Fe2O3 was selected to impart magnetic character in rGO composite for an easy removal of the rGO/γ-Fe2O3 adsorbent from the reaction mixture. Selecting MG as a target pollutant refers to its toxicity, persistent structure, and most importantly among the model pollutants which make it a valuable benchmark to study the optimization of adsorption process using RSM. The RSM model fitting study and model statistics were also carried out to optimize adsorption parameters effectively using central composite design (CCD) with reduced adsorption experiments. Additionally, MG dye adsorption process on rGO/γ-Fe2O3 nanocomposite was investigated using kinetic models and adsorption isotherms. The nanocomposite was synthesized by just mixing rGO and γ-Fe2O3 using ultrasonication and the prepared adsorbent was characterized with different analytical techniques to explore its structure, surface morphology, and surface charge.
Experimental
Materials
Iron (II) sulfate heptahydrate (FeSO4.7H2O), potassium hydroxide (KOH), sodium hydroxide (NaOH), hydrochloric acid (HCl), ethanol (C2H5OH), sodium nitrate (NaNO3), ascorbic acid (C6H8O6), 30% H2O2 and triton (X-100) were supplied by Sigma-Aldrich whereas malachite green (MG) dye was provided by VWR. Furthermore, all studies used freshly obtained distilled water, and no further purification was applied to any other compounds.
Synthesis of reduced graphene oxide (rGO)
The technique described in our earlier report was used to manufacture graphene oxide (GO) prior to the production of rGO22. The synthesized GO was further reduced to rGO using ascorbic acid (AA)23. In a typical experiment, GO and AA (1:1) were mixed in 100 mL distilled H2O under continuous stirring for 30 min at 25 °C. Then, H2O2 was inserted to the mixture monitored by the mixture’s sonication at 60 °C for 30 min. The homogeneous suspension was centrifuged to separate black colored rGO. The achieved rGO was cleaned with ethanol and distilled H2O followed by drying in an oven at 100 °C.
Synthesis of rGO/γ-Fe2O3 nanocomposite
In a typical synthesis, 15 g FeSO4.7H2O was suspended completely in 100 mL distilled H2O at 25 °C under continuous stirring. The clear solution was then mixed with 2 mL of the surfactant TX-100, and the mixture was agitated for two hours. The reaction mixture was hydrolyzed slowly by adding 0.1 M KOH till the formation of greenish-black precipitates under vigorous stirring. Ordinary filter paper was employed to filter out the precipitates, which were then rinsed with distilled H2O and a solution of distilled water and ethanol until a neutral pH was achieved. The precipitates were further dried at 100 °C in an oven. The dried substance, or γ-Fe2O3, was pulverized into a fine powder. Then, 95% as prepared γ-Fe2O3 and 5% rGO were suspended in distilled water and mixed ultrasonically to prepare rGO/γ-Fe2O3 nanocomposite. Finally, the mixture was filtered, washed, oven-dried, ground and then saved in a sample vial for further use.
Characterization of rGO/γ-Fe2O3 nanocomposite
X’pert X-ray powder diffractometer (PW1398, Philips, Netherland) was used to determine the microstructural properties of the nanocomposite while using the preferred orientation in the XRD pattern. The presence of Fe, oxygen and carbon in the sample was verified using an x-ray photoelectron spectrometer (PHI 5000 VersaProbe II, ULVAC-PHI, Japan). FTIR spectrum of the nanocomposite was recorded from 650 to 4000 using Bruker alpha II FTIR spectrometer. SDT Q600 TGA/DSC analyzer was used to investigate the thermal response of the rGO/γ-Fe2O3 nanocomposite. Moreover, the surface morphology of the nanocomposite was evaluated using a scanning electron microscope (JEOL JSM-IT700HR, Tokyo, Japan). The pH at point of zero charge (PZC) was also estimated using the salt addition technique to know the variation in surface charge of the rGO/γ-Fe2O3 nanocomposite while varying the pH24. A UV-visible spectrophotometer (UV-1800, Shimadzu, Japan) was employed to monitor the adsorption of MG dye on the prepared nanocomposite.
Adsorption study
Malachite green dye supplied by SIGMA was used as an adsorbate to prepare its solutions with different concentrations varying from 20 ppm to 100 ppm by dissolving respective mg of dye in the one L solution prepared in distilled water. Moreover, factors involved in the MG dye removal study were pH (2, 4, 6, 8, 10), contact time (60, 90, 30, 120 and 150 min.), adsorbent amount (50, 100, 150, 200 and 250 mg) and dye concentration (20, 40, 60, 80 and 100 ppm). In a typical experiment, 150 mg rGO/γ-Fe2O3 was added to 100 mL of 20 ppm MG dye solution. The pH of the prepared suspension was adjusted at 6 followed by shaking of the suspension for 90 min at 25 °C and 150 rpm using a mechanical shaker. To determine the MG dye λmax, or absorbance at a wavelength of 617 nm, the sample was filtered using a syringe filter and then placed through a UV-visible spectrophotometer when the shaking operation was complete. Moreover, the following Eqs. 1 and 2 were used to determine the % removal and adsorption ability (mg/g), respectively25.
where,” C₀” is the initial dye concentration, “C” is the final concentration of the dye after the adsorption process, “V” is the volume of dye solution (L) and “m” is the amount of adsorbent (g) used in a typical experiment. Moreover, a similar adsorption procedure was adopted for all runs provided in Table 1 while varying the factors given in Table 2. In the adsorption experiment, 0.1 M NaOH and 0.1 M HCl were used to maintain the pH.
Experimental design and optimization of parameters through RSM
RSM is being extensively used to optimize an experiment or process. This methodology explores relationships between the response and independent variables while utilizing valid statistical tools and mathematical models through distinctive experimental designs. Among these designs, a CCD has been used widely to optimize a process comprising five levels of each independent factor18,26. In this study, CCD was applied using Design –Expert 13.0.1.0 software to understand the main, quadratic and interaction effects of four factors (Table 2) on removal (%) of MG dye by rGO/γ-Fe2O3 nanocomposite. Experimental runs were generated using Eq. 3 and tabulated in Table 127. Among these 30 runs, 16, 8, and 6 runs were assigned to factorial, axial, and center points, respectively in the CCD.
Where “N” is the total number of runs in CCD, “K” is the number of total factors and “C” denotes center points. Furthermore, adsorption experiments related to the removal of MG dye by rGO/γ-Fe2O3 were conducted for each run based on the assigned factors’ level mentioned in Table 1 and their respective response (% removal) was determined as actual values. The observed (actual) response for each experimental run was further analyzed using the following mathematical model to study the effect of each variable on it27.
Where “Y” is the response variable, “Ai” and “Aj” are coded independent variables and bo is regression intercept, while bi, bii and bij signify the linear, quadratic and interaction coefficients. Moreover, ANOVA test was also performed, and different parameters were recorded to assess the fitness of the model. Based on the fitness of the model, the predicted values were tabulated (Table 1) and optimization was processed to get the optimized value for each factor. The interaction of two factors while keeping the other two optimized parameters fixed was presented in the form of 3D surface plots. Moreover, the optimized parameters were validated by conducting an actual experiment.
Adsorption isotherms and thermodynamic studies
In isotherm investigations, 100 mL of a 20 ppm MG dye solution was mixed with 0.2 g of rGO/γ-Fe2O3 nanocomposite. 0.1 M NaOH was added to the solution to bring its pH down to 8. The suspensions were then subjected to a mechanical shaker at 25 °C and 150 rpm. The similar experiments were performed for 40, 60, 80 and 100 ppm MG dye solutions. A UV-visible spectrophotometer was used to determine the absorbance of the unadsorbed MG dye concentration after the samples had been shaken for 113 min and collected via a syringe filter. Furthermore, several adsorption isotherm models, including the Dubinin-Radushkevich, Temkin, Freundlich, and Langmuir models, were used to examine the MG dye adsorption process on the rGO/γ-Fe2O3 nanocomposite28. Moreover, thermodynamic study was conducted at 25–50 °C with an interval of 5 °C while keeping other parameters fixed. This study helped in assessing the feasibility, spontaneity, and nature of the MG dye adsorption on the heterogenous surface of rGO/γ-Fe2O3 nanocomposite29.
Adsorption kinetic studies
In an adsorption kinetic study, 0.2 g rGO/ γ-Fe2O3 nanocomposite was added to 80 ppm MG dye solution. 0.1 M NaOH was added to the solution to bring its pH down to 8. The suspension was subjected to a mechanical shaker at room temperature and 150 rpm. The sample was collected through a syringe filter after 10 min followed by absorbance measurement of unadsorbed MG dye concentration using a UV-visible spectrophotometer. A similar procedure was adopted for other shaking time intervals such as 20, 30, 40, 50 and 60 min. Moreover, the adsorption process was shown using the Pseudo 1st order, Pseudo 2nd order, Elovich, and Intraparticle diffusion models28.
Results and discussion
Characterization of rGO/ γ-Fe2O3 nanocomposite
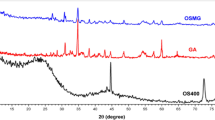
Figure 1a shows the XRD patterns of the nanocomposite, rGO and γ-Fe2O3, wherein the diffractions corresponding to γ-Fe2O3 and rGO are observable in rGO/γ-Fe2O3 which further confirm the composite nature of the prepared sample. The sharp diffractions appeared at 2θ values of 18.36° (111), 30.17° (220), 35.54° (311), 43.22° (400), 53.75° (422), 57.38° (511) and 62.78° (440) are corresponded with cubic γ-Fe2O3 (JCPDS# 39-1346) with good crystallinity and have close agreement with literature30,31. In addition, the diffraction peak at 26.45° with 002 plane is clear evidence for the presence of rGO in the nanocomposite32,33. Moreover, the shifting of γ-Fe2O3 preferential plane (311) in nanocomposite to the lower 2θ value was also noticed. This decrease in 311 plane position was mainly attributed to the possible interaction of rGO with γ-Fe2O3 which consequently increased the interplanar distance from 2.50 Å to 2.52 Å. The microstructural parameters due to γ-Fe2O3 and rGO in the nanocomposite were determined while selecting the preferred diffractions appeared at 2θ values of 35.54° and 26.45°, respectively. Table 3 displays these characteristics, which were determined using the formulas provided in our earlier correspondence34.
The typical XPS survey scan of the nanocomposite in 0-1200 eV binding energy range is specified in Fig. 1b, wherein the peaks corresponding to Fe, O and C can be seen. The presence of Fe was confirmed due to the existence of two Fe2p split peaks i.e. Fe2P3/2 and Fe2p1/2 at 708 eV and 722 eV binding energy, respectively. The assignment of these two peaks is correlated with the literature and the higher binding energy of Fe2p1/2 as compared to Fe2p3/2 is mainly due to the lower j-value and then kinetic energy of the ejected core-electron35,36. Moreover, an intense peak at 527 eV is attributed to O1s present in both rGO and Fe2O3. Another intense peak at 282 eV binding energy due to C1s confirms the presence of carbon-based species i.e. rGO in the nanocomposite. Figure 1c presents the FTIR spectrum of the nanocomposite to understand the functional group’s vibrations due to respective components in rGO/ γ-Fe2O3. The particular Fe-O stretching vibration of γ-Fe2O3 is observed at 554.50 cm− 1 which has close agreement with the literature37. Moreover, the appearance of two prominent absorptions at 795.45 cm− 1 and 892.12 cm− 1 which are in the Fe-O range can be attributed to the engaging of Fe with oxygen containing functional groups in rGO38. The vibration band at 1133.21 cm− 1 is attributed to the C-OH stretching present in rGO39. The smaller broad band at 1633.45 cm− 1 is credited to the C=C present in rGO33. A broad band at 2087. 27 cm− 1 in nanocomposite is also observed in the FTIR spectrum of rGO. However, a broad band between 3042 and 3559 that further splits into two smaller bands at 3147.64 and 3442.90 presents the stretching vibrations of OH at the surface of γ-Fe2O3 and rGO, respectively38,41. Moreover, the prepared nanocomposite presents excellent thermal stability, wherein only ~ 9% weight loss was noticed till 580 °C as shown in the thermogravimetric curve (Fig. 1d). The first stage showed a weight loss of ~ 2.6% with a temperature range of 40–200 °C. The elimination of oxygen functionality and water molecules from the nanocomposite are attributed to this loss. A notable weight loss of ~ 5% was estimated between 190 °C and 280 °C. After the 2nd stage, no significant weight loss (~ 1.6%) was noticed till 580 °C which further supports an excellent thermal stability posed by the rGO/ γ-Fe2O3 nanocomposite. Moreover, the inset of Fig. 1d shows the DSC curve of the nanocomposite with two broad peaks of the endothermic and exothermic processes occur while varying temperature from 40 to 580 °C. A broad endothermic peak (60–270 °C) indicates heat absorption in order to remove oxygen holding functional groups from the surface of rGO mainly and trapped H2O molecules in nanocomposite42. However, a broad exothermic peak (300–450 °C) describes the heat released during the transitioning of γ-Fe2O3 phase into thermodynamically stable α-Fe2O3 phase and decomposition of carbon skeleton of rGO42,43. Moreover, a steady decrease in heat flow at elevated temperature in Fig. 1d (inset) presents the decomposition of rGO. The magnetic properties of γ-Fe2O3 and rGO/γ-Fe2O3 were assessed through vibrating sample magnetometer analysis (VSM) conducted at 25 °C (Fig. S1). The VSM results indicated that the magnetization for γ-Fe2O3 was ~ 58 emu g− 1, demonstrating its ferrimagnetic property (Fig. S1). Following the functionalization of γ-Fe2O3 to create rGO/γ-Fe2O3, the saturation magnetization value decreased to ~ 28 emu g− 1 (Fig. S1) which presents the superparamagnetic property of the nanocomposite. This variation in magnetic property also validates the effective integration of γ-Fe2O3 with non-magnetic rGO and consequently, the synthesis of nanocomposite (Fig. S1).
A SEM was used to assess the nanocomposite’s surface shape, as seen in Fig. 2. The dispersions of cubic γ-Fe2O3 are noticed on the surface of rGO nanosheet. It is also observed in Fig. 2a that the dispersion of γ-Fe2O3 is not homogeneous everywhere on rGO. In addition to the cubic crystals of γ-Fe2O3, a few orthorhombic crystals of γ-FeO(OH), known as lepidocrocite, are observable in Fig. 2b. The possibility of the existence of γ-FeO(OH) during the preparation of maghemite in an aqueous system at alkaline pH is also reported44,45,46. It is worth mentioning here that the reflection pertaining to γ-FeO(OH) were not clearly observable in the XRD patterns due to its very low concentration relative to γ-Fe2O3 phase.
The adsorbent’s surface charge has a significant role in determining the mechanism of the adsorption process. In this context, the point of zero charge (PZC) which refers to pH where rGO/ γ-Fe2O3 nanocomposite’s surface net charge equals zero was estimated (pHPZC = ~ 7.1) from a graph between ∆pH and pHi as shown in Fig. 3. In a particular experiment, 100 mg rGO/ γ-Fe2O3 nanocomposite was combined with 50 mL 0.1 M NaNO3 solution in 100 mL conical flask at initial pH (pHi) 2. The prepared suspension was subjected to a mechanical shaker for 24 h at room temperature and 150 rpm to record the final pH (pHf). Moreover, a similar procedure was adopted to determine pHf values at 4, 6, 8, 10 and 12 initial pH values. Then the pHi and pHf values were used to find the ∆pH for the PZC plot as shown in Fig. 3. It is well documented that the adsorbent’s surface at pH > pH(PZC) becomes negatively charged owing to the adsorption of more OH⁻⁻ ions whereas surface becomes positively charged at pH < pH(PZC) due to greater H3O+ ions26,28. Hence, the surface of rGO/ γ-Fe2O3 nanocomposite in this study became positive below 7.1 and negative above 7.1 pH. Moreover, the N2 adsorption/desorption isotherms and surface parameters for γ-Fe2O3 and rGO/γ-Fe2O3 are also presented in Fig. S2 and Table S1. The increased BET surface area of 123 m2 g− 1 for rGO/γ-Fe2O3 compared to 31 m2 g− 1 for γ-Fe2O3 (Fig. S2 and Table S1) could improve the adsorption capacity of rGO/γ-Fe2O3.
Response surface modeling (RSM) and optimization of adsorption parameters
To understand the individual and combined effects of independent variables on the response variable, response surface modeling is used, which is based on a fit model. The model fitting study and model statistics analysis suggested a quadratic model (P < 0.0001) due to its higher adjusted and predicted R2, and lower standard deviation and PRESS in comparison to linear, 2FI, and cubic models as presented in Tables 4 and 5. Further evidence of the model’s importance comes from Table 6’s 318.44 F-value and P-value less than 0.0001. A P-value better than 0.05 further confirms the quadratic model’s fitness in this investigation for the percentage of MG dye removed by the rGO/γ-Fe2O3 nanocomposite. The lack of fit F-value of 9.58 is not significant, the non-significant lack of fit is excellent. The fact that the fit F-value is 9.58 indicates that there is a 24.68% possibility that noise is the cause of a fit F-value this low. The corrected R2 value of 0.9944 and the projected R2 value of 0.9859 vary by less than 0.2, indicating a satisfactory agreement. Furthermore, the model’s acceptable precision ratio (63.069 > 4) and low (5.90%) coefficient of variation (C.V.) values demonstrate its repeatability and dependability, respectively, and validate that it may be utilized to traverse the design space18,28.
In this work, response surface modeling was used in central composite design to examine the effects of independent variables (factors) on the percentage removal of MG dye by rGO/γ-Fe2O3 nanocomposite. These factors included adsorbent dosage (A), beginning dye concentration (B), pH (C), and contact duration (D). The suggested quadratic model was applied, and the contribution of factors was explored using ANOVA results presented in Table 6. The resultant quadratic regression expression based on significant (P < 0.05) model terms (Table 6) for % removal of MG dye in terms of coded values is provided in Eq. 5:
This regression equation was used to predict the response. This equation showed that all the factors have a positive influence except A2 and D2 on the % removal. The terms included in the above equation show that pH-related terms have a greater effect in removing MG dye. The model suggests that higher pH may result in a higher % removal of dye. The evaluation of the second-order quadratic regression model was done through statistical analysis of variance (ANOVA) as shown in Table 6. The model terms with P-values less than 0.0500 such as A, B, C, D, AB, AC, BC, CD, A2, C2, and D2 have a significant impact on % removal of MG dye. However, the model terms are declared insignificant in predicting response if their P value is greater than 0.05.
The diagnostics plots (Fig. 4) also show the effectiveness of the suggested model. Figure 4a shows the closeness of observed % removal values to the model-predicted values. It is inferred from the predicted vs. actual plot that the experimental and predicted values have an excellent correlation with smaller residuals. The Box-Cox plot (Fig. 4b) is used to assess the normal distribution of data from the lambda value. It is reported that lambda value close to 1 indicates the normal distribution which does not require any power transformation18,47. The Box-Cox plot verifies the normal distribution in this study for the removal of MG dye because of having a lambda value close to 1. Figure 4c and d show the spreading of all residual points within the upper and lower boundary lines, which further confirm the appropriateness of the model for the data. These diagnostics plots suggest that this model is equally good for the prediction, therefore, the predicted values based on second order quadratic model are tabulated in Table 1.
In an optimization analysis, CCD was developed using Design –Expert 13.0.1.0 for optimizing 4 different independent variables for the removal of MG dye by rGO/ γ-Fe2O3 nanocomposite at room temperature and 150 rpm. Wherein, the numerical optimization revealed 100 different solutions and based on Derringer’s desirability function as shown in Fig. 5, the optimal adsorption parameters were found to be 200 mg/100 mL, 80 ppm, 7.99 pH and 112.68 min. for nanocomposite dose, MG dye initial concentration, pH and contact time, respectively. Moreover, these optimal parameters were in the range of 90.86% dye removal based on a 0.923 desirability function. An experiment of the rounded optimized values (200 mg, 80 ppm B, 8 pH and 113 min.) was also conducted to validate the predicted value. The new observed % removal at optimized conditions was calculated to be ~ 88%. Moreover, a perturbation plot in Fig. 5 is also presented to assess the impact of all four parameters on the % removal of MG dye. Wherein, parameter “C” is more deviated from the reference point indicating a highly significant effect on the response than other factors. The decreasing order of impact of four studied parameters based on their deviation from the reference point in the perturbation plot and F-values given in Table 6:
Effects of interaction parameters on MG removal by rGO/ γ-Fe2O3
The effects of interaction parameters (P < 0.05) such as AB, AC, BC and CD present in regression Eq. 5 were explored using 3D response surface plots for the % removal of MG dye as shown in Fig. 6. The plots were constructed to investigate the effect of two parameters within the model-designated range on the percentage removal of dye while keeping the other two parameters fixed at their optimized values at 150 rpm and room temperature. It is inferred from Fig. 6a that at high nanocomposite dose, the % dye removal increases with the increase in dye concentration at 8 pH and 113 min. contact time. At the initial stage, the AB interaction response is found linear and then changes to quadratic by increasing dose and dye concentration. Figure 6b shows how the BC interaction affects dye removal at the ideal dosage and contact duration. A linear response is shown for all values of B and C. It is also evident from the plot that at lower pH, the dye removal increases slowly while increasing dye concentration and reaches ~ 20% at high dye concentration. Whereas the dye removal increases from ~ 63 to ~ 90% at a high pH value i.e. 8 pH with the increase in dye content from 40 to 80 ppm, respectively. Whereas, a major influence has been noticed by varying pH as shown in Fig. 6c (AC interaction). Where a significant increase in % removal is observable at all doses of nanocomposite at fixed dye concentration (80 ppm) and contact time (113 min.). A quadratic effect is observable in Fig. 6d while analyzing DC interaction on dye removal at fixed optimized A and B parameters. At lower pH, a very small change in dye removal (0 to 20%) is noticed by increasing contact time whereas at higher pH value (8 pH), the response is increased from ~ 77% to ~ 90% while increasing contact time from 60 to 120 min, respectively. The analysis of 3D response surface plots revealed the highly significant impact of pH on MG dye removal in compression to other parameters. The decreasing order of impact of four interaction terms such as AB, AC, BC and CD based on their P-values given in Table 6:
It is worth noting that the optimization analysis based on the 0.923 desirability function generated 90.87% dye removal at optimized parameters which is also verified through actual experiment with an acceptable difference of 2.86%. However, our ultimate purpose is to optimize parameters to achieve 100% MG dye removal by rGO/ γ-Fe2O3 nanocomposite at room temperature and 150 rpm mixture shaking. In this context, the 3D response surface response of statistically significant interaction terms beyond the designated ranges of the parameters is also investigated and presented in Fig. 7. Wherein, the red flattened portion in plots presents the region in the response surface where the 100% dye removal can be achieved based on RSM suggested model and consequently the values of interaction parameters can also be estimated and which are tabulated in Table 7.
The significance of pH greater than pH (PZC) for the efficient removal of MG dye is obvious from the perturbation and response surface plots. In the present study the recorded point zero charge value for absorbent was 7.1. When the pH of absorbent was greater than 7.1 the surface of rGO/γ-Fe2O3 became negatively charged and can attract the positively charged MG dye. When the pH was increased to 10, greater forces of attraction became operative between negatively charged adsorbent and cationic MG eventually leading to maximum adsorption of dye and thus maximum response was recorded. Whereas at pH lower than pH (PZC), low % removal was observed due to positively charged adsorbent surface which led to repulsive forces between rGO/γ-Fe2O3 nanocomposite and MG dye. Moreover, based on highly significant (P < 0.0001) interaction among the terms, AC interaction has been used to verify while performing an actual experiment at pH 10, 113 min. contact time, 122 mg/100 mL rGO/γ-Fe2O3 dose and 80 ppm MG dye solution. The obtained MG dye removal (~ 98%) was very close to 100% predicted from the response surface plot as shown in Fig. 8.
The comparison of UV-visible absorption spectra of MG dye before and after adsorption on rGO/γ-Fe2O3 nanocomposite at predicted values (pH 10, 113 min contact time, 122 mg/100 mL rGO/γ-Fe2O3 dose and 80 ppm MG dye solution) of parameters from AC interaction by RSM suggested model to achieve 100% MG dye removal at 25 °C.
Adsorption isotherm and thermodynamic studies
The adsorption process requires a detailed interpretation of adsorption isotherms to develop the mechanism of MG dye removal by rGO/γ-Fe2O3 nanocomposite. Each isotherm model assesses specific physical and chemical parameters that are important to explain an adsorption process. Therefore, Langmuir, Freundlich, Temkin and Dubinin-Radushkevich models have been applied using Eqs. 6–9, respectively27,48,49:
where “Ce” and “qe” are the MG dye concentration in mg/L and amount (mg/g) of MG dye adsorbed per unit mass of rGO/γ-Fe2O3 nanocomposite at equilibrium between adsorption and desorption. Whereas, “qm” represents the maximum adsorption capacity in mg/g units, and “KL” is known as the Langmuir constant which also provides information about adsorption energy. Moreover, “KF” and “1/n” are Freundlich constants, which help in estimating the adsorption capacity and intensity, respectively. The favorability of the adsorption process and heterogeneity of the adsorbent’s surface are usually assessed by the value of “n” which lies between 1 and 10. In Eq. 8, “B” is a Temkin constant that is used to estimate the heat of adsorption in J/mole, whereas “AT” (L/g) relates to the equilibrium binding constant. “KDRK” (mol.2/kJ2) is the Dubinin-Raduskavich-Kaganer constant for adsorption energy determination, whereas “qd” (mg/g) is the theoretical adsorption capacity. Moreover, \(\:\in\:\) is Polyani potential which describes the interaction energy between MG dye and rGO/γ-Fe2O3 nanocomposite48.
Moreover, the data have been analyzed through the isotherms presented in Fig. 9a-d, and the parameters obtained from the Langmuir (Fig. 9a), Freundlich (Fig. 9b), Temkin (Fig. 9c) and DRK (Fig. 9d) adsorption isotherms are given in Table 8. It is noticed that most of the parameters are determined from the slope and intercept of the respective isotherms except separation factor “RL” and the mean adsorption energy “E”. These parameters have been calculated by applying the equations used by Al-Saeedi et al.48. It is inferred from the isotherms’ analysis that the n-value (1.60) greater than 1 and RL (0.26) between 0 and 1 favors the adsorption of MG dye on the surface of rGO/γ-Fe2O3 nanocomposite adsorbent28. Moreover, the correlation coefficient of Freundlich isotherm is found to be (R2 = 0.947) highest among others, which further confirms the removal of MG dye by the heterogenous surface of rGO/γ-Fe2O3 nanocomposite26,28. The suitability of Freundlich isotherm also favors the multilayered adsorption of dye on the prepared nanocomposite of the heterogeneous surface. Moreover, the obtained free adsorption energy (E = 1 kJ/mol.) from the DRK isotherm is less than 8 kJ/mol. which further confirms the physical adsorption of MG dye. In order to assess the adsorption capacity of MG on rGO/γ-Fe2O3, DRK model has been preferred because the Freundlich and Temkin isotherms cannot directly provide the adsorption capacity whereas Langmuir isotherm is not considered due to its lower R2 value than DRK isotherm. The calculated adsorption capacity (qm = 37.39 mg g− 1) has a close agreement with experimental adsorption capacity i.e., 40.64 mg g− 1. The decreasing trend of isotherms’ fitting based on correlation coefficient (R2) for the removal of MG dye by rGO/γ-Fe2O3 nanocomposite is.
The adsorption isotherms (a) Langmuir (b) Freundlich (c) Temkin and (d) Dubinin-Raduskavich-Kaganer with their correlation coefficient values obtained at within-range optimized fixed parameters (pH 8, 113 min contact time, and 122 mg/100 mL rGO/γ-Fe2O3 dose) with 20, 40, 60 80 and 100 ppm MG dye solution at 25 °C.
The thermodynamic parameters such as ∆H°, ∆S° and ∆G° were determined using the equations provided by Narasimharao et al., and Sadoq et al.50,51. Furthermore, Van’t-Hoff equation was used to estimate the ∆H° and ∆S° from the slope and intercept, respectively while drawing a linear graph between ln KL and 1/T as shown in the Fig. 10. The estimated thermodynamic parameters such as ∆H° (-30.43 kJ/mol.), ∆S° (-0.07 kJ/mol.K) and average ∆G° (-8.42 kJ/mol.) demonstrate the favorability of exothermic adsorption of MG dye with decreased randomness on the heterogenous surface of rGO/γ-Fe2O3 nanocomposite. Previously, Sundararaman also reported the negative values of ∆H°, ∆S° and ∆G° for the adsorption of MG dye which favored the spontaneous adsorption of MG dyes molecules with decreasing randomness due to organized arrangement of dye molecules on the surface of adsorbent at lower temperature52.
Adsorption kinetic studies
Adsorption kinetics describe the rate of adsorption, the interaction between adsorbate and adsorbents, and the steps involved in an adsorption process. In this context, different adsorption kinetics models such as pseudo-1st order, pseudo-2nd order, Elovich and intraparticle diffusion models have been applied to explore the mechanism for the removal of MG dye through adsorption on the heterogenous surface of rGO/γ-Fe2O3 nanocomposite. Moreover, the suitability of these adsorption models was assessed through their correlation coefficient (R2) values. The mathematical expressions of these kinetic models in the form of linear equations are presented below28:
Where, “qt” and “qe” are the adsorption capacity at time any time t and equilibrium time, respectively. Moreover, “k1” and “k2” are the rate constants for pseudo-1st and 2nd -order reactions, respectively in Eqs. 10 and 11. Equations 12 and 13 represent Elovich and intraparticle diffusion models, respectively. Wherein, “\(\:\alpha\:\)” and “\(\:\beta\:\)” are the initial adsorption rate constant and desorption constant, respectively, whereas “\(\:{k}_{int}\)” presents an intraparticle diffusion constant. Furthermore, the adsorption data have been analyzed through the kinetic plots presented in Fig. 11a-d and the kinetic parameters obtained from the pseudo-1st order (Fig. 11a), pseudo-2nd order (Fig. 11b), Elovich (Fig. 11c) and intraparticle diffusion (Fig. 11d) models are provided in Table 9. The kinetic analysis revealed the fitness of the pseudo-2nd order model based on the highest R2 value (0.978) as shown in Table 9 for the adsorption of MG dye by rGO/γ-Fe2O3 nanocomposite. The decreasing order of kinetics models’ fitting based on the correlation coefficient is:
The suitability of the pseudo-2nd order kinetic model describes that this adsorption mechanism depends on the amount of MG dye and rGO/γ-Fe2O349. The adsorption of MG on rGO/γ-Fe2O3 follows Freundlich adsorption isotherm and pseudo-2nd order kinetics in a better way, which is consistent with the studies reported by Dada et al., Debnath et al., Sadoq et al., and Kandasamy et al.29,49,51,53. Furthermore, the Elovich model is also applied to study the adsorption mechanism of MG dye on a heterogenous adsorbent surface as shown in Fig. 11c27. To explore the steps involved in the adsorption of MG dye, the intraparticle diffusion model (Fig. 11d) has been studied. It is established that the intraparticle diffusion model is a robust model to explore the stages involved in an adsorption process28. It is evident from the two linear portions in a plot (Fig. 11d) that the adsorption of MG dye onto rGO/γ-Fe2O3 nanocomposite is a two-stage process. Wherein, the first stage corresponds to the fast movement of MG molecules from the bulk to the external surface of the rGO/γ-Fe2O3 nanocomposite possibly due to H-bonding whereas in the 2nd stage, dye molecules diffuse into the internal pores of the nanocomposite due to due to Van der Waals interactions at a slower rate. Therefore, the kinetics of adsorption in this study is mainly controlled by the 2nd step as shown in the Fig. 11d.
The adsorption kinetics models (a) Pseudo-1st order (b) Pseudo-2nd order (c) Elovich and (d) intraparticle diffusion model with their correlation coefficient values obtained at within-range optimized fixed parameters (pH 8, 122 mg/100 mL rGO/γ-Fe2O3 dose and 80 ppm MG dye solution) while varying contact time 10–60 min with an interval of 10 min at 25 °C.
A comparison of adsorption capacities observed in this study with the previously reported studies is challenging due to differences in adsorption conditions such as adsorbent dose, pH, contact time, dye concentration and temperature. However, Table 10 displays the comparison of the adsorption capacity for MG on heterogenous surface of rGO/γ-Fe2O3 nanocomposite with the few metal oxides based reported adsorbents along with their adsorption conditions.
Desorption of MG dye and regeneration and reusability of rGO/γ-Fe2O3 nanocomposite
After completing the adsorption study at 25 °C, the mixture was heated on hot plate at 50 °C for 60 min to desorb the physically adsorbed MG dye from the surface of rGO/γ-Fe2O3 nanocomposite and during this process, nanocomposite was settled down. The supernatant was decanted, and 50 mL (1:0.5) C2H5OH-H2O mixture was added to the beaker containing the settled rGO/γ-Fe2O3 nanocomposite. Then, the suspension was kept on mechanical shaker for 60 min at 150 rpm to further facilitate the desorption of MG dye by ethanol. Then, the rGO/γ-Fe2O3 nanocomposite was separated from the mixture through a simple magnet bar. The recovered adsorbent was further washed with distilled water followed by drying at 50 °C. The structure and surface morphology of the regenerated adsorbent was also explored through XRD pattern and SEM image as shown in Fig. 12a-b, respectively. Wherein, a minor decrease in peak intensity of few diffraction peaks without significant changes in the principal diffractions of the regenerated sample in XRD pattern confirms the stability and effectiveness of rGO/γ-Fe2O3 for MG dye removal. The effectiveness of the adsorbent is also validated from the scanning electron micrograph presenting insignificant variation in surface morphology. Moreover, the regenerated rGO/γ-Fe2O3 nanocomposite was further used four times to assess its dye removal ability in successive cycles using 244 mg/200 mL nanocomposite, 80 ppm MG dye solution, 10 pH, and contact time 113 min. at room temperature (25 °C) and 150 rpm shaking speed. After the 4th cycle, ~ 10% less adsorbent efficiency and ~ 8% loss in the recovered amount of rGO/γ-Fe2O3 nanocomposite has been observed as shown in Fig. 12c in comparison to 1st use of the nanocomposite for the removal of MG dye.
Conclusions
Reduced graphene oxide and maghemite (γ-Fe2O3) were mixed successfully using ultrasonication to prepare rGO/γ-Fe2O3 nanocomposite. The presence of diffractions related to cubic γ-Fe2O3 and rGO in representative XRD pattern verified the composite nature of the adsorbent. The peaks of Fe2p3/2, Fe2p1/2, O1s, C1s in XPS survey scan and Fe-O stretching, C=C and C=O vibrations in the FTIR spectrum also verified the successful formation of rGO/γ-Fe2O3 nanocomposite. Moreover, an inhomogeneous dispersion of cubic γ-Fe2O3 and a small number of orthorhombic γ-FeO(OH) was noticed on the surface of rGO in scanning electron micrographs. The nanocomposite posed excellent stability with ~ 9% weight loss to 580 °C. The pH (PZC) analysis showed that the surface of rGO/γ-Fe2O3 nanocomposite becomes negatively charged above pH 7.1 and positively charged below pH 7.1. The model fitting and statistics analysis revealed the appropriateness of the quadratic model (P < 0.0001) with a non-significant lack of fit and 0.9944 adjusted R2 value. The optimal adsorption parameters obtained from the within-range numerical optimization were 200 mg/100 mL dose, 80 ppm dye solution, 7.99 pH and 112.68 min. contact time. The effects of significant (P < 0.05) interaction parameters unveiled the most significant impact of pH followed by the rGO/γ-Fe2O3 dose for the removal of MG dye. Moreover, 3D response surface plots beyond the designated values of the adsorption parameters predicted the different combinations of parameters based on the RSM suggested model to achieve 100% dye removal. The multilayer adsorption of MG dye on the heterogenous surface of the rGO/γ-Fe2O3 nanocomposite was shown by fitting adsorption isotherms and kinetic models. Moreover, thermodynamic study unveiled the favorability of MG dye adsorption on the rGO/γ-Fe2O3 surface at lower temperature.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Anju, A., Ravi, S. & Bechan, P. S., Water pollution with special reference to pesticide contamination in India. J. Water Resour. Protect. 2010. (2010).
Xu, F., Bai, W., Zhang, J. & Jin, L. The synthesis and application of MRI-fluorescence dual mode materials with absorption ability on quantitative analysis of malachite green. Phys. Scr. 99 (10), 105575 (2024).
Jin, L., Li, L., Xu, F. & Zhang, J. The synthesis and application of L-cysteine graphene quantum dots for quantitative analysis of nitrite content in water. Phys. Scr. 99 (6), 065124 (2024).
Xu, X., Zhang, S., Wang, Y., Wang, N., Jiang, Q., Liu, X. & Zhang, W. 2D surfaces twisted to enhance electron freedom toward efficient advanced oxidation processes. Appl. Catal. B Environ. Energy, 345, 123701 (2024).
Zhuang, Q. et al. Catalysis enhancement of Co3O4 through the Epitaxial Growth of Inert ZnO in Peroxymonosulfate activation: The catalytic mechanism of surface hydroxyls in singlet oxygen generation. Cryst. Growth. Des. https://doi.org/10.1021/acs.cgd.4c01357 (2024).
Shan, Z. et al. Hollow dodecahedra graphene oxide-cuprous oxide nanocomposites with effective photocatalytic and bactericidal activity. Front. Chem. 9, 755836 (2021).
Liu, W., Huang, F., Wang, Y., Zou, T., Zheng, J. & Lin, Z. Recycling Mg(OH)2 nanoadsorbent during treating the low concentration of CrVI. Environ. Sci. Technol. 45 (5), 1955–1961 (2011).
Shoaib, M. et al. Magnesium doped mesoporous bioactive glass nanoparticles: A promising material for apatite formation and mitomycin c delivery to the MG-63 cancer cells. J. Alloys Compd. 866, 159013. (2021).
Bahadur, A., Saeed, A., Shoaib, M., Iqbal, S. & Anwer, S. Modulating the burst drug release effect of waterborne polyurethane matrix by modifying with polymethylmethacrylate. J. Appl. Polym. Sci. 136 (13), 47253. (2019).
Mondal, B., Hasan, M. M. & Islam, M. D. Nano-Adsorbent based solutions for wastewater treatment—an overview. Dev. Wastewater Treat. Res. Process. 401–424. (2024).
Lingamdinne, L. P. et al. Biogenic reductive preparation of magnetic inverse spinel iron oxide nanoparticles for the adsorption removal of heavy metals. Chem. Eng. J. 307, 74–84 (2017).
Bahadur, A. et al. Biocompatible waterborne polyurethane-urea elastomer as intelligent anticancer drug release matrix: A sustained drug release study. React. Funct. Polym. 119, 57–63 (2017).
Bichave, M. S. et al. Nano-metal oxides-activated carbons for dyes removal: A review. Mater. Today Proc. 77, 19–30. (2023).
GadelHak, Y., El-Azazy, M., Shibl, M. F. & Mahmoud, R. K. Cost estimation of synthesis and utilization of nano-adsorbents on the laboratory and industrial scales: A detailed review. Sci. Total Environ. 875, 162629 (2023).
Fazal, T. et al. Recent developments in natural biopolymer based drug delivery systems. RSC Adv. 13 (33), 23087–23121 (2023).
Umeh, C. T. et al. Recent approach in the application of nanoadsorbents for malachite green (MG) dye uptake from contaminated water: A critical review. Environ. Nanatechnol. Monit. Manag. 100891. (2023).
Tatarchuk, T., Soltys, L. & Macyk, W. Magnetic adsorbents for removal of pharmaceuticals: A review of adsorption properties. J. Mol. Liq. 384, 122174 (2023).
Hussain, W. et al. Synthesis of MnS from single-and multi-source precursors for photocatalytic and battery applications. J. Electron. Mater. 48, 2278–2288 (2019).
Dang, A. et al. High-efficient adsorption for versatile adsorbates by elastic reduced graphene oxide/Fe3O4 magnetic aerogels mediated by carbon nanotubes. J. Hazard. Mater. 457, 131846 (2023).
Narayana, P. et al. Predictive capability evaluation and optimization of pb (II) removal by reduced graphene oxide-based inverse spinel nickel ferrite nanocomposite. Environ. Res. 204, 112029 (2022).
Lingamdinne, L. P., Koduru, J. R., Chang, Y. Y. & Karri, R. R. Process optimization and adsorption modeling of pb (II) on nickel ferrite-reduced graphene oxide nano-composite. J. Mol. Liq. 250, 202–211 (2018).
Manzar, R. et al. Graphene oxide-mediated green synthesis of Ag, Co3O4 and ZnO nanocomposites for multifunctional antimicrobial applications. J. Solgel Sci. Technol. 1–19. (2024).
Faniyi, I. et al. The comparative analyses of reduced graphene oxide (RGO) prepared via green, mild and chemical approaches. SN Appl. Sci. 1, 1181 (2019).
Areej, I. et al. Fabrication of New Hyper-cross-linked polymer for efficient heavy metal Adsorption from Industrial Wastewater. Arab. J. Sci. Eng. 1–16. (2024).
Gohari, R. M., Safarnia, M., Koohi, A. D. & Salehi, M. B. Adsorptive removal of cationic dye by synthesized sustainable xanthan gum-g p (AMPS-co-AAm) hydrogel from aqueous media: Optimization by RSM-CCD model. Chem. Eng. Res. Des. 188, 714–728 (2022).
Shoaib, M. et al. Sustained drug delivery of doxorubicin as a function of pH, releasing media, and NCO contents in polyurethane urea elastomers. J. Drug Deliv. Sci. Technol. 39, 277–282 (2017).
Onu, C. E. et al. Decolourization of bromocresol green dye solution by acid functionalized rice husk: artificial intelligence modeling, GA optimization, and adsorption studies. J. Hazard. Mater. Adv. 9, 100224 (2023).
Gholamiyan, S., Hamzehloo, M. & Farrokhnia, A. RSM optimized adsorptive removal of erythromycin using magnetic activated carbon: Adsorption Isotherm, kinetic modeling and thermodynamic studies. Sustain. Chem. Pharm. 17, 100309 (2020).
Dada, A. O. et al. Zinc oxide decorated plantain peel activated carbon for adsorption of cationic malachite green dye: Mechanistic, kinetics and thermodynamics modeling. Environ. Res. 252, 119046 (2024).
Li, D. et al. Carbon quantum dots as fluorescent probes in the measurement of the critical micelle concentration of surfactants. Colloids Surf. A. 679, 132584 (2023).
Qamar, M. T., Aslam, M., Ismail, I. M., Salah, N. & Hameed, A. The assessment of the photocatalytic activity of magnetically retrievable ZnO coated γ-Fe2O3 in sunlight exposure. Chem. Eng. J. 283, 656–667 (2016).
Saleem, Q. et al. Synchronized electrochemical detection of hydroquinone and catechol in real water samples using a Co@ SnO 2–polyaniline composite. RSC Adv. 13 (15), 10017–10028 (2023).
Sengunthar, P., Bhavsar, K., Balasubramanian, C. & Joshi, U. Physical properties and enhanced photocatalytic activity of ZnO-rGO nanocomposites. Appl. Phys. A. 126 (7), 567 (2020).
Mateen, S. et al. Integration of WO3-Doped MoO3 with ZnO photocatalyst for the removal of 2-Nitrophenol in natural sunlight illumination. Catalysts 13 (9), 1262 (2023).
Javed, M. et al. Integration of Mn-ZnFe2O4 with S-g-C3N4 for boosting spatial charge generation and separation as an efficient photocatalyst. Molecules 27(20), 6925 (2022).
Grosvenor, A., Kobe, B., Biesinger, M. C. & McIntyre, N. Investigation of multiplet splitting of Fe2p XPS spectra and bonding in iron compounds. Surf. Interface Anal. Int. J. Devoted Dev. Appl. Techn. Anal. Surf. Interfaces Thin Films. 36 (12), 1564–1574 (2004).
Sobhanardakani, S., Jafari, A., Zandipak, R. & Meidanchi, A. Removal of heavy metal (hg (II) and cr (VI)) ions from aqueous solutions using Fe2O3@ SiO2 thin films as a novel adsorbent. Process Saf. Environ. Prot. 120, 348–357 (2018).
Abouelkheir, S. S., Ibrahim, H. A. & Beltagy, E. A. Functionalized maghemite superparamagnetic iron oxide nanoparticles (γ-Fe2O3-SPIONs)-amylase enzyme hybrid in biofuel production. Sci. Rep. 13 (1), 11117 (2023).
Naseem, T. et al. Adsorption and kinetics studies of cr (VI) by graphene oxide and reduced graphene oxide-zinc oxide nanocomposite. Molecules 27 (21), 7152 (2022).
Elbasuney, S., El-Sayyad, G. S., Yehia, M. & Abdel Aal, S. K. Facile synthesis of RGO-Fe2O3 nanocomposite: A novel catalyzing agent for composite propellants. J. Mater. Sci. Mater. Electron. 31 (23), 20805–20815 (2020).
Khoshnam, M., Farahbakhsh, J., Zargar, M. & Mohammad, A. α-Fe2O3/graphene oxide powder and thin film nanocomposites as peculiar photocatalysts for dye removal from wastewater. Sci. Rep. 11 (1), 20378 (2021).
Mahendran, G. B. et al. Green preparation of reduced graphene oxide by Bougainvillea glabra flower extract and sensing application. J. Mater. Sci. Mater. Electron. 31, 14345–14356 (2020).
Tian, R. et al. Ru-functionalized Ni-doped dual phases of α/γ-Fe2O3 nanosheets for an optimized acetone detection. J. Nanostruct. Chem. 13 (6), 577–589 (2023).
Zhang, T., Zhao, Y., Kang, S., Li, Y. & Zhang, Q. Formation of active Fe(OH)3 in situ for enhancing arsenic removal from water by the oxidation of Fe (II) in air with the presence of CaCO3. J. Clean. Prod. 227, 1–9 (2019).
Yousefi, A., Seyyed Ebrahimi, S., Seyfoori, A. & Mahmoodzadeh Hosseini, H. Maghemite nanorods and nanospheres: Synthesis and comparative physical and biological properties. BioNanoScience 8, 95–104 (2018).
Sarfraz, S. et al. Removal of hexavalent chromium ions using micellar modified adsorbent: Isothermal and kinetic investigations. RSC Adv. 12 (37), 23898–23911 (2022).
Laxmi Deepak Bhatlu, M., Athira, P., Jayan, N., Barik, D. & Dennison, M. S. Preparation of breadfruit leaf biochar for the application of Congo red dye removal from aqueous solution and optimization of factors by RSM-BBD. Adsorp. Sci. Technol. 2023, 7369027. (2023).
Al-Saeedi, S. I. et al. Isotherm and kinetic studies for the adsorption of methylene blue onto a novel Mn3O4-Bi2O3 composite and their antifungal performance. Front. Environ. Sci. 11, 1156475 (2023).
Debnath, S. & Das, R. Strong adsorption of CV dye by Ni ferrite nanoparticles for waste water purification: Fits well the pseudo second order kinetic and Freundlich isotherm model. Ceram. Int. 49 (10), 16199–16215 (2023).
Narasimharao, K. et al. Synthesis and characterization of hexagonal MgFe layered double hydroxide/grapheme oxide nanocomposite for efficient adsorptive removal of cadmium ion from aqueous solutions: Isotherm, kinetic, thermodynamic and mechanism. J. Water Process. Eng. 47, 102746 (2022).
Sadoq, M. et al. Elimination of crystal violet from aqueous solution by adsorption on naturel polysaccharide: Kinetic, isotherm, thermodynamic studies and mechanism analysis. Arab. J. Chem. 17 (1), 105453 (2024).
Sundararaman, T. et al. Adsorptive removal of malachite green dye onto coal-associated soil and conditions optimization. Adsorp. Sci. Technol. 2021, 5545683. (2021).
Kandasamy, S. et al. Adsorption of methylene blue dye by animal dung biomass–derived activated carbon: Optimization, isotherms and kinetic studies. Biomass Convers. Biorefinery 1–15. (2023).
Muinde, V. M., Onyari, J. M., Wamalwa, B. & Wabomba, J. N. Adsorption of malachite green dye from aqueous solutions using mesoporous chitosan–zinc oxide composite material. Environ. Chem. Ecotoxicol. 2, 115–125 (2020).
Ejeta, B. A., Aaga, G. F., Fereja, W. M. & Mengesha, B. Biofabrication of highly effective and easily regenerated CuO nanoparticles as adsorbents for Congo Red and malachite green removal. Sci. Rep. 14 (1), 24116 (2024).
Syal, A. & Sud, D. Sonochemically synthesized mesoporous pyrophanite-MnTiO3/TiO2 nanoparticles: Adsorbent for removal of commercial malachite green dye. Iran. J. Chem. Chem. Eng. Res. 41 (8). (2022).
Khalatbary, M., Sayadi, M. H., Hajiani, M. & Nowrouzi, M. Adsorption studies on the removal of malachite green by γ-Fe2O3/MWCNTs/Cellulose as an eco-friendly nanoadsorbent. Biomass Convers. Biorefinery. 14 (2), 2495–2513 (2024).
Alkhudaydi, A. M., Danish, E. Y. & Abdel Salam, M. Magnetite/MXene (Fe3O4/Ti3C2) nanocomposite as a novel adsorbent for environmental remediation of malachite green dye. Molecules 29 (6), 1372 (2024).
Acknowledgements
This work was financially supported by the Guangdong Province Key Construction Discipline Research Ability Improvement Project (2021ZDJS081, 2022ZDJS052, 2022ZDJS053 and 2022ZDJS054). Key Research Platforms and Projects for Ordinary Universities of Guangdong Provincial Department of Education (2022ZDZX3029). Researchers Supporting Project number (RSP2025R242), King Saud University, Riyadh, Saudi Arabia. This research was supported by the International Collaborative Research Program (ICRP2023008), Internal Faculty/Staff Research Support Programs (IRSPC2024007) at Wenzhou Kean University.
Author information
Authors and Affiliations
Contributions
The manuscript was written with the contributions of all authors. All authors have approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhu, L., Sana, A., Qamar, M.T. et al. Response surface methodology for the design of malachite green dye removal by γ-Fe2O3 dispersed on reduced graphene oxide sheets. Sci Rep 15, 4402 (2025). https://doi.org/10.1038/s41598-025-88072-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-88072-7
Keywords
This article is cited by
-
Green synthesis of CaO-Fe₃O₄ composites for photocatalytic degradation and adsorption of synthetic dyes
Environmental Science and Pollution Research (2025)