Abstract
To report the results of a single-arm, prospective partial breast irradiation (PBI) trial from China mainland using a dose of 40.05 Gy in 15 fractions delivered with intensity-modulated radiation therapy (IMRT) technique for patients with early stage breast cancer. Patients aged ≥ 50 years who underwent breast-conserving surgery for unifocal non-lobular invasive breast cancer, with pathological T1 disease, clear margins, negative axillary nodes, and positive hormonal receptors, were recruited. The primary endpoint was 3-year cosmetic deterioration, and secondary endpoints included adverse events, ipsilateral breast tumor recurrence (IBTR), regional recurrence, and survivals. This trial is registered with ClinicalTrials.gov (registration No. NCT03411174). From Jan of 2015 to July of 2018, 208 out of 222 patients recruited were evaluable and included in final analysis. The median follow-up was 66.3 (range: 42.0-105.4) months. The 3-year overall cosmetic deterioration rate was 3.5%. The rates of grade 2 radiation dermatitis and breast induration was 5.8% and 1.5%, respectively. No one experienced ≥ grade 2 breast pain, edema, or telangiectasia. The 5-year cumulative incidence of IBTR and RR was 0.5%. No one developed DM. The 5-year DFS was 99.0%. Four patients died from non-breast cancer causes, and the 5- year OS was 97.9%. In conclusion, we observed lower rates of cosmetic deterioration, IBTR, and ≥ grade 2 acute/late normal tissue effects following PBI with a moderately hypofractionated regimen delivered with IMRT technique. Therefore, this regimen represents an attractive option when an external beam PBI approach is chosen to treat a patient with low-risk early breast cancer.
Similar content being viewed by others
Background
Adjuvant breast radiation therapy (RT) after breast-conserving surgery (BCS) has been shown to reduce the risk of any recurrence and improve survival in patients with early stage breast cancer1. Whole breast irradiation (WBI) is the most widely used treatment in China and internationally so far. With the accumulating evidence from large-scale randomized clinical trials (RCTs) that various partial breast irradiation (PBI) regimens provide local control that is not inferior to WBI at long-term follow-up2,3,4,5, PBI is becoming an alternative treatment of WBI for suitable patients6,7,8,9. In addition to brachytherapy2 and intraoperative RT (IORT) techniques10,11, external beam RT (EBRT)-based PBI has also been thoroughly investigated in several RCTs3,4,5,12,13,14. However, the optimal schedule of EBRT-based PBI to achieve the best balance between disease control and adverse effects including toxicity and cosmesis remains to be a challenge given some conflicting results3,4,5,12. Therefore, efforts should be made to identify a suitable, safe PBI prescription and delivery technique to avoid the documented cosmetic deterioration and more late toxicity observed in the RAPID and the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-39/Radiation Therapy Oncology Group (RTOG) 0413 (NSABP B-39/RTOG 0413) trials4,5.
We present the results of a single-arm, prospective PBI trial from China mainland using 40.05 Gy in 15 fractions delivered with inverse intensity-modulated RT (IMRT) technique for patients with early stage breast cancer.
Materials and methods
Patients
Female patients were considered eligible for the trial if they were aged ≥ 50 years, underwent breast-conserving surgery for newly diagnosed, unilateral, unifocal, non-lobular invasive breast cancer, with pathologically confirmed primary tumor size ≤ 20 mm in maximum diameter (pT1), estrogen receptor (ER) positive (> 1%), surgical margins ≥ 2 mm in any directions, tumor bed labeled with clips, and nodes negative (pN0). Adjuvant systemic therapies including chemotherapy were allowed if indicated according to international and/or institutional treatment protocols. Patients were excluded if they were aged < 50 years, with serious comorbidities, with previous breast cancer or other malignant tumor history, previously received radiotherapy to the breast or thorax, or underwent neoadjuvant chemotherapy. A written informed consent was obtained for each patient.
Radiotherapy
At computed-tomography (CT) simulation, patient was positioned supine on a breast board with arms fully abducted and head secured. The tumor bed (TB) was delineated under the guidance of surgical clips placed in the lumpectomy cavity during surgery, seroma if visible, and preoperative imaging. The clinical target volume (CTV) was created by adding a uniform 15 mm margin to each TB in any directions, limited deeply by chest wall and superficially by 3–5 mm beneath skin surface. Planning target volume (PTV) was created by expanding a 5 mm margin to the CTV, but was limited to 3–5 mm within the skin. The bilateral breasts, bilateral lungs, heart, and spinal cord were contoured as organs at risk.
The PBI plan was developed using Philips Pinnacle (version 9.10) or Varian Eclipse (version 11.0) treatment planning system (TPS). A dose of 40.05 Gy was prescribed to PTV in 15 fractions over three weeks. The PTV was to receive 95% of the prescription dose to 98% of the volume using a class solution, 5–7 beam angle, IMRT technique. Dosimetric characteristics including PTV coverage, dose conformity, and dose to ipsilateral breast, lung and heart were evaluated. The treatment was performed using Elekta or Varian linear accelerator, and patient positioning was verified using cone beam CT (CBCT) before each fraction. Corrections were executed if set-up error was 5 mm or more.
PBI was started within 12 weeks from the surgery; if adjuvant chemotherapy was administered, radiotherapy was started after 4 weeks from the last chemotherapy cycle. Anti-HER2 targeted therapy was allowed to use concurrently with PBI if indicated.
Outcomes
Primary endpoint of the study was to evaluate the 3-year cosmetic deterioration; secondary endpoints included acute and late normal tissue effects, 5-year ipsilateral breast tumor recurrence (IBTR), regional recurrence (RR), distant metastasis (DM), disease-free survival (DFS), overall survival (OS), and second primary cancers.
All patients were evaluated at baseline, and followed up every 3 months after PBI for 2 years, every 6 months for the next 3 years, and annually thereafter. Clinical examinations were performed at each follow-up visit; mammography was typically done annually.
Cosmetic outcome was assessed by the physician using 4-category Harvard Breast Cosmesis Scale15 based on clinical examination and photograph taken at each follow-up visit. An excellent cosmetic score was assigned when the treated breast looked like the contralateral one; a good score was assigned for minimal but identifiable radiation effects of the treated breast; a fair score was used if significant radiation effects were readily observable; a poor score was used for severe sequelae due to radiation effects. The Cosmetic deterioration was defined to be a change of overall cosmetic score from excellent or good at baseline to fair or poor at 1, 2, and 3 year post-PBI.
Acute radiation toxicity refers to the immediate or early harmful effects on healthy tissues that occur after exposure to radiation, typically within hours or a few weeks. Specifically, acute radiation dermatitis was assessed weekly within one month of PBI completion using common terminology criteria for adverse events (CTCAE) version 4.0. Late radiation toxicity refers to the delayed, often chronic damage to healthy tissues that occurs months to years after exposure to radiation. Specifically, at 3 years post-PBI, breast pain, edema, telangiectasia, and induration were assessed using the late radiation morbidity scoring scheme from the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC)16; and lymphedema of ipsilateral upper arm was assessed using the International Society of Lymphology Staging System17.
Local recurrence (LR) was defined as the reappearance of disease in the index quadrant, and a new primary (NP) was defined as in other quadrants of the same breast. The sum of LR and NP was defined as IBTR. Regional recurrence (RR) was defined as any recurrence in regional nodes including the ipsilateral axilla, supraclavicular region, and internal mammary chains. Distant metastasis (DM) was defined as any recurrence to distant organs.
The study was approved by the institutional ethnical committee, and registered with ClinicalTrials.gov on 26/01/2018 (registration number NCT03411174). All methods were performed in accordance with the relevant guidelines and regulations.
Statistical analysis
Patient characteristics were described as medians and ranges for continuous variables, and as frequencies and percentages for categorical variables using descriptive method. Nonparametric estimates of the IBTR, RR, LRR, DM, and DFS were estimated using the Kaplan-Meier method. A p-value of less than 0.05 was considered statistically significant. All the analyses were performed using the statistical software SPSS 25.0.
Results
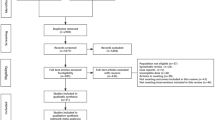
From Jan of 2015 to July of 2018, 222 patients were enrolled onto this trial. 14 patients were excluded because of protocol violations, withdrawal of consent or loss of follow-up, leaving 208 patients (93.6%) for final analyses of cosmesis, morbidity, and any cancer event.
The median age for patients included in the analysis was 62 (range: 50–77) years. The majority were post-menopausal, underwent BCS without oncoplastic surgery (OPS), and axillary sentinel lymph node biopsy (SLNB). Most patients had T1b-c, histological grade 1–2 disease, with negative lymph-vascular invasion (LVI). All patients had positive hormone receptor and negative nodal status. Following surgery, 31.3% of patients received adjuvant chemotherapy; all patients received adjuvant endocrine therapy; and 6 out of 7 HER2 positive patients received adjuvant trastuzumab. Of the 65 patients who received chemotherapy, 27 had grade 3 histology, 10 were LVI positive, 55 had high Ki-67 index, and 7 were HER2 positive. Patient, tumor, and treatment-related characteristics at baseline were shown in Table 1. As of Jan 1, 2024, the median time of follow-up was 66.3 (range: 42.0–105.4) months.
Cosmetic outcome
Table 2 shows physician-assessed cosmetic outcome. Data for physician-rated cosmesis at baseline, 1, 2, and 3 year follow-up were available for 199 patients. Overall cosmesis was excellent or good in 178 patients (89.4%), and fair or poor in 21 patients (10.6%) at baseline. Cosmetic deterioration was observed in 2 patient at 1 year, 4 patients at 2 years, and 1 patient at 3 years post-PBI. The 3-year overall cosmetic deterioration rate was 3.5%. Table 3 shows the background of patients with deteriorated cosmesis.
Acute/late normal tissue effects
Table 4 shows acute and late normal tissue effects. Data for acute skin toxicity, i.e., radiation dermatitis within 1 month of PBI completion, were available for 206 patients. Grade 2 radiation dermatitis was reported in 12 patients (5.8%). No Grade 3–4 radiation dermatitis was observed.
Data for late toxicities at 3 years post-PBI were available for 199 patients. None of them experienced grade 2 or above breast pain, edema, or telangiectasia. Grade 2 breast induration was observed in 3 patients (1.5%). Stage 0 lymphedema was observed in 12 patients, including 10 undergoing SLNB and 2 undergoing axillary lymph node dissection (ALND); and Stage 1 lymphedema was observed in 1 patient (0.5%) who underwent ALND.
Disease control and survival
At 5-year follow-up, one patient was reported to develop a LR, and underwent salvage mastectomy; another patient was reported to have axillary recurrence, and underwent salvage ALND. The cumulative incidence of IBTR and RR at 5 years was 0.5%. The overall 5-year LRR rate was 1%. No DM was recorded. The 5-year DFS was 99.0% (95% CI: 97.5-99.7%).
At the time of analysis, four patients (1.9%) had died, all from non-breast cancer causes, including 2 from cerebrovascular accident, 1 from lung cancer, and 1 from COVID-19 (Corona Virus Disease 2019). No one had died from breast cancer. The median time of survival was 60.2 (IQR: 51.3–71.3) months, and the 5-year OS was 97.9% (95% CI: 96.5-99.4%).
Second primary malignancies
There were 3 patients with second primary cancer reported, including 1 with lung cancer, 1 with rectal cancer, and 1 with gastric cancer. No contralateral BC was noted.
Discussions
With the rapid advances in breast radiotherapy, the paradigm of local management of breast cancer has changed drastically, and moved toward individualized precision medicine strategies according to risk stratification18,19,20,21,22. In this context, PBI represents one of de-escalation schemes of RT by limiting the irradiated volume to around the TB and completing treatment within a shorter duration. A suitable PBI regimen is expected to maintain equivalent local control to WBI but without jeopardizing the cosmetic outcome and increasing normal tissue effects.
Main evidence shows that local control of disease following PBI is closely related to an adequate selection of patients. Looking at the baseline characteristics in the GEC-ESTRO2, IMPORT-LOW3, RAPID4, NSABP B-39/RTOG 04135, and APBI-IMRT Florence trials12, which demonstrated the noninferiority of PBI versus WBI in preventing LR, most women who were recruited had small, low-grade, ER-positive, and node-negative tumors. However, in the ELIOT and TARGIT trials, which investigated the application of IORT techniques for delivery of APBI10,11, no strict and clear selection criteria for patients could be used because no final pathology report was available at the time of IORT. This drawback might in part account for the significantly higher event rate for IBTR at 5 years with IORT than with WBI in the ELIOT trial (4.4% vs. 0.4%, hazard ratio 9.3) as well as TARGIT trail (3.3% vs. 1.3%, p = 0.042)11. These data highlight the importance of proper selection of patients. Our trial was designed to recruit low-risk patients largely per the ESTRO and the ASTRO recommendations, and demonstrated a very low IBTR rate with only one event at the 5-year median follow-up. This finding supports that the IMRT-based moderately hypofractionated regimen is oncologically safe for selected patients.
Whereas the cosmetic outcomes and treatment-related normal tissue effects seem to be strongly associated with schedule of choice and delivery technique. The RAPID trial reported that accelerated PBI (APBI) with a dose of 38.5 Gy in 10 fractions delivered twice per day over 5–8 days using 3-dimensional conformal RT (3D-CRT) technique significantly increased the rates of adverse cosmesis defined as fair or poor at 3 years as assessed by trained nurses (29% vs. 17%, p = 0.001) and by patients (26% vs. 18%, p = 0.002), and ≥ grade 2 late radiation toxicity as compared to WBI4. The NSABP B-39/RTOG 0413 trial utilizing an identical APBI regimen to treat the majority (70%) of patients in their APBI arm was published alongside the RAPID trial, and demonstrated similar treatment-related toxicities between the two arms, but greater rate than expected in the APBI arm5. The increased risk of adverse cosmesis and toxicities was probably due to twice-daily therapy allowing insufficient time for repair of normal tissue effects, and inhomogeneous dose distribution associated with 3D-CRT treatment planning.
In contrast, favorable toxicity profile and cosmesis was noted in multiple PBI trials using IMRT technique. The Randomized Phase III APBI-IMRT Florence trial employing a prescription of 30 Gy in 5 nonconsecutive once-daily fractions, displayed significantly better results with respect to acute toxicity (2% vs. 31.2%), late toxicity (0% vs. 2.7%), and adverse cosmesis as evaluated by both physicians (0% vs. 1.9%) and patients (0.8% vs. 14.6%) in the APBI arm as compared to WBI arm12. The UK IMPORT LOW trial explored a moderately hypofractionated PBI regimen with a dose of 40 Gy in 15 daily fractions over 3 weeks using standard tangential fields and forward field-in-field IMRT technique, and showed significantly lower rates of 5-year breast appearance changed (35.1% in PBI vs. 47.7% in WBI, p < 0.0001) and breast harder or firmer (15.3% in PBI vs. 35.3% in WBI, p = 0.024) in the PBI group compared with WBI group3. The Danish Breast Cancer Group (DBCG) PBI trial tested whether PBI 40 Gy/15 fractions was noninferior to WBI 40 Gy/15 fractions in terms of breast induration using similar delivery technique, and reported a significantly lower rate of grade 2–3 breast induration at 3 years in the PBI arm compared with WBI arm (5.1% in PBI vs. 9.7% in WBI, p = 0.014)13. These favorable outcomes might be ascribed to improved dose homogeneity by IMRT treatment planning. Our trial employed a PBI regimen of 40.05 Gy/15 fractions delivered by IMRT technique, and reported very low rates of cosmetic deterioration and ≥ grade 2 acute/late normal tissue effects. Our finding supports that the IMRT-based moderately hypofractionated regimen is cosmetically acceptable, and clinically safe. Therefore, this moderately hypofractionated schedule represents one of attractive options when an external beam PBI approach is chosen to treat a patient with low-risk early breast cancer. The latest ASTRO PBI guideline recommends 40.05 Gy in 15 daily fractions over 3 weeks or 30 Gy in 5 once daily fractions delivered on nonconsecutive days within 2 weeks for patients with early-stage invasive breast cancer or DCIS receiving external beam PBI23.
Less skin toxicity and improved cosmesis was also noted in GEC-ESTRO trial, which used multicatheter interstitial brachytherapy (MIB) for PBI and allowed both pulsed-dose-rate (PDR) and high-dose-rate (HDR) regimens of 7 and 8 twice daily fractions2,24. The 5-year results demonstrated that MIB is associated with a lower incidence of mild (grade 1–2) and moderate (grade 3) acute dermatitis compared with WBI2; whereas the 10-year results demonstrated that MIB had comparable or higher rates of “good/excellent” cosmesis compared with WBI24. These favorable outcomes might be ascribed to less dose to skin, smaller volume irradiated, and homogeneous dose distribution associated with brachytherapy. The NSABP B-39/RTOG 0413 trial used a regimen of 3400 cGy in 10 fractions delivered twice daily with brachytherapy, but has not yet reported outcomes of the brachytherapy subgroup5. According to the latest ASTRO PBI guideline, 3010 cGy in 7 fractions, 3200 cGy in 8 fractions, or 3400 cGy in 10 fractions delivered twice daily is recommended for patients with early-stage invasive breast cancer or DCIS receiving PBI with HDR brachytherapy23.
Undoubtedly, appropriate PBI techniques (e.g., modality, treatment technique, fractionation regimen, dose per fraction, and total dose) are crucial to improve cosmetic outcomes after PBI23. Other factors, including smoking history, obesity, located in the lower inner quadrant or near the skin surface, larger breast size or lumpectomy cavity, persistent seroma, and acute skin reactions or late fibrosis post PBI, might impact cosmetic outcomes as well25,26. We therefore should consider patient-related factors such as breast size and tumor ___location in treatment planning, encourage smoking cessation and weight management. For patients with larger surgical defects, oncoplastic surgical techniques should be employed to improve breast contour27.
Although this study showed excellent results regarding cosmesis, toxicity, and tumor control, we identified some potential limitations. First, it was based on a relatively small number of patients with a shorter follow-up period. Second, the duration of this moderately hypofractionated PBI regimen lasts 3 weeks, which is relatively long, compared with EBRT-based WBI schedule of 26 Gy in 5 fractions or brachytherapy-based PBI schedule of 34 Gy in 10 fractions delivered in 5–7 days. Our future research will focus on whether a shorter course of PBI maintains comparable toxicity profile and cosmetic outcome for Chinese women patients. Third, due to the limited number of patients with cosmetic deterioration, it’s not feasible to conduct a multivariate analysis to find risk factors for worse cosmesis.
Conclusions
In conclusion, we observed lower rates of cosmetic deterioration, IBTR, and ≥ grade 2 acute/late normal tissue effects following PBI with a dose of 40.05 Gy in 15 fractions delivered with IMRT technique. Therefore, this moderately hypofractionated regimen represents an attractive option when an external beam PBI approach is chosen to treat a patient with low-risk early breast cancer in China mainland. However, we admit that the result was based on a relatively small number of patients with a shorter follow-up period, and the schedule is relatively long. Our future research will focus on whether a shorter PBI schedule maintains comparable toxicity profile and cosmetic outcome for Chinese women patients.
Data availability
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Abbreviations
- LVI:
-
Lymph-vascular invasion
- ER:
-
Estrogen receptor
- PR:
-
Progesterone receptor
- OPS:
-
Oncoplastic surgery
- SLNB:
-
Sentinel lymph node biopsy
- ALND:
-
Axillary lymph node dissection
- HER2:
-
Human epidermal receptor 2
References
Early Breast Cancer Trialists’ et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 378(9804), 1707–1716 (2011).
Strnad, V. et al. 5-year results of accelerated partial breast irradiation using sole interstitial multicatheter brachytherapy versus whole-breast irradiation with boost after breast-conserving surgery for low-risk invasive and in-situ carcinoma of the female breast: A randomised, phase 3, non-inferiority trial. Lancet 387(10015), 229–238 (2016).
Coles, C. E. et al. Partial-breast radiotherapy after breast conservation surgery for patients with early breast cancer (UK IMPORT LOW trial): 5-year results from a multicentre, randomised, controlled, phase 3, non-inferiority trial. Lancet 390(10099), 1048–1060 (2017).
Whelan, T. J. et al. External beam accelerated partial breast irradiation versus whole breast irradiation after breast conserving surgery in women with ductal carcinoma in situ and node-negative breast cancer (RAPID): A randomised controlled trial. Lancet 394(10215), 2165–2172 (2019).
Vicini, F. A. et al. Long-term primary results of accelerated partial breast irradiation after breast-conserving surgery for early-stage breast cancer: A randomised, phase 3, equivalence trial. Lancet 394(10215), 2155–2164 (2019).
Correa, C. et al. Accelerated partial breast irradiation: Executive summary for the update of an ASTRO evidence-based Consensus Statement. Pract. Radiat. Oncol. 7(2), 73–79 (2017).
Strnad, V. et al. DEGRO practical guideline for partial-breast irradiation. Strahlenther Onkol. 196(9), 749–763 (2020).
Smith, B. D. et al. Accelerated partial breast irradiation consensus statement from the American Society for Radiation Oncology (ASTRO). Int. J. Radiat. Oncol. Biol. Phys. 74(4), 987–1001 (2009).
Polgar, C. et al. Patient selection for accelerated partial-breast irradiation (APBI) after breast-conserving surgery: Recommendations of the Groupe Europeen De Curietherapie-European Society for Therapeutic Radiology and Oncology (GEC-ESTRO) breast cancer working group based on clinical evidence (2009). Radiother Oncol. 94(3), 264–273 (2010).
Vaidya, J. S. et al. Effect of delayed targeted intraoperative Radiotherapy vs whole-breast radiotherapy on local recurrence and survival: Long-term results from the TARGIT-A randomized Clinical Trial in early breast Cancer. JAMA Oncol. 6(7), e200249 (2020).
Veronesi, U. et al. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): A randomised controlled equivalence trial. Lancet Oncol. 14(13), 1269–1277 (2013).
Meattini, I. et al. Accelerated partial-breast irradiation compared with whole-breast irradiation for early breast Cancer: Long-term results of the Randomized Phase III APBI-IMRT-Florence Trial. J. Clin. Oncol. 38(35), 4175–4183 (2020).
Offersen, B. V. et al. Partial breast irradiation Versus whole breast irradiation for early breast Cancer patients in a Randomized Phase III Trial: The Danish breast Cancer Group partial breast irradiation trial. J. Clin. Oncol. 40(36), 4189–4197 (2022).
Livi, L. et al. Accelerated partial breast irradiation using intensity-modulated radiotherapy versus whole breast irradiation: 5-year survival analysis of a phase 3 randomised controlled trial. Eur. J. Cancer. 51(4), 451–463 (2015).
Trombetta, M., Julian, T. B., Kim, Y., Werts, E. D. & Parda, D. The allegheny general modification of the Harvard Breast Cosmesis Scale for the retreated breast. Oncol. (Williston Park). 23(11), 954–956 (2009).
Cox, J. D., Stetz, J. & Pajak, T. F. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int. J. Radiat. Oncol. Biol. Phys. 31(5), 1341–1346 (1995).
Executive Committee of the International Society of L. The diagnosis and treatment of peripheral lymphedema: 2020 Consensus Document of the International Society of Lymphology. Lymphology 53(1), 3–19 (2020).
Haviland, J. S. et al. The UK standardisation of breast radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 14(11), 1086–1094 (2013).
Offersen, B. V. et al. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer, version 1.1. Radiother Oncol. 118(1), 205–208 (2016).
Strnad, V. et al. Recommendations from GEC ESTRO breast Cancer Working Group (I): target definition and target delineation for accelerated or boost partial breast irradiation using multicatheter interstitial brachytherapy after breast conserving closed cavity surgery. Radiother Oncol. 115(3), 342–348 (2015).
Kunkler, I. H., Williams, L. J., Jack, W. J., Cameron, D. A. & Dixon, J. M. investigators PI: Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): A randomised controlled trial. Lancet Oncol 16(3), 266–273 (2015).
Murray Brunt, A. et al. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet 395(10237), 1613–1626 (2020).
Shaitelman, S. F. et al. Partial breast irradiation for patients with early-stage invasive breast Cancer or Ductal Carcinoma in situ: An ASTRO Clinical Practice Guideline. Pract. Radiat. Oncol. 14(2), 112–132 (2024).
Strnad, V. et al. Accelerated partial breast irradiation using sole interstitial multicatheter brachytherapy compared with whole-breast irradiation with boost for early breast cancer: 10-year results of a GEC-ESTRO randomised, phase 3, non-inferiority trial. Lancet Oncol. 24(3), 262–272 (2023).
Goldberg, M. & Whelan, T. J. Accelerated partial breast irradiation (APBI): Where are we now? Curr. Breast Cancer Rep. 12(4), 275–284 (2020).
Peterson, D. et al. Predictors of adverse cosmetic outcome in the RAPID trial: An exploratory analysis. Int. J. Radiat. Oncol. Biol. Phys. 91(5), 968–976 (2015).
Turgeon, M. K., Willcox, L. M., Styblo, T. M. & Losken, A. Impact of oncoplastic surgery on oncologic outcomes in patients with breast Cancer. Plast. Reconstr. Surg. Glob Open. 12(1), e5561 (2024).
Acknowledgements
We appreciate the research nurse, Li Shen, who did excellent work throughout the study.
Author information
Authors and Affiliations
Contributions
JMa designed the trial; JMa, XC, XM, ZY, XY, JMeng, WS, LZ, and ZZ recruited and treated patients; XZ, XW, and ZX contributed equally in data collection, analysis, and manuscript drafting, and shared the first authorship; MM was involved in survival analysis; GL, JW, ZS, XG, and ZZ reviewed the manuscript; JMa reviewed and edited the manuscript; all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, X., Wang, X., Xu, Z. et al. Hypofractionated partial breast irradiation after breast-conserving surgery for patients with early stage breast cancer in China Mainland: a single-arm prospective trial. Sci Rep 15, 3869 (2025). https://doi.org/10.1038/s41598-025-88600-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-88600-5