Abstract
Interstitial lung disease (ILD) leads to worse outcomes in subjects with rheumatoid arthritis (RA). Few large-scale longitudinal studies have provided comprehensive data on the incidence and risk of ILD in RA compared with non-RA populations. We compare the risk of incident ILD in subjects with RA and matched controls, while also evaluating the impact of RA serologic status. Using the Korean National Health Insurance Service Data between 2010 and 2017, we identified 52,325 individuals with RA and 261,625 without RA. During a median follow-up period of 4.4 years, 3.7% of the RA cohort and 0.5% of the matched cohort developed ILD, with incidence rates of 8.30 and 1.01 per 1,000 person-years. Compared to controls, the risk of incident ILD was significantly higher in the RA cohort (adjusted hazard ratio [aHR] 7.84 [7.29–8.44]), and seropositive and seronegative RA exhibited aHRs of 9.00 (8.34–9.72) and 4.81 (4.25–5.44). Although the risk of ILD was higher in seropositive RA than seronegative RA, the risk of developing ILD was also higher in subjects with seronegative RA than in matched controls, suggesting that close monitoring for ILD should be performed in this population.
Similar content being viewed by others
Introduction
Rheumatoid arthritis (RA) is the most common inflammatory arthritis disease and is characterized by systemic inflammation that progressively damages joints, particularly the small joints in the wrists, hands, and feet, often resulting in severe disability1. RA is associated with various extra-articular manifestations, including interstitial lung disease (ILD), which is a serious condition linked to increased morbidity and mortality rates1,2,3. Furthermore, ILD in subjects with RA is correlated with a decline in quality of life, limitations in functional abilities, and increased utilization of healthcare services, leading to higher costs4,5. The heterogeneity and variable course of ILD in subjects with RA present an ongoing challenge for clinicians.
Due to the clinical importance of ILD in RA patients, numerous previous studies have focused on its prevalence, incidence, and associated risk factors2,6,7,8. The prevalence of ILD ranges from approximately 4–8% in surveyed RA populations2,6,7,8and the incidence varies widely from 1.8 to 7.1 per 1,000 person-years8. Despite ongoing efforts, there remains a shortage of large-scale longitudinal studies that provide comprehensive data on the incidence and risk of ILD, especially in comparison to the non-RA population2. This is primarily because most prior studies did not include a control group. While one study did compare the risk of ILD between RA and non-RA populations, it had a relatively small sample size and did not fully adjust for potential confounders2. Therefore, to accurately assess the risk of ILD in the RA population relative to controls, further longitudinal cohort studies that thoroughly address these factors are essential.
Seropositivity for rheumatoid factor (RF) or anti-cyclic citrullinated peptide antibodies (anti-CCP) indicates high disease activity and thereby increases the risk of extra-articular manifestations9,10. The development of ILD was found to be related to RF or anti-CCP positivity in previous studies9,11,12,13. Given that confirmation of seropositivity is a standard practice at RA diagnosis, data on the association between seropositivity and ILD risk would be clinically very informative. However, few studies have conducted additional analyses to determine differences in the incidence and risk factors of ILD based on serological status.
Therefore, the objective of this study was to compare the incidence and risk of ILD between a cohort of individuals with RA and age- and sex-matched controls drawn from a large, nationally representative longitudinal database in Korea. Additionally, to assess whether RA serologic status affects the risk of developing incident ILD, we compared ILD risk between seropositive and seronegative populations with RA and compared these groups with the non-RA population.
Results
Baseline characteristics
The mean age of the study population was 58.5 years (standard deviations [SD], 10.0 years) and 24.5% were male. The proportions of pulmonary and extra-pulmonary comorbidities were higher in the RA cohort than the matched cohort (p < 0.001 for all), except for diabetes mellitus (p = 0.288). Additionally, compared to the matched cohort, there were fewer never-smokers, alcohol drinkers, and regular exercisers in the RA cohort (p < 0.001). Body mass index (BMI) was lower in the RA cohort than in the matched cohort (p < 0.001).
The seropositive rheumatoid arthritis (SPRA) group had a higher proportion of males, was younger, and had fewer never-smokers than subjects with seronegative rheumatoid arthritis (SNRA) (p < 0.001 for all). Additionally, subjects with SPRA consumed more alcohol, engaged in regular exercise more frequently, and had a higher BMI than those with SNRA (p < 0.001 for all). All comorbidities, except for myocardial infarction (p = 0.150), airway diseases (p = 0.242), and tuberculosis were more frequently observed in individuals with SPRA than those with SNRA. However, tuberculosis was more common in subjects with SNRA compared to those with SPRA (p = 0.005) (Table 1).
Incidence and risk of ILD according to the presence or absence of RA
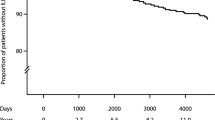
During the median follow-up period of 4.4 (interquartile range, 2.6–6.4) years, 3.7% (n = 1,921/52,325) of the RA cohort and 0.5% (n = 1,197/261,625) of the matched cohort developed ILD, with incidence rates of 8.30 and 1.01 per 1,000 person-years, respectively (p < 0.001 for both). Even after adjusting for potential confounders, the risk of incident ILD was also significantly higher in the RA cohort than in the matched cohort: unadjusted hazard ratio (HR) 8.25, 95% confidence interval [CI] 7.68–8.87; adjusted HR in Model 1 8.19, 95% CI 7.62–8.81; adjusted HR in Model 2 7.97, 95% CI 7.41–8.58; subdistribution HR [sHR] in Model 3 7.84, 95% CI 7.29–8.44). Similarly, the cumulative incidence plot depicts a significantly higher incidence of ILD in the RA cohort than in the matched cohort (log-rank p < 0.001; Fig. 1A).
Incidence and risk of ILD according to RA serologic status
When RA was classified according to serologic status, 2.1% (n = 323/15,094) of the SNRA cohort and 4.3% (n = 1,598/37,231) of the SPRA cohort developed ILD, with incidence rates of 4.88 and 9.66 per 1,000 person-years, respectively (p < 0.001 for both) (Table 2). In model 3, both SNRA (sHR 4.81, 95% CI, 4.25–5.44) and SPRA cohorts (sHR 9.00, 95% CI, 8.34–9.72) were at higher risk of developing ILD compared to matched controls. When the risk of ILD was compared between SPRA and SNRA cohorts, the SPRA cohort exhibited higher risk of ILD than the SNRA cohort (sHR 1.90, 95% CI, 1.68–2.14) (Table 2). Figure 1B shows the cumulative incidence plot according to RA serologic status.
Subgroup analysis
Sex, age, and smoking status had significant interactions with the association between RA and ILD development (p for interaction < 0.001 for all). The association between RA and ILD was more prominent in females compared to males (adjusted HR 9.37, 95% CI 8.52–10.30 vs. 6.24, adjusted HR 95% CI 5.57–7.00) and in younger individuals compared to older individuals (adjusted HR 11.0, 95% CI 9.91–12.20 vs. adjusted HR 5.62, 95% CI 5.06–6.24). Regarding smoking status and ILD risk, the risk of ILD was especially high in never-smokers (adjusted HR 8.78; 95% CI, 8.03–9.60) and current smokers (adjusted HR 8.37; 95% CI, 6.18–11.33 and adjusted HR 6.99; 95% CI, 5.62–8.69) compared to ex-smokers (adjusted HR 5.87, 95% CI 4.50–7.64 for < 20 pack-years (PY) and adjusted HR 5.42, 95% CI 4.23–6.93 for ≥ 20 PY) (p for interaction < 0.001). Income, alcohol drinking, regular exercise, BMI, and tuberculosis did not have significant effect modifications on the association between RA and ILD development (Table 3). Similar results were observed in both SPRA and SNRA cohorts (Supplementary Table 1).
Discussion
This study is the largest comprehensive study to evaluate the incidence of ILD in individuals with RA compared with a non-RA population using national cohort data. Furthermore, we conducted additional analyses by subdividing the cohort into SPRA and SNRA groups. Individuals with RA had an ILD incidence rate of 8.30 per 1,000 person-years, which is approximately 8-fold higher than those without RA. Additionally, the increased risk of developing ILD was significant in both SPRA and SNRA, although it was especially high in SPRA. Sex, age, and smoking status had significant interactions with the association between RA and ILD development.
Since ILD is a serious extra-articular manifestation of RA related to increased morbidity and mortality14, previous epidemiological studies have focused on ILD incidence and related risk factors. One recent population-based study in Germany (n= 40,686 in 2007 to 85,175 in 2020) investigated the prevalence and incidence of ILD in RA patients between 2007 and 2020. They reported that incident ILD ranged from 0.13 to 0.21% per year (0.17% in SPRA and 0.07% in SNRA in 2020)6. One recent Canadian RA population-based study (n= 184,400) using repeated cross-sectional analyses from 2000 to 2018 reported that ILD incidence rose from 1.6 (95% CI 1.3–2.0) to 3.3 (95% CI 3.0–3.6) per 1,000 RA patients during the study period7. Another study with nationwide longitudinal data from the USA from 2008 to 2017 (n= 509,787) reported that the incidence rate of ILD was 7.14 (95% CI 7.02–7.26) per 1,000 person-years among subjects with RA8. Although many studies have been conducted, these previous studies have limitations with due to the lack of a control group. Thus, the relative risk of ILD in individuals with RA versus those without RA could not be adequately evaluated.
To the best of our knowledge, only one USA population-based study (subjects with RA, n = 582; subjects without RA, n= 603) compared RA and non-RA cohorts, and reported that the risk of developing ILD was 7.7% for subjects with RA and 0.9% for those without, showing an approximately 9-fold increase in risk2. However, the number of subjects with RA and controls was relatively small (about 600 in each group), and only age, sex, and smoking status were adjusted for in this study. Additionally, RA was not analyzed by serologic status. After addressing these limitations by including a larger study population and adjusting for potential biases, our study demonstrated that patients with RA have approximately 8-fold higher risk of developing ILD than those without RA, which is consistent with the results of the abovementioned study2. Our study also has the advantage of performing subgroup analysis according to seropositivity.
Similar to our results showing higher risk of ILD in SPRA than in SNRA, previous studies showed that RF or anti-CCP positivity was related to the development of ILD9,11,12,13. For example, the Veterans Affairs Rheumatoid Arthritis prospective registry (n= 2,328) reported that higher concentrations of RF and anti-CCP were independently associated with the presence of RA-ILD13. In addition, a Canadian cohort study (n= 184,400) found trends toward an association between RF seropositivity and ILD, although the results did not achieve statistical significance7. Supporting this, translational research reported higher concentrations of anti-CCP in bronchoalveolar lavage fluid in individuals with RA with lung abnormalities, and suggested that RA-related autoantibodies are produced in the lungs and may promote ILD-related inflammation.
While it is well established that the risk of ILD is higher in SPRA than in SNRA13, it remains largely unclear whether the risk of ILD in SNRA is higher compared to the non-RA population. In previous studies, individuals with SNRA were used as controls to estimate the risk of ILD in individuals with SPRA. Another previous study that evaluated the risk of ILD in individuals with RA versus controls did not subdivide RA into SPRA and SNRA2. Overcoming these limitations by comparing the relative risk of ILD in RA to matched controls according to seropositivity, we were able to determine that SNRA carries approximately 5-fold higher risk of developing ILD compared to age- and sex-matched controls.
In our study, the association between RA and ILD was especially prominent among females, younger individuals, and never- or current smokers. The reasons for these phenomena could not be fully explained considering the observational nature of our study. One consideration is RA disease severity, which is significantly associated with increased risk of ILD. Studies have shown that RA disease activity measures are worse in females than in males and in patients with younger disease onset15,16. However, due to lack of data on disease activity other than seropositivity, we could not adjust for disease severity. Interestingly, smoking status also had a significant influence on the relationship between RA and the risk of ILD. This association was more prominent among never-smokers and current smokers compared to among ex-smokers. Smoking is not only a risk factor of idiopathic ILD, including idiopathic pulmonary fibrosis17,18, but is also an important risk factor for developing ILD in RA19. Therefore, it would be plausible for the risk of ILD to be higher in currently smoking RA individuals compared to ex-smokers, since quitting smoking could attenuate the risk of ILD20. However, unexpectedly, the risk of ILD in never-smokers was as high as that in current smokers. Currently, it is difficult to provide plausible explanations for these phenomena, and thus, future prospective studies, including genetics, are needed.
Our study results provide valuable insights for clinicians and basic researchers. First, the higher risk of ILD not only in SPRA but also SNRA versus matched controls suggests that monitoring for ILD should be considered even in SNRA patients. Unfortunately, there is no standard method used for the early screening of ILD in patients with RA. Chest imaging (CT or lung ultrasonography), pulmonary function tests (e.g., spirometry and diffusion capacity of carbon monoxide), or some biomarkers (e.g., matrix metalloproteinase, pulmonary, activation-regulated chemokine, surfactant protein D, or KL-6) might be helpful for the early detection of ILD21,22. Future studies are needed. Second, our study results could be helpful for researchers exploring the pathogenesis of RA-ILD, especially in those with SNRA. While most studies have evaluated the role of anti-CCP in the pathogenesis of RA-ILD, there is a large area of uncertainty regarding how ILD develops in SNRA.
Our study has some limitations. First, since this study was conducted with a Korean dataset, studies performed in other countries or ethnicities are needed to generalize our study results. Also, healthy user bias can occur by including only individuals with health screening data in our analyses. Second, although we used a validated definition of RA based on International Classification of Disease 10th revision (ICD-10) codes and medications, ICD-10 code-based definition of RA may over- or underestimate RA. Similarly, other comorbidities were determined using ICD-10 codes, so there might be over- or underestimations of diagnosis. For ILD, the definition of ILD has evolved over time with various patterns of ILD including interstitial lung abnormality and interstitial pneumonia with autoimmune features. Such patterns of ILD have not been included in the ICD-10 codes used in our study. This could have affected the incidence of ILD in our study. Third, due to lack of laboratory, pulmonary function, and radiologic data, we could not incorporate these factors into our analyses. We also did not have clinical data to determine the severity of RA and ILD, so we were unable to conduct an analysis that reflected disease severity. Future studies are needed to incorporate these data. Fourth, unlike previous studies showing a male predominance in RA-ILD, in our study, the risk of developing RA-ILD was higher in females than in males. This might be attributable to environmental or ethnic influences; however, we could not verify this.
In conclusion, we determined the incidence of ILD in patients with RA based on a nationwide dataset. The incidence of ILD in individuals with RA was higher than in individuals without RA. Although the risk of ILD was higher in individuals with SPRA than SNRA, the risk of developing ILD was also significantly higher in individuals with SNRA than in matched controls, suggesting that close monitoring for ILD should be performed in the RA population as a whole.
Methods
Data source
This is a retrospective cohort study. For this study we used a dataset provided by the Korean National Health Insurance Service (NHIS), a universal insurance provider managed by the government that covers 97% of the Korean population, approximately 50 million people. The NHIS dataset includes information on socioeconomics, demographic variables, healthcare utilization, health screening examination findings, disease diagnosis based on ICD-10 codes, and medical treatment and procedures23. The NHIS database includes a variety of medical and health information and has been widely used in various epidemiologic studies to identify risk factors for diseases24,25,26,27,28,29.
Data from annual or biennial health screening examination programs for all adults, offered free of charge by the Ministry of Health and Welfare, constitute the health screening database. Commencing in 2009, the health screening examination encompasses anthropometric measurements like BMI, questionnaires pertaining to smoking, alcohol consumption, and physical activity, blood tests including lipid levels, and chest radiographs. With a current participation rate of 70–80%, the health screening examinations are representative of the Korean population. Furthermore, the Korean government furnishes anonymized health examination data to researchers23.
Our study protocol was approved by the Institutional Review Board of Samsung Medical Center (IRB No. SMC 2022-06-141). The requirement for informed consent was waived by the IRB of Samsung Medical Center because the NHIS database uses an anonymous patient identification system. Our study complied with the Declaration of Helsinki and is reported according to the STROBE reporting guidelines for epidemiologic studies.
Study population and characteristics
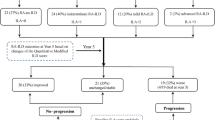
To establish the RA cohort, from among 119,788 individuals diagnosed with RA between 2010 and 2017, we initially included 64,457 individuals who were diagnosed with RA and had health screening examination data within 2 years before RA diagnosis or follow-up between 2010 and 2017 for comprehensive analyses considering various confounders (e.g., BMI, personal habits including smoking status, alcohol consumption, or physical activity). We exclude those with other rheumatic diseases (n = 213) and those with missing health screening examination data (n= 2,321). Since the development of RA-ILD is relatively rare in young individuals30,31, and health screening examinations were only offered to individuals aged 40 years or older prior to 201923, we excluded those younger than 40 years (n = 5,489). After further excluding those who had previously been diagnosed with cystic fibrosis (n = 18) or ILD (n = 2,253) and those who were diagnosed with ILD within 1 year after RA diagnosis (n = 1,072) to minimize the risk of reverse causality, we identified 53,091 individuals for the RA cohort. Of these, 52,325 individuals were eligible for 1:5 age- and sex-matching.
To establish age- and sex-matched controls, of the 1,207,831 individuals who were approximately 1:10 age-and sex-matched to the 119,788 individuals with RA, we included 677,322 who underwent health screening examinations in the same year that matched RA patients received health screening examinations. After excluding (1) 193,902 individuals aged < 40 years and 30,705 with missing health screening examination data, (2) 51 individuals with cystic fibrosis, (3) 20 individuals with other rheumatic diseases, (4) 2,084 individuals diagnosed with ILD before the index date, and (5) 2,529 individuals diagnosed with ILD within 1 year after the index date, 448,031 individuals remained in the matched controls. Of these, 261,625 individuals were eligible for 1:5 age and sex matching for the RA cohort (Fig. 2).
Study exposure
The exposure of this study was RA, which included both SPRA and SNRA. To identify subjects with SPRA and SNRA, a separate operational definition was applied to each group32,33,34,35. The NHIS operates the Rare and Intractable Disease (RID) program for patients with certain diseases, which provides beneficial cost reduction for relevant medical expenses. For subjects with RA, SPRA are eligible to be registered in the RID program only if the following requirements are satisfied: a positive result of RF or anti-CCP and a clinician’s official document certifying that the patient meets the classification criteria for RA. SPRA patients were defined based on whether their claim records included the ICD-10 diagnostic code M05, the RID registration code V223, and a record of prescriptions for any disease-modifying anti-rheumatic drugs (DMARDs), including conventional synthetic DMARDs (methotrexate, hydroxychloroquine, leflunomide, sulfasalazine, tacrolimus, cyclosporine, D-penicillamine, bucillamine, azathioprine, minocycline, or mizoribine), biologic DMARDs (adalimumab, etanercept, infliximab, golimumab, rituximab, abatacept, tocilizumab), or targeted synthetic DMARDs (tofacitinib) for at least 180 days. For SNRA, subjects who visited the hospitals with diagnostic codes of ICD-10 M06, except M06.1 and M06.4, and had a prescription record of DMARDs for ≥ 180 days were defined as patients with newly diagnosed SNRA32,33,34,35. The index date was defined as when the RA-related diagnostic code was first registered.
Study outcome
The outcome of this study was the incidence of ILD. ILD was defined as claims under the ICD-10 diagnosis code J8436,37,38. The study participants were followed from 1 year after RA diagnosis (or corresponding index date for matched controls) to the date of ILD diagnosis, censored date, or 31 December 2019.
Covariates
Data for basal characteristics of age, sex, BMI, smoking status, alcohol status, physical activity, income status, and comorbidities were collected from the dataset. Household income was categorized into quartiles based on insurance premium levels (in Korea, insurance premiums are determined by income level), with those covered by Medical Aid (poorest 3%) being merged into the lowest income quartile39,40. The lowest income quartile group was defined as “low income”. Personal behaviors, including smoking status, alcohol consumption, and physical activity were determined based on a self-reported questionnaire. Smoking status was divided into never, ex-, and current smoker41, and subjects with a history of smoking were subdivided into groups based on 20 PY. Alcohol consumption was divided into none, 1–2 times a week, 3–4 times a week, and almost every day. “Regular exercise” was defined as a moderate-intensity exercise for more than 5 days per week or vigorous exercise for more than 3 days per week26,42,43. BMI was calculated as body weight divided by the square of height (kg/m2) and classified into four groups as follows: low (< 18.5 kg/m2), normal (18.5–22.9 kg/m2), overweight (23.0–24.9 kg/m2), obese (≥ 25 mg/k2)44,45,46. The definitions of comorbidities (diabetes mellitus, hypertension, dyslipidemia, chronic kidney disease, ischemic heart disease, and airway diseases [asthma or chronic obstructive pulmonary disease]) were based on ICD-10 codes as previously described47,48,49,50,51,52,53,54,55,56. Additionally, tuberculosis and other rheumatic diseases, including systemic sclerosis, systemic lupus erythematosus, dermatomyositis, mixed connective tissue diseases, and polymyalgia rheumatica, were defined using ICD-10 codes as well as registration with the national RID supporting program25,27,29,33,34,35,39,47,57 (Supplementary Table 2).
Statistical analysis
Descriptive statistics are presented as number (percentage) for categorical variables and mean ± SD for continuous variables. We compared the two groups using the χ2 test for categorical variables, and t-tests for continuous variables. The incidence rates of ILD were calculated by dividing the number of incident events by the total follow-up period (1,000 person-years). A cumulative incidence plot was used to compare the incidence of ILD between the RA and matched cohorts, and a log-rank test was used to evaluate significant differences between the groups.
The risk of incident ILD in the RA cohort compared to the matched cohort was estimated using univariable and multivariable Cox proportional hazards regression analyses. In multivariable models, potential variables that may affect the development of RA as well as ILD were adjusted; model 1 was adjusted for age, sex, low income, smoking, alcohol drinking, physical activity, and body mass index that can be obtained from health screening examination. Model 2 was further adjusted for common medical comorbid conditions (diabetes mellitus, hypertension, dyslipidemia, chronic kidney disease, myocardial infarction, airway diseases, and tuberculosis). Model 3 used a fine gray competitive risk model, calibrating all the variables that were adjusted in Model 2.
Stratified analyses were performed by basal demographics (sex, age, body mass index [BMI], and income status), personal habits (smoking, alcohol drinking, and regular exercise), and tuberculosis. Additionally, all these analyses were performed in equally divided groups according to RA serologic status. A two-sided p-value < 0.05 was considered statistically significant. All statistical analyses were performed using Statistical Analysis System (SAS) version 9.4 (SAS Institute Inc., Cary, NC, USA. http://www.sas.com).
Ethical approval and consent to participate
The study protocol was approved by the Institutional Review Board of Samsung Medical Center (IRB No. SMC 2022-06-141). The requirement for informed consent was waived by the IRB of Samsung Medical Center because the NHIS database uses an anonymous patient identification system.
Data availability
The data that support the findings of this study are available from the Korean National Health Insurance Service. Restrictions apply to the availability of these raw data, which were used under license for the current study, and so are not publicly available. Data are however available from the corresponding authors upon reasonable request and with permission of the Korean National Health Insurance Service.
Abbreviations
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- DM:
-
Diabetes mellitus
- DMARD:
-
Disease-modifying antirheumatic drug
- HR:
-
Hazard ratio
- ICD-10:
-
International Classification of Disease 10th revision
- ILD:
-
Interstitial lung disease
- NHIS:
-
National Health Insurance Service
- RA:
-
Rheumatoid arthritis
- RID:
-
Rare intractable disease
- SD:
-
Standard deviation
- SNRA:
-
Seronegative rheumatoid arthritis
- SPRA:
-
Seropositive rheumatoid arthritis
References
Shaw, M., Collins, B. F., Ho, L. A. & Raghu, G. Rheumatoid arthritis-associated lung disease. Eur. Respir Rev. 24, 1–16 (2015).
Bongartz, T. et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum. 62, 1583–1591 (2010).
Hyldgaard, C., Ellingsen, T., Hilberg, O. & Bendstrup, E. Rheumatoid arthritis-Associated interstitial lung disease: clinical characteristics and predictors of Mortality. Respiration 98, 455–460 (2019).
Doyle, T. J. et al. Functional impact of a spectrum of interstitial lung abnormalities in rheumatoid arthritis. Chest 146, 41–50 (2014).
Raimundo, K. et al. Rheumatoid arthritis-interstitial lung disease in the United States: prevalence, incidence, and Healthcare costs and mortality. J. Rheumatol. 46, 360–369 (2019).
Albrecht, K., Strangfeld, A., Marschall, U. & Callhoff, J. Interstitial lung disease in rheumatoid arthritis: incidence, prevalence and related drug prescriptions between 2007 and 2020. RMD Open. 9, e002777 (2023).
Fidler, L. et al. Rheumatoid arthritis associated interstitial lung disease: Trends in epidemiology and mortality in Ontario from 2000 to 2018. Respir Med. 107282. https://doi.org/10.1016/j.rmed.2023.107282 (2023).
Sparks, J. A. et al. Prevalence, incidence and cause-specific mortality of rheumatoid arthritis-associated interstitial lung disease among older rheumatoid arthritis patients. Rheumatol. (Oxford). 60, 3689–3698 (2021).
Kim, S. K., Park, S. H., Shin, I. H. & Choe, J. Y. Anti-cyclic citrullinated peptide antibody, smoking, alcohol consumption, and disease duration as risk factors for extraarticular manifestations in Korean patients with rheumatoid arthritis. J. Rheumatol. 35, 995–1001 (2008).
Nyhäll-Wåhlin, B. M. et al. High disease activity disability burden and smoking predict severe extra-articular manifestations in early rheumatoid arthritis. Rheumatol. (Oxford). 48, 416–420 (2009).
Yang, J. A. et al. Clinical characteristics associated with occurrence and poor prognosis of interstitial lung disease in rheumatoid arthritis. Korean J. Intern. Med. 34, 434–441 (2019).
Aubart, F. et al. High levels of anti-cyclic citrullinated peptide autoantibodies are associated with co-occurrence of pulmonary diseases with rheumatoid arthritis. J. Rheumatol. 38, 979–982 (2011).
Natalini, J. G. et al. Autoantibody Seropositivity and Risk for interstitial lung disease in a prospective male-predominant rheumatoid arthritis cohort of U.S. Veterans. Ann. Am. Thorac. Soc. 18, 598–605 (2021).
Hyldgaard, C. et al. A population-based cohort study of rheumatoid arthritis-associated interstitial lung disease: comorbidity and mortality. Ann. Rheum. Dis. 76, 1700–1706 (2017).
Sokka, T. et al. Women, men, and rheumatoid arthritis: analyses of disease activity, disease characteristics, and treatments in the QUEST-RA study. Arthritis Res. Therapy. 11, R7 (2009).
Innala, L. et al. Age at onset determines severity and choice of treatment in early rheumatoid arthritis: a prospective study. Arthritis Res. Ther. 16, R94 (2014).
Alarcon-Calderon, A., Vassallo, R., Yi, E. S. & Ryu, J. H. Smoking-related interstitial Lung diseases. Immunol. Allergy Clin. North. Am. 43, 273–287 (2023).
Zhu, J., Zhou, D., Yu, M. & Li, Y. Appraising the causal role of smoking in idiopathic pulmonary fibrosis: a mendelian randomization study. Thorax. 79, 179–181 (2023).
Zhang, M., Yin, J. & Zhang, X. Factors associated with interstitial lung disease in patients with rheumatoid arthritis: a systematic review and meta-analysis. PLoS One. 18, e0286191 (2023).
Sokolove, J. et al. Increased inflammation and disease activity among current cigarette smokers with rheumatoid arthritis: a cross-sectional analysis of US veterans. Rheumatology 55, 1969–1977 (2016).
Doyle, T. J. et al. Detection of rheumatoid arthritis-interstitial lung disease is enhanced by serum biomarkers. Am. J. Respir Crit. Care Med. 191, 1403–1412 (2015).
Wang, Y. et al. The role of lung ultrasound B-lines and serum KL-6 in the screening and follow-up of rheumatoid arthritis patients for an identification of interstitial lung disease: review of the literature, proposal for a preliminary algorithm, and clinical application to cases. Arthritis Res. Ther. 23, 212 (2021).
Shin, D. W., Cho, J., Park, J. H. & Cho, B. National General Health Screening Program in Korea: history, current status, and future direction a scoping review. Precis Future Med. 6, 9–31 (2022).
Kim, B. G. et al. Increased risk of New-Onset Asthma after COVID-19: a Nationwide Population-based Cohort Study. J. Allergy Clin. Immunol. Pract. 12, 120–132e125 (2024).
Choi, H. et al. Long-term mortality of tuberculosis survivors in Korea: a Population-based Longitudinal Study. Clin. Infect. Dis. 76, e973–e981 (2023).
Lee, H. et al. Overall and respiratory mortality reduction with physical activity in subjects with and without asthma. Allergy 78, 1677–1680 (2023).
Yoo, J. E. et al. Tuberculosis and risk of Parkinson’s disease: a nationwide cohort study. Pulmonology 29, 250–252 (2023).
Kim, Y. et al. Gastroesophageal reflux disease increases susceptibility to Nontuberculous Mycobacterial Pulmonary Disease. Chest 163, 270–280 (2023).
Lee, H. R. et al. Tuberculosis and the risk of ischemic heart disease: a Nationwide Cohort Study. Clin. Infect. Dis. 76, 1576–1584 (2023).
Kelly, C. A. et al. Rheumatoid arthritis-related interstitial lung disease: associations, prognostic factors and physiological and radiological characteristics–a large multicentre UK study. Rheumatol. (Oxford). 53, 1676–1682 (2014).
Mohning, M. P. et al. Duration of rheumatoid arthritis and the risk of developing interstitial lung disease. ERJ Open. Res. 7, 00633–2020 (2021).
Cho, S. K. et al. Development of an algorithm for identifying rheumatoid arthritis in the Korean National Health Insurance claims database. Rheumatol. Int. 33, 2985–2992 (2013).
Choi, H. et al. Impact of rheumatoid arthritis and seropositivity on the risk of Non-cystic Fibrosis Bronchiectasis. Chest. 165, 1330–1340 (2024).
Chung, C. et al. Rheumatoid arthritis increases the risk of COPD: a Nationwide Retrospective Cohort Study. Chest. 165, 1362–1371 (2024).
Cho, M. H. et al. Rheumatoid arthritis and risk of Lung Cancer: a Nationwide Cohort Study. J. Thorac. Oncol. 19, 216–226 (2024).
Taehee, K. et al. Does COVID-19 vaccination increase the risk of ILD in a population level? ERJ Open. Res. 10, 00690–02023 (2024).
Kim, B. G. et al. Risk of newly diagnosed interstitial lung disease after COVID-19 and impact of vaccination: a nationwide population-based cohort study. Front. Public. Health. 11, 1295457 (2023).
Lee, H. et al. Interstitial lung disease increases susceptibility to and severity of COVID-19. Eur. Respir J. 58, 2004125 (2021).
Moon, S. M. et al. Increased Lung Cancer Risk and Associated Risk factors in tuberculosis survivors: a Korean Population-based study. Clin. Infect. Dis. 77, 1329–1339 (2023).
Yoo, J. E. et al. Diabetes Status and Association with risk of tuberculosis among Korean adults. JAMA Netw. Open. 4, e2126099 (2021).
Park, H. Y. et al. Impact of chronic obstructive pulmonary disease on mortality: a large national cohort study. Respirology 25, 726–734 (2020).
Yang, B. et al. Impacts of regular physical activity on hospitalisation in chronic obstructive pulmonary disease: a nationwide population-based study. BMJ Open. Respir Res. 11, e001789 (2024).
Yoo, J. E. et al. Anemia, Sarcopenia, physical activity, and the risk of tuberculosis in the older population: a nationwide cohort study. Ther. Adv. Chronic Dis. 12, 20406223211015959 (2021).
Kim, S. H. et al. Association between non-cystic fibrosis bronchiectasis and the risk of incident dementia: a nationwide cohort study. Chron. Respir Dis. 20, 14799731231222282 (2023).
Choi, H. et al. Body Mass Index, Diabetes, and risk of tuberculosis: a retrospective cohort study. Front. Nutr. 8, 739766 (2021).
Yang, B. et al. Being Underweight increases the risk of non-cystic fibrosis bronchiectasis in the Young Population: a Nationwide Population-based study. Nutrients. 13, 3206 (2021).
Yang, B. et al. Systemic sclerosis and risk of bronchiectasis: a nationwide longitudinal cohort study. Arthritis Res. Ther. 25, 209 (2023).
Kim, T. et al. Impact of allergic disease on the risk of Mycobacterial Disease. J. Allergy Clin. Immunol. Pract. 11, 2830–2838e2834 (2023).
Kim, B. G. et al. Risk of Ischemic Heart Disease in Chronic Obstructive Pulmonary Disease: a Nationwide Cohort Study. J. Korean Med. Sci. 38, e344 (2023).
Yeo, Y. et al. Risk of dementia in survivors of active tuberculosis in Korea: a nationwide cohort study. J. Infect. Public. Health. 17, 286–292 (2024).
Lee, H. et al. Impact of air pollution on healthcare utilization in patients with bronchiectasis. Front. Med. (Lausanne). 10, 1233516 (2023).
Choi, H. et al. Increased mortality in patients with non cystic fibrosis bronchiectasis with respiratory comorbidities. Sci. Rep. 11, 7126 (2021).
Choi, H. et al. Bronchiectasis and increased mortality in patients with corticosteroid-dependent severe asthma: a nationwide population study. Ther. Adv. Respir Dis. 14, 1753466620963030 (2020).
Yang, B. et al. Increased incidence and Associated Risk factors of aspergillosis in patients with Bronchiectasis. J. Pers. Med. 11, 422 (2021).
Lee, H. et al. Coexisting COPD increases mortality in patients with corticosteroid-dependent asthma: a Nationwide Population-based study. Allergy Asthma Immunol. Res. 12, 821–831 (2020).
Yang, B. et al. Risk of Coronavirus Disease 2019 occurrence, severe presentation, and mortality in patients with Lung Cancer. Cancer Res. Treat. 53, 678–684 (2021).
Jo, Y. S. et al. Relationship between total cholesterol level and tuberculosis risk in a nationwide longitudinal cohort. Sci. Rep. 11, 16254 (2021).
Funding
No funding.
Author information
Authors and Affiliations
Contributions
H.K. and D.W.S. are the guarantors of the manuscript and take responsibility for the content of the manuscript, including the data and analysis.B.-G.K., H.L., K.H., H.K., and D.W.S. contributed to the conception and design of the study.B.-G.K., H.L., Y.E., K.H., J.-H.J., H.C., H.K., and D.W.S. were involved in the collection and interpretation of the data.K.H. and J.-H.J. were involved in statistical analyses.B.-G.K. and H.L. were major contributors to writing the manuscript.All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kim, BG., Lee, H., Eun, Y. et al. Association between rheumatoid arthritis and interstitial lung disease and impact of serologic status: a large-scale longitudinal study. Sci Rep 15, 4885 (2025). https://doi.org/10.1038/s41598-025-88323-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-88323-7