Abstract
Toxic elements exposure and imbalance in essential element homeostasis remain incomprehensive in esophageal squamous cell carcinoma (ESCC) carcinogenesis, especially in tumor progression. To reveal the toxic and essential elements inside body associated with ESCC occurrence, aggressive features and outcomes, whole blood concentrations of eight trace elements were quantified in 150 ESCC cases and 177 controls using inductively coupled plasma-mass spectrometry (ICP-MS). Concentrations of cadmium (Cd), lead (Pb), chromium (Cr), copper (Cu), arsenic (As), and selenium (Se) showed significant differences between the case and control subjects. The restricted cubic spline (RCS) analysis showed As, Zinc (Zn), and manganese (Mn) was linked with ESCC risk in a U-shaped pattern, whereas an inverted U-shaped curve for Cd (all P-non-linear < 0.05). Contrary to Se, the elements Pb, Cr and Cu were positively associated with ESCC risk. By Bayesian Kernel Machine Regression models, the mixtures of the eight trace elements were found to be significantly associated with ESCC risk and metastasis, with Cr, Mn, Cu, Zn, and Pb having a PIP of 1.000 for occurrence risk and Mn being the main contributor for metastatic risk (PIP = .6570). The weighted quantile sum (WQS) model consistently showed that Cu, Cr, Pb, and Cd ranked as the top four positive elements for ESCC risk. Multivariable logistic regression analysis indicated Pb and As were positively associated with tumor invasion (adjusted OR 3.024 [1.053–8.689]; OR 4.385 [1.271–15.126], respectively), whereas Se had the opposite trend (adjusted OR 0.261 [0.074–0.927). Patients with high Cr, Mn, or Pb showed worse overall survival (OS), and high Mn were linked to inferior progression-free survival (PFS) (all P < 0.05). Zn and Pb, and Mn and Cu were identified as independent prognostic factors for OS and PFS, respectively. This study suggests trace element disbalance in human body contributes to the risk of onset and progression of ESCC, especially in a high-incidence region. Further epidemiological and experimental studies were needed to clarify the probable pathogenic processes underpinning the potential link between trace element mixtures and ESCC.
Similar content being viewed by others
Introduction
Esophageal cancer, a formidable malignancy of the upper digestive tract, is characterized by its aggressive nature and poor prognosis. According to the Global Cancer Statistics for 2022, esophageal cancer ranks as the 11th most commonly diagnosed cancer and the seventh in terms of mortality worldwide, among which esophageal squamous cell carcinoma (ESCC) accounts for nearly 90% of all cases, especially in East Asia1. Approximately half of the global new cases and deaths of ESCC occur in China2. Other areas with high ESCC rates are mainly distributed in East and Central Asia, as well as Africa (2020). Even within China, the incidence of esophageal cancer can vary up to tenfold regionally3. Moreover, the incidence is higher in males than in females, accounting for nearly 70% of all esophageal cancer cases4. Accordingly, geographic and gender variations in incidence is believed to be the most salient epidemiological features of ESCC, strongly implying the specificity in etiology of the disease.
It has been widely recognized that carcinogen exposure and nutritional deficiency are critical risk factors for ESCC, especially in areas with high incidence5,6,7. Trace elements, including essential and non-essential (toxic) elements, have been suspected to either inhibit or accelerate the development of some cancers8,9,10. Extensive research has raised concerns about the contribution of heavy metals, such as arsenic (As), cadmium (Cd), chromium (Cr), and lead (Pb), to esophageal cancer11,12,13. Conversely, the beneficial effects of supplementation of certain essential trace elements, such as zinc (Zn), copper (Cu), iron (Fe), and selenium (Se), have been suggested in the prevention of esophageal cancer14. Notably the epidemiological evidence for specific element in relation to incidence and mortality of ESCC remains controversial. For instance, an inverse association of toenail Se and serum Se levels with ESCC risk as well as death from ESCC has been reported in the Netherlands cohort study15 and the Nutrition Intervention Trial in Linxian, China16, respectively. Nevertheless, the Golestan Cohort study claimed that no significant association between Se concentration in toenails and the risk of ESCC17. Additionally, the correlation between Cd and OS of ESCC patients has yielded inconsistent results in two studies, with one showing a positive association and the other finding no statical significance18. However, to date the potential impact of imbalance in trace element homeostasis on ESCC progression has received little attention. Although causation can only be established through experimental models, an observational study is essential to initially explore the association betweentrace element profiles in human body and the occurrence, aggressiveness features, andoutcomes of ESCC.
The Chaoshan region, comprising four cities (Shantou, Chaozhou, Jieyang and Shanwei) in southeastern China, is considered to be the sole coastal area with a high prevalence of ESCC in China, as its age-standardized incidence rate is higher than that of surrounding region and the rest of China6. More particularly, Nan’ao island, one of the towns in this area, has a much higher age-standardized incidence rate than the national level2,19. Some issues in this region have attracted widespread concerns, including a particular patrilineal background, special eating and lifestyle habits, and environmental heavy metal contamination in the local ___domain20. For instance, it has been demonstrated that the residents of the Chaoshan region share a similar matrilineal genetic background with another high-risk area, the Taihang Mountain area21,22. Moreover, the residents have perennial habits of hot tea drinking and Chinese herb consumption, which are believed to induce esophageal inflammation23 and heavy metal exposure24, respectively. Significantly, the concentrations of multiple heavy metals in the environmental media and human body in this typical area have been addressed to be excessive20,25,26,27,28. Considering the above reasons, assessing the joint and interactive effects of trace element mixtures on ESCC occurrence and progression in this special population will shed light on the implication of trace elements in etiology and carcinogenic mechanism of ESCC.
Superior to linear and logistic regression models, the restricted cubic spline (RCS) can evaluate dose–response associations and assess possible non-linear associations between variables and outcomes. In addition, given the collinearity and potential interaction between multiple elements that should be taken seriously, there is an increasing necessity to use novel statistical approaches, such as Bayesian kernel machine regression (BKMR) and weighted quantile sum (WQS) regression, to estimate the overall effect of a mixture and the exposure–response function containing complicated interactions between elements. Therefore, we comprehensively applied the aforementioned analytical models to evaluate the single and combined effects of trace elements in whole blood on the occurrence, aggressiveness and prognosis of ESCC, and to characterize the dose–response relationship.
Materials and methods
Study population
The current study conducted a case–control study comprising 150 ESCC cases and 177 controls, matched for age and gender, with participants meeting the following inclusion criteria: age 18 or above, permanent residency in the Chaoshan region for at least 10 years, and similar socioeconomic status and dietary habits. Inclusion criteria for the patients were as follows: (1) pathologically diagnosed ESCC; (2) complete clinicopathological and follow-up information. The patients who did not have electronic medical records in our institution or could not be contacted by telephone or community to confirm survival status were excluded. The controls underwent gastroscopy or upper gastrointestinal barium meal examination to exclude malignant tumors of the upper digestive tract. The blood samples of cases were collected from May 2001 to June 2020 at the Cancer Hospital of Shantou University Medical College, while the controls were gathered over the same period at the First Affiliated Hospital of Shantou University Medical College. Before enrollment, all participants were aware of the research content and potential consequences, and then signed an informed consent for this investigation. All patients were interviewed regularly via phone or in-person after confirming the diagnosis of ESCC until June 2020. This study was approved by the Medical Ethics Committee of the Cancer Hospital of Shantou University Medical College (No. 2019026). All participants gave their informed written consent after being notified of the details and potential consequences of the study.
Sample collection and trace element measurement
Whole blood samples of the cases were collected at the time of diagnosis before any treatment. Two milliliters of peripheral venous blood were collected into an EDTA-K2 microtube in the morning and stored at − 80 °C. A conventional wet acid digestion method was utilized for sample pretreatment. Briefly, a total of 100 μL of blood samples were baked at 80 °C for 6 h, and then digested with 65% nitric acid (200 μL) and 30% hydrogen peroxide (100 μL). Subsequently, the sample was diluted to a final volume of 5 mL with 3% dilute nitric acid. The concentrations of eight trace elements, including Cr, manganese (Mn), Cu, Zn, As, Se, Cd, and Pb, were determined by inductively coupled plasma-mass (ICP-MS) method using ICP-MS spectrometer (Agilent Technologies, Santa Monica, CA, United States) with 99.999% argon as described previously29. Internal standards scandium, germanium, indium, and bismuth were employed for quality control. Additionally, and calibration curves were created for element quantification, and the repeatedly measured standards were utilized for monitoring system stability. The regression coefficients of calibration curves were at or above 0.999. A solvent blank was routinely analyzed to ensure the instrument performance remained within acceptable parameters. An internal standard solution with 45Sc, 72Ge, 115In, 209Bi was accounted for the accuracy of our measurement. The reference material Seronorm “Elements in Whole Blood” (Seronorm, Norway) was utilized as a quality control to ensure stability of the detection system. To monitor and maintain analytical consistency, a control sample was systematically integrated into every batch of 15 samples processed.
Data collection and outcome ascertainment
General demographic information and clinical data covering age, gender, native place, clinicopathological characteristics, disease history, family history, body mass index (BMI), treatment modalities and follow-up were obtained from the medical records system. Tumor aggressiveness was defined based on tumor invasion in-depth and metastatic status (lymph node or distant metastasis) according to the 8th edition of the American Joint Committee on Cancer (AJCC) staging system. In multivariable logistic regression analysis and multi-element models, the ESCC risk (yes/no, binary), invasion depth of primary tumor (superficial/deep, binary), and metastases (yes/no, binary) of ESCC were employed as outcome variables. ESCC risk was ascertained by the diagnosis of ESCC within the study population. The current study classified tumor invasion depth as superficial (T1 + T2) and deep (T3 and beyond), and patients without regional lymph node metastasis (N0) and distant metastasis (M0) were defined as “non-metastases”, while patients with regional lymph node metastasis (N1/N2/N3) and/or distant metastasis (M1) were defined as “metastases”. OS was defined as the duration from the date of diagnosis to the time of death, measured in months, and PFS was calculated as the interval from the baseline to the time of death or tumor progression event e.g. locoregional recurrence or distant metastasis (in months).
Statistical analysis
The mean and standard deviation (SD) were used to describe data with a for normal distributions, while median with inter-quartile range (IQR) were used for data with a non-normal distribution. The Mann–Whitney U test (two-tailed) was employed to compare trace element concentrations between the case and control groups. Additionally, Spearman’s rank correlation analysis was used to assess bivariate correlations between each pair of trace element. The association between blood trace element levels and ESCC risk was evaluated by RCS with optimal knots using the median of each trace element concentration in controls as the inflection point. The spline model was adjusted for age and gender. Moreover, two complementary mixture approaches, WQS and BKMR models were established to examine the mixed effects of eight trace elements on three outcome variables, with all models adjusted for age and gender.
For WQS score evaluation, the division of the subjects into training (40%) and testing (60%) sets to calculate the weights and assess the statistical significance was based on the proportions reported in previous literature30.
The current study performed 1000 bootstrap samplings at each iteration to enhance the stability of the model, constraining both positive and negative directionality modes for the mixed effects31. Trace elements were ranked by probability, with estimated weights exceeding 0.125 (1/8) considered significant contributors to the WQS score.
Probit extension of Bayesian kernel machine regression (BKMR-P) was used for comprehensive evaluation of the impact of the eight trace elements on ESCC risk and clinical outcomes. Before BKMR analysis, all trace element concentrations were further standardized after ln-transformation using the z-score method, and the Markov chain Monte Carlo (MCMC) sampler was run for 10,000 iterations32. Posterior inclusion probability (PIP), in a range of 0 to 1 yielded by BKMR, exceeding 0.5 was typically considered as indicative of importance for the outcomes33. The BKMR model was represented by the equation below.
The function \(h( )\) represents the exposure–response association that incorporates considerations for nonlinearity and/or interaction between the mixture by using kernel machine regression, and \(Zi\hspace{0.17em},{\beta }^{T} and ei\) represent the vector of covariates, the corresponding vector of the \(Zi\)-coefficient and the residual, respectively.
Based on the median concentration of each trace element in each group, ESCC patients were classified into higher- and lower-level groups. Then, multivariable logistic regression analysis was performed to estimate the contribution of blood trace element burdens to clinicopathological features of ESCC by odds ratios (ORs) and corresponding 95% confidence intervals (CIs), using the lower-level group as a reference.
R package “survminer” was used to determine the optimal truncation values for each trace element level, with which all cases were divided into a high- and low- burden group of trace element levels. Kaplan–Meier log-rank tests were performed to compare the OS and PFS of ESCC patients with variant trace element burdens and clinicopathological characteristics. A multivariable Cox proportional hazard regression model was then constructed to calculate the hazard ratios (HRs) and 95% CIs for trace elements and clinicopathological features. Univariate model covariates with a p-value < 0.2 were added to the multivariable model.
SPSS (25.0) and R software (Version 4.2.3) were utilized for statistical RCS, WQS, and BKMR were implemented with the R packages “rms” (version 6.5.0), “gWQS” (version 3.0.4), and “bkmr” (version 0.2.2), respectively. Differences with P < 0.05 were considered statistically significant.
Results
Characteristics of the participants
A total of 150 ESCC patients and 177 controls were enrolled in this study. There was no significant difference in gender and age between the cases and controls (P > 0.05). The demographic characteristics are presented in Table S1.
Distribution of blood trace element levels among subjects
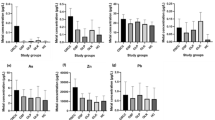
Among all the trace elements, blood Cd, Pb, Cr, Cu, As, and Se showed significant differences between the ESCC cases and controls (P < 0.01). Levels of Cd, Pb, Cr, and Cu in the cases were higher than controls, while a significant decrease in blood As and Se levels was observed in the case group (Fig. 1). Blood Cr and Zn both showed significant differences between the patients with different clinicopathological features (Table S2). Most of the trace elements exhibited positive correlations with each other, with correlation coefficients ranging from 0.21 to 0.64 (Fig. S1). Specifically, Se and As were strongly correlated (r = 0.64), and Cr-Mn and Mn-Zn showed moderate correlations with correlation coefficient values of 0.50 and 0.45, respectively.
Comparison of eight trace element concentrations between cases and controls. Statistical analysis was performed using a two-tailed Mann–Whitney U test. The concentration thresholds for each element were determined based on the interquartile range (IQR) of the control group. Specifically, the lower and upper thresholds were defined as Q1 − 1.5IQR and Q3 + 1.5IQR, respectively. Abbreviations: Cr, chromium; Mn, manganese; Cu, copper; Zn, zinc; As, arsenic; Se, selenium; Cd, cadmium; Pb, lead; ns, not significant.
Blood trace element burdens associated with risk for ESCC
RCS analyses showed that the associations of blood As, Zn, and Mn with ESCC risk was U-shaped (P-non-linear = 0.0148; P-non-linear < 0.0001; P-non-linear < 0.0001, respectively) (Fig. 2F-H). Conversely, blood Cd levels gave an inverted U-shaped trend with the risk of ESCC (P-non-linear = 0.001) (Fig. 2A). High levels of Pb, Cr, and Cu were associated with increased ESCC risk (all P-overall < 0.0001), though only blood Pb level was non-linearly related to ESCC risk (P-non-linear = 0.0028). (Fig. 2B-D). Blood Se showed an inverse linear relationship with risk of ESCC (P-non-linear = 0.8887), albeit with a wide 95% CI (Fig. 2E).
Restricted cubic spline multivariable logistic model of the association between blood levels of trace elements and ESCC risk. The solid red line represents the multivariable-adjusted odds ratio (OR), with the shaded portions showing the 95% confidence interval (CI). The black dashed line represents the reference line for no association at a OR of 1.0. The inflection points are the medians of the levels of trace elements in controls. Models were adjusted for gender and age.
For estimation of the overall effect, the mixtures of total trace elements were associated with ESCC risk with both positive and negative weights in the WQS regression model (all P < 0.05, Table S3). To identify the individual elements with major contributions in the mixture, the eight trace elements were separated into positive and negative weights for ESCC risk (Fig. 3). As a result, Cu ranked as the top element with the most positive weight, followed by Cr, Pb, and Cd, while the top three negative elements for ESCC risk were As, Mn, and Zn, with gradually decreasing weights (Fig. 3). Similarly, for the BKMR model, exposure to the mixture showed increased ESCC risk when the overall mixture was above the 50th percentile (Fig. 4A). All of the trace elements had high posterior inclusion probabilities (PIP) in relation to ESCC risk (all PIP > 0.8), especially for Cr, Mn, Cu, Zn, and Pb (all PIP = 1.00, Table S4). Furthermore, significant dose–response associations of Cr, Mn, Cu, and Pb with risk for ESCC were observed when the concentrations of the others were fixed at their 25th, 50th, and 75th percentile (Fig. 4B).
WQS regression identifies relevant trace elements in the blood for ESCC risk, tumor invasion depth, and metastatic risk. Panel A bar plot depicts the positive weights of each element attributed to the mixture, while Panel B depicts the negative weights. The red dashed line represents the significant thresholds (equal to the reciprocal of the number of elements in the mixture). Models were adjusted for age and gender.
Overall and individual exposure effects of trace element mixtures with 95% CIs on ESCC risk, tumor invasion depth and metastasis risk using the BKMR model. All estimates were adjusted for age and gender. (A) Change in outcome when all metals were at their median by either increasing or decreasing all metals in decile increments. (B) Estimated values of exposure–response were calculated by comparing the risk for individual trace elements at its 75th percentile as compared to when the mixtures of other elements were fixed at their 25th (red line), 50th (green line), or 75th (blue line) percentile.
Association between whole blood trace element levels and ESCC aggressiveness
Based on the medians of trace element concentrations in cases, the associations between trace elements and tumor invasion depth and metastases were evaluated using multivariable logistic regression (Fig. 5). Lower level of Se and higher burdens of Pb and As were found in ESCC patients with a greater degree of tumor invasion for both unadjusted (OR 0.280, 95% CI 0.083–0.949; OR 2.998, 95% CI 1.059–8.489; OR 4.224, 95% CI 1.247–14.315) and adjusted (OR 0.261, 95% CI 0.074–0.927; OR 3.024, 95% CI 1.053–8.689; OR 4.385, 95% CI 1.271–15.126) models. However, there was no significant association for any trace elements with metastatic risk. Moreover, WQS models, where we fitted the clinicopathological features, showed the associations for tumor invasion or metastasis were not statistically significant (Table S3). Moreover, for the BKMR models, though no significant association could be found for tumor invasion depth, the impact of the trace element mixture showed a positive association with metastatic risk. Among them, Mn was the most relevant trace element for metastatic risk (Table S4). Nevertheless, all trace elements did not exhibit any statistical effect on tumor invasion or metastasis whether their concentrations were fixed at the 25th, 50th, or 75th percentile (Fig. 4B).
Prognostic risk factors for ESCC progression (K-M + COX)
Kaplan–Meier analysis indicated that high whole blood burdens of Pb, Cr and Mn, as well as patients being male or with tumor metastases, had shorter OS (all P < 0.05; Fig. 6A-E). Subsequently, multivariable Cox proportional hazard regression analysis identified blood levels of Mn, Pb and Zn, as well as gender, as independent prognostic factors for OS with hazard ratios (HR) of 2.66 (95% CI 1.28–5.52), 2.13 (95% CI 1.06–4.27), 0.19 (95% CI 0.07–0.51) and 0.37 (95% CI 0.14–0.93), respectively (Fig. 7A). Whole blood Mn levels, gender, and tumor metastases were all associated with PFS (log-rank test; all P < 0.05; Fig. 6F-H). The Cox proportional hazard regression model revealed that whole blood levels of Mn (HR 2.01; 95% CI 1.08–3.73) and Cu (HR 0.35; 95% CI 0.15–0.79) acted as independent prognostic predictors for PFS (Fig. 7B). The rest results of Kaplan–Meier analysis are presented Supplemental Material (Figs. S2 and S3).
Discussion
With the development of industry and agriculture and the concurrent shift in lifestyle patterns, environmental pollution and dietary imbalances inevitably come with abnormal accumulation or depletion states trace elements, contributing to the onset of cancer34. Available epidemiological studies have revealed variations in trace element profiles between ESCC patients and healthy individuals11,12,35. Regarding the accumulating evidence linking trace element burdens to cancer risk, some investigators proposed that trace elements could serve as potential biomarkers for the diagnosis of cancer, ESCC included11,36. However, there has been an absence of research focusing on the alterations in trace element levels as potential predictors of disease progression in patients with ESCC. The current study was the first to explore the prognostic prediction of whole blood trace element levels in ESCC risk, aggressiveness and outcomes.
In line with previous reports, our findings indicated distinct element profiles between cases and controls. Of particular interest, the findings of Zn, Cd, Pb and Cu accumulating in the whole blood of ESCC patients, such as Zn, Cd, Pb and Cu were not entirely consistent with that was reported in serum or tumor tissues in other studies35;. While the contradictions may be ascribed to regional and demographic disparities, it is also plausible that divergence in the specimen types employed for assessing body burden levels constitutes a substantial contributing factor to these discrepancies. It is well-known that trace elements are not uniformly distributed in tissues and organs, and even accumulate differentially between cellular blood components and plasma/serum11,37,38,39. Recently, Cao et al. compared the trace element levels in paired serum, whole blood, and tissue of ESCC patients, and demonstrated that certain elements (Cu, As, Se, and Sr) in blood could be used for element exposure burdens assessment. They also suggested a favorable correlation between serum and whole blood for Cu, As, Se, and Sr11. Therefore, more compelling biological evidence should be required to validate the ideal biological sample type for evaluation of toxic and essential elements exposure.
The BKMR and WQS models consistently yielded the trace element mixture was associated with ESCC risk, among which Cr, Pb, Cu and Cd were the most relevant elements. This result was supported by the findings of the RCS model, which showed that Cr, Pb and Cu displayed a positive trend with ESCC risk, accompanied by an inverted U-shaped for Cd. In accordance with our results, a large-scale study conducted in Spaniard indicated esophageal cancer was associated with soils containing higher Pb content40. Although no significant association was reported between Cr concentrations in toenails and the risk of ESCC in a population from a region with high ESCC incidence in Golestan17, hexavalent Cr was unraveled to heighten risk of colorectal cancer41. Albeit with dual effect on regulating cancer development, excess Cu was claimed to cause cellular injury and contribute to the angiogenesis of tumor42. Consistent with our observations, elevated plasma levels of Cu were observed in an area of Kashmir, India with high esophageal cancer incidence43, indicating an excess Cu burden may be responsible for the development of this malignancy. Given the nonlinear association between Cu intake and ESCC risk44 and the potential therapeutic benefits of nanoparticles loaded with Cu ions in esophageal cancer treatment45, further research is needed to elucidate the role of Cu burden in ESCC development fully.
While the carcinogenesis of Cd has been consistently verified, our results indicate an inverted U-shaped relationship between blood Cd content and the risk of ESCC, which underscores a dual and somewhat paradoxical role of Cd in the etiology of ESCC. It is worth noted that the non-linear association with ESCC risk is not unique to Cd, as similar U-shape trends were observed for Zn, Mn, and As in relation to ESCC risk within the subjects. The U-shaped relationships observed between certain elements and ESCC risk are consistent with the view that both deficiency and excess of specific elements, particularly essential trace elements, have the potential to impact cellular processes related to cancer development. For example, low levels of Zn can lead to a weakened antioxidant defense, increasing the risk of oxidative stress and DNA damage, which contribute to the mechanisms of cancer initiation46. Conversely, excessive Zn disrupt cellular homeostasis and stimulate the overproduction of reactive oxygen species, which can also promote cancer progression47. Similarly, both low and high levels of Mn can disrupt the balance of pro- and anti-oxidant status, thereby indirectly contributing to cancer risk48. These intriguing findings imply the intricate nature of certain toxic elements (Cd, As) and specific essential elements (Zn, Mn) in potential carcinogenicity and anti-carcinogenicity, with a dose–response relationship and an optimal concentration for their most significant effect on ESCC risk. Hence, caution should be exercised when considering nutritional supplementation of trace elements in the general population or application of metal-based materials/strategy in cancer patients, especially for individuals with a high baseline level.
Our analysis revealed a negative association between whole blood Se and ESCC risk in a linear dose–response manner, which supports the results claimed in earlier studies showing Se levels in scalp hair and serum display a negative trend with risk for esophagus cancer12,35. A randomized trial conducted in high incidence areas for esophageal cancer indicated that Se supplementation might prevent esophageal cancer44,49. The preventive and anticarcinogenic effects of Se, including regulating the antioxidant defense, influencing cell signaling and autophagy, inhibiting cancer metastasis and the angiogenic processes, and stimulating the antitumor immunity, have been extensively demonstrated50,51. However, another nutrition intervention trial conducted in a selenium-deficient, high-ESCC incidence district of China showed no protective effect against esophageal cancer following supplementation of Se52. Interestingly, a cohort study indicated a U-shaped relationship between dietary Se supplementation and risk for ESCC44. It is worth noted that U-shaped trends were also displayed for Zn, Mn, and As with ESCC risk in the current study. In general, these exposure–response shapes provide specific information about the complicated associations between trace element burdens and ESCC risk, suggesting the carcinogenic potential or antineoplastic effect of certain trace elements in a dose-dependent manner. Hence, caution should be exercised when considering nutritional supplementation of trace elements in the general population or application of metal-based materials/strategy in cancer patients, especially for individuals with a high baseline level.
Se and multiple Se-containing compounds have been shown to slow down cancer progression by reducing inflammation and oxidative DNA damage, thereby exerting chemo-preventive effects53. Furthermore, the inhibition of the beta-catenin/TCF pathway, attenuating cell viability and increasing cell apoptosis, have been suggested as potential mechanisms for Se-induced ESCC suppression54,55. The same views have emerged that Mn inhibited tumor progression by mediating multiple cancer physiological processes56. As expected, our multivariate logistic regression analysis showed that individuals with lower blood Se and Mn are more likely to have deeper invasion of the primary tumor. This result reinforces the beneficial effect of adequate status of Se and Mn during tumor progression. However, we are cautious about encouraging supplementation of these two elements in ESCC patients, given that Se was claimed to be a toxic mineral with a rather small therapeutic window57 and Mn was implicated in tumor progression by accumulating in Mn-rich niches and enhancing cell migration58.
Conversely, we showed As and Pb were positively associated with tumor invasion, which supports the evidence provided from published epidemiological and toxicological research in several types of cancer. An in vitro study revealed that six-month exposure to inorganic As at a concentration relevant to contaminated drinking water could promote the malignant characteristics of colon cancer cells59. Likewise, chronic As exposure was confirmed to induce basal characteristics and increase invasiveness in papillary bladder cancer cells via upregulating basal transcriptional factors60. Furthermore, high As was found to be associated with increased risk of recurrence in bladder patients in an epidemiological study61. It was also noted that our BKMR analysis showed the trace element mixture was positively associated with metastatic risk of ESCC. These findings collectively suggest the implication of single and combined effects of trace elements in ESCC aggressiveness.
The effects of plasma trace elements on OS of patents with ESCC have been determined in a recent study, in which Cd was identified as a risk factor for shorter OS18. Unexpectedly, no significant association between whole blood level of Cd and OS was observed in the current study. The inconsistent results might be due to the differential distribution of trace elements in serum and whole blood11. While Cd appears to be associated with increased cancer mortality in a systematic review and meta-analysis with 1332 cancer deaths62, the implication of Cd in ESCC prognosis needs to be further verified in population investigation with larger sample size.
To the best of our knowledge, the current study is first to discuss the associations of single-element and mixed trace elements with OS and PFS in patients with ESCC simultaneously. Our study presented that Cr, Mn, and Pb were correlated with shorter OS, among which Mn and Pb were independent prognostic factors for OS. These results are consistent with the reported relative mortality rates in mining areas, in which environmental exposure to Pb was related to a greater mortality of carcinogenic tumors63. A positive correlation between Mn concentrations in drinking water and cancer mortality was also reported in cities along Chinese Huai’an River64. Besides, our results showed Zn has a protective effect on the OS in patients with ESCC, which supporting the findings that dietary Zn intake was inversely related to esophageal mortality65. Notably, Zn treatment has been confirmed to be effective therapeutic strategy for ESCC in rodent model66.
The presence of Cr and Mn in tumor tissues has been associated with recurrence and recurrence-free survival in patients with oral cavity squamous cell carcinoma67. Consistent with the findings mentioned above, on another scale, Mn and Cu were inferred as independent prognostic predictors for PFS, and patients with high blood Mn, Cr, and Cu levels were revealed to have shorter PFS, albeit with marginal significance in the latter two. It is noteworthy that the distinct prognostic implications of trace element levels for OS and PFS is aligns with Cihan’ findings in non-small cell lung cancer, where hair levels of Aurum (Au) and Cu were significantly associated with PFS rather than OS68. This suggests that certain trace elements might exert a more pronounced influence on the process of cancer growth, spread or metastasis, instead of being the immediate cause of mortality. Interestingly, no significant association between As, Cd levels and poor OS or PFS was found in the current study, which might be attributed to the specific cut off values employed in our statistical model. Notably, only the highest tertile of Cd levels was identified as being linked to poor adverse OS and PFS outcomes in ESCC in previous research18,29. These findings collectively underscore the potential predictive significance of trace element burden assessment in ESCC prognosis.
In the current study, the multiple models provide a comprehensive understanding the overall mixture effect of trace elements, which are more consistent with the real situation of the human exposure. However, some limitations should be considered. First, we could not establish causality between altered blood trace element contents and ESCC development and progression based on the observational study. Moreover, the relatively small sample size may impair the stability and validity of our estimates. In any case, our findings may provide some clues for further epidemiological surveys or experimental studies to explore the mechanisms for ESCC progression induced by specific elements. Secondly, inherent to observational studies, there inevitably exist some residual confounding events, although we adjusted for age and gender in the analytical models. To some extent, the adjustment factors included in the model may still be incomplete. For instance, genetic susceptibility, including gene mutation69, and patrilineal genetic structures, such as the non-recombining portion of the Y chromosome22 have been implicated in the etiology of ESCC. However, due to the limited number of cases that underwent gene mutation detection or genotyping of the Y chromosome, this information was not included in our research. Other confounding factors, such as family history of cancer, drinking and smoking history for cancer risk and specific therapeutic programs, nutritional status, and family finances for prognosis cannot be neglected. Another potential source of limitation is that the estimation of trace element burdens in ESCC patients relies on single-time-point blood samples taken at diagnosis, before clinical intervention. The predictive value of dynamic change of trace element levels is yet to be comprehensively evaluated. Furthermore, concurrent assessments of trace element concentrations in tumor tissues or urine are absent from our study. Admittedly, blood concentrations of trace elements generally represent short-term body burdens for recent exposure and are easily subject to dietary and disease-related fluctuations. Meanwhile, certain elements with unique characteristics, are quickly eliminated from the blood (Yüksel). However, a universally accepted and precise standard for accurately gauging trace element accumulation in the human body is still lacking. Moreover, trace element concentrations in human populations are indeed influenced by dietary structure, environmental conditions, socioeconomic status, which potentially have temporal trends. However, due to the practical difficulties in collecting clinical samples with complete follow-up information, the duration of sample collection extended over 20 years, potentially introducing biases into the results. Last but not least, the imbalance in the ratio between the training and testing sets in WQS model could potentially introduce uncertainties that affect the reliability and validity of the model’s evaluation. In summary, it is essential to acknowledge that our findings necessitate further validation through rigorous experimental studies and comprehensive epidemiological investigations encompassing larger, more homogeneous patient cohorts, particularly considering the confounders such as genetic background, smoking status and history, occupational exposure, and dietary habits. Such endeavors will be instrumental in elucidating the complex relationships between trace element homeostasis and ESCC progression and outcomes, ultimately contributing to precision prevention and control strategies for this disease.
Conclusions
The current work delineates the specific blood trace element profiles of ESCC patients by comparing to healthy controls. The coexistence of linear, U-shaped, and inverted U-shaped relationships between trace elements and ESCC risk, as observed in our study, highlights the multifaceted role of trace elements in cancer etiology. Particularly, the carcinogenic or anticarcinogenic effects of specific elements may be contingent upon their dose–response relationships with ESCC risk. Moreover, the findings of element mixture and certain trace elements associated with aggressiveness and OS/PFS provide new insights into the impact of trace elements on the outcome of patients with ESCC. These insights offers a meaningful contribution to the understanding of trace elements in ESCC etiology and prognosis.. Consequently, screening for trace element concentrations in whole blood could potentially facilitate the identification of individuals at high-risk and enable the prediction of clinical outcomes prior to the initiation of treatment. It should also be pointed out that nutritional supplementation of essential trace elements in the general population or application of heavy metal-based materials/strategy in cancer patients should be considered prudently.
Data availability
The datasets generated during and/or analysed during the current study are not publicly available due to patient privacy concerns but are available from the corresponding author on reasonable request.
Abbreviations
- ESCC:
-
Esophageal squamous cell carcinoma
- Cr:
-
Chromium
- Mn:
-
Manganese
- Cu:
-
Copper
- Zn:
-
Zinc
- As:
-
Arsenic
- Se:
-
Selenium
- Cd:
-
Cadmium
- Pb:
-
Lead
- BMI:
-
Body mass index
- ICP-MS:
-
Inductively coupled plasma-mass spectrometry
- RCS:
-
Restricted cubic spline
- BKMR:
-
Bayesian kernel machine regression
- WQS:
-
Weighted quantile sum
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- SD:
-
Standard deviation
- IQR:
-
Inter-quartile range
- OR:
-
Odds ratio
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
References
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. https://doi.org/10.3322/caac.21834 (2024).
Li, S. et al. Changing trends in the disease burden of esophageal cancer in China from 1990 to 2017 and its predicted level in 25 years. Cancer Med. 10, 1889–1899. https://doi.org/10.1002/cam4.3775 (2021).
Abnet, C. C., Arnold, M. & Wei, W. Q. Epidemiology of esophageal squamous cell carcinoma. Gastroenterology 154, 360–373. https://doi.org/10.1053/j.gastro.2017.08.023 (2018).
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. https://doi.org/10.3322/caac.21660 (2021).
Li, P. et al. The associations of air pollution and socioeconomic factors with esophageal cancer in China based on a spatiotemporal analysis. Environ. Res. 196, 110415. https://doi.org/10.1016/j.envres.2020.110415 (2021).
Tang, W. R., Chen, Z. J., Lin, K., Su, M. & Au, W. W. Development of esophageal cancer in Chaoshan region, China: Association with environmental, genetic and cultural factors. Int. J. Hyg. Environ. Health 218, 12–18. https://doi.org/10.1016/j.ijheh.2014.10.004 (2015).
Uhlenhopp, D. J., Then, E. O., Sunkara, T. & Gaduputi, V. Epidemiology of esophageal cancer: Update in global trends, etiology and risk factors. Clin. J. Gastroenterol. 13, 1010–1021. https://doi.org/10.1007/s12328-020-01237-x (2020).
Chanihoon, G. Q., Afridi, H. I., Kazi, T. G., Talpur, F. N. & Baig, J. A. Evaluation of zinc and cadmium levels in the biological samples of Ewing sarcomas patients and healthy subjects. Clin. Chim Acta 522, 1–7. https://doi.org/10.1016/j.cca.2021.08.002 (2021).
Kazi, T. G. et al. Interaction of cadmium and zinc in biological samples of smokers and chewing tobacco female mouth cancer patients. J. Hazard Mater. 176, 985–991. https://doi.org/10.1016/j.jhazmat.2009.11.139 (2010).
Rodríguez-Tomàs, E. et al. Trace elements under the spotlight: A powerful nutritional tool in cancer. J. Trace Elem. Med. Biol. 68, 126858. https://doi.org/10.1016/j.jtemb.2021.126858 (2021).
Cao, K. et al. Associations of trace element levels in paired serum, whole blood, and tissue: An example of esophageal squamous cell carcinoma. Environ. Sci. Pollut. Res. Int. 30, 38052–38062. https://doi.org/10.1007/s11356-022-24960-z (2023).
Kazi, T. G. et al. Comparison of essential and toxic elements in esophagus, lung, mouth and urinary bladder male cancer patients with related to controls. Environ Sci Pollut Res Int. 22, 7705–7715. https://doi.org/10.1007/s11356-014-3988-z (2015).
Sohrabi, M. et al. Evaluating tissue levels of the eight trace elements and heavy metals among esophagus and gastric cancer patients: A comparison between cancerous and non-cancerous tissues. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. 68, 126761. https://doi.org/10.1016/j.jtemb.2021.126761 (2021).
Ma, J. et al. Increased total iron and zinc intake and lower heme iron intake reduce the risk of esophageal cancer: A dose-response meta-analysis. Nutr. Res. 59, 16–28. https://doi.org/10.1016/j.nutres.2018.07.007 (2018).
Steevens, J., van den Brandt, P. A., Goldbohm, R. A. & Schouten, L. J. Selenium status and the risk of esophageal and gastric cancer subtypes: The Netherlands cohort study. Gastroenterology 138, 1704–1713. https://doi.org/10.1053/j.gastro.2009.12.004 (2010).
Wei, W. Q. et al. Prospective study of serum selenium concentrations and esophageal and gastric cardia cancer, heart disease, stroke, and total death. Am. J. Clin. Nutr. 79, 80–85. https://doi.org/10.1093/ajcn/79.1.80 (2004).
Hashemian, M. et al. Toenail mineral concentration and risk of esophageal squamous cell carcinoma, results from the Golestan Cohort Study. Cancer Med. 6, 3052–3059. https://doi.org/10.1002/cam4.1247 (2017).
Yu, K. et al. Effect of trace element mixtures on the outcome of patients with esophageal squamous cell carcinoma: A prospective cohort study in Fujian, China. BMC Cancer 24, 24. https://doi.org/10.1186/s12885-023-11763-9 (2024).
Tian, H. et al. Estimating cancer incidence based on claims data from medical insurance systems in two areas lacking cancer registries in China. EClinicalMedicine 20, 100312. https://doi.org/10.1016/j.eclinm.2020.100312 (2020).
Huang, W., Shi, X. & Wu, K. Human body burden of heavy metals and health consequences of Pb exposure in Guiyu, an e-waste recycling town in China. Int. J. Environ. Res. Public Health 18, 12428. https://doi.org/10.3390/ijerph182312428 (2021).
Guohong, Z. et al. Genetic heterogeneity of oesophageal cancer in high-incidence areas of southern and northern China. PloS one 5, e9668. https://doi.org/10.1371/journal.pone.0009668 (2010).
Huang, H. et al. Y-chromosome evidence for common ancestry of three Chinese populations with a high risk of esophageal cancer. PloS one 5, e11118. https://doi.org/10.1371/journal.pone.0011118 (2010).
Zhong, Y. et al. Hot tea drinking and the risk of esophageal cancer: A systematic review and meta-analysis. Nutr. Cancer 74, 2384–2391. https://doi.org/10.1080/01635581.2021.2007963 (2022).
Yao, T., Jiang, S., Hou, K., Sun, H. & Wang, H. Cadmium (Cd) accumulation in traditional Chinese medicine materials (TCMMs): A critical review. Ecotoxicol. Environ. Saf. 242, 113904. https://doi.org/10.1016/j.ecoenv.2022.113904 (2022).
Guo, Y., Yang, G.-Y., Dong, Q.-X. & Huang, C.-J. Distribution of heavy metals in soils from the typical regions of Shantou and their environmental pollution assessment. Huan Jing Ke Xue 28, 1067–1074 (2007).
Mao, Z. et al. Pollution characteristics, sources, and risks of 23 metallic elements in sediments from Guiyu and its upstream and downstream. Geochimica 05, 513–524. https://doi.org/10.19700/j.0379-1726.2021.05.007 (2021).
Zhang, Y. et al. Exposure to multiple heavy metals associate with aberrant immune homeostasis and inflammatory activation in preschool children. Chemosphere 257, 127257. https://doi.org/10.1016/j.chemosphere.2020.127257 (2020).
Zhao, P. et al. Accumulation of nutrients and potentially toxic elements in plants and fishes in restored mangrove ecosystems in South China. Sci. Total Environ. 838, 155964. https://doi.org/10.1016/j.scitotenv.2022.155964 (2022).
Chen, J. et al. Environmental cadmium exposure promotes the development, progression and chemoradioresistance of esophageal squamous cell carcinoma. Front. Cell Dev. Biol. 10, 792933. https://doi.org/10.3389/fcell.2022.792933 (2022).
Sanders, A. P. et al. Combined exposure to lead, cadmium, mercury, and arsenic and kidney health in adolescents age 12–19 in NHANES 2009–2014. Environ. Int. 131, 104993. https://doi.org/10.1016/j.envint.2019.104993 (2019).
Carrico, C., Gennings, C., Wheeler, D. C. & Factor-Litvak, P. Characterization of weighted quantile sum regression for highly correlated data in a risk analysis setting. J. Agric. Biol. Environ. Stat. 20, 100–120. https://doi.org/10.1007/s13253-014-0180-3 (2015).
Bobb, J. F. et al. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics (Oxford, England) 16, 493–508. https://doi.org/10.1093/biostatistics/kxu058 (2015).
Barbieri, M. M. & Berger, J. O. Optimal predictive model selection. Ann. Stat. 32, 870–897 (2004).
Venturelli, S. et al. Minerals and cancer: Overview of the possible diagnostic value. Cancers (Basel) 14, 1256. https://doi.org/10.3390/cancers14051256 (2022).
Zhang, J. et al. Association between serum level of multiple trace elements and esophageal squamous cell carcinoma risk: A case-control study in China. Cancers (Basel) 14, 4239. https://doi.org/10.3390/cancers14174239 (2022).
Lossow, K., Schwarz, M. & Kipp, A. P. Are trace element concentrations suitable biomarkers for the diagnosis of cancer?. Redox. Biol. 42, 101900. https://doi.org/10.1016/j.redox.2021.101900 (2021).
Azevedo, R., Gennaro, D., Duro, M., Pinto, E. & Almeida, A. Further Evidence on Trace Element Imbalances in Haemodialysis Patients-Paired Analysis of Blood and Serum Samples. Nutrients. 15, 1912. https://doi.org/10.3390/nu15081912 (2023).
Manton, W. I., Rothenberg, S. J. & Manalo, M. The lead content of blood serum. Environ. Res. 86, 263–273 (2001).
Milne, D. B., Sims, R. L. & Ralston, N. V. Manganese content of the cellular components of blood. Clin. Chem. 36, 450–452 (1990).
López-Abente, G. et al. Compositional analysis of topsoil metals and its associations with cancer mortality using spatial misaligned data. Environ. Geochem. Health 40, 283–294. https://doi.org/10.1007/s10653-016-9904-3 (2018).
Bonfiglio, R. et al. The impact of toxic metal bioaccumulation on colorectal cancer: Unravelling the unexplored connection. Sci. Total Environ. 906, 167667. https://doi.org/10.1016/j.scitotenv.2023.167667 (2023).
Yang, X., Tang, Z., Li, J. & Jiang, J. Esophagus cancer and essential trace elements. Front. Public Health 10, 1038153. https://doi.org/10.3389/fpubh.2022.1038153 (2022).
Dar, N. A. et al. Association between copper excess, zinc deficiency, and TP53 mutations in esophageal squamous cell carcinoma from Kashmir Valley, India–a high risk area. Nutr. Cancer 60, 585–591. https://doi.org/10.1080/01635580802290231 (2008).
Hashemian, M. et al. Dietary intake of minerals and risk of esophageal squamous cell carcinoma: results from the Golestan Cohort Study. Am. J. Clin. Nutr. 102, 102–108. https://doi.org/10.3945/ajcn.115.107847 (2015).
Xu, R. et al. Hyaluronic acid/polyethyleneimine nanoparticles loaded with copper ion and disulfiram for esophageal cancer. Carbohydr. Polym. 261, 117846. https://doi.org/10.1016/j.carbpol.2021.117846 (2021).
Alam, S. & Kelleher, S. L. Cellular mechanisms of zinc dysregulation: A perspective on zinc homeostasis as an etiological factor in the development and progression of breast cancer. Nutrients 4, 875–903. https://doi.org/10.3390/nu4080875 (2012).
Coradduzza, D. et al. Heavy metals in biological samples of cancer patients: A systematic literature review. Biometals 37, 803–817. https://doi.org/10.1007/s10534-024-00583-4 (2024).
Shen, F. et al. The association between deficient manganese levels and breast cancer: A meta-analysis. Int. J. Clin. Exp. Med. 8, 3671–3680 (2015).
Jessri, M., Rashidkhani, B., Hajizadeh, B., Jessri, M. & Gotay, C. Macronutrients, vitamins and minerals intake and risk of esophageal squamous cell carcinoma: A case-control study in Iran. Nutr. J. 10, 137. https://doi.org/10.1186/1475-2891-10-137 (2011).
Domínguez-Álvarez, E. et al. Selenium and tellurium in the development of novel small molecules and nanoparticles as cancer multidrug resistance reversal agents. Drug Resist. Updat. 63, 100844. https://doi.org/10.1016/j.drup.2022.100844 (2022).
Razaghi, A., Poorebrahim, M., Sarhan, D. & Björnstedt, M. Selenium stimulates the antitumour immunity: Insights to future research. Eur. J. Cancer 155, 256–267. https://doi.org/10.1016/j.ejca.2021.07.013 (2021).
Wang, S.-M. et al. Effects of nutrition intervention on total and cancer mortality: 25-year post-trial follow-up of the 5.25-year Linxian Nutrition Intervention Trial. J. Natl. Cancer Inst. 110, 1229–1238. https://doi.org/10.1093/jnci/djy043 (2018).
Ahsan, A. et al. Potential chemotherapeutic effect of selenium for improved canceration of esophageal cancer. Int. J. Mol. Sci. 23, 5509. https://doi.org/10.3390/ijms23105509 (2022).
Liu, T., Sun, Y., Yang, S. & Liang, X. Inhibitory effect of selenium on esophagus cancer cells and the related mechanism. J. Nutr. Sci. Vitaminol. (Tokyo) 66, 456–461. https://doi.org/10.3177/jnsv.66.456 (2020).
Zhang, W. et al. beta-Catenin/TCF pathway plays a vital role in selenium induced-growth inhibition and apoptosis in esophageal squamous cell carcinoma (ESCC) cells. Cancer Lett. 296, 113–122. https://doi.org/10.1016/j.canlet.2010.04.001 (2010).
Rozenberg, J. M. et al. The role of the metabolism of zinc and manganese ions in human cancerogenesis. Biomedicines 10, 1072. https://doi.org/10.3390/biomedicines10051072 (2022).
Rayman, M. P. The importance of selenium to human health. Lancet 356, 233–241. https://doi.org/10.1016/s0140-6736(00)02490-9 (2000).
Stelling, M. P. et al. Manganese systemic distribution is modulated in vivo during tumor progression and affects tumor cell migration and invasion in vitro. Sci. Rep. 11, 15833. https://doi.org/10.1038/s41598-021-95190-5 (2021).
Chen, J. et al. Inorganic arsenic exposure promotes malignant progression by HDAC6-mediated down-regulation of HTRA1. J. Appl. Toxicol. JAT 43, 1214–1224. https://doi.org/10.1002/jat.4457 (2023).
Mehus, A. A. et al. Chronic arsenic exposure upregulates the expression of basal transcriptional factors and increases invasiveness of the non-muscle invasive papillary bladder cancer line RT4. Int. J. Mol. Sci. 23, 12313. https://doi.org/10.3390/ijms232012313 (2022).
Ghosh, S. et al. Arsenic level in bladder tumor of patients from an exposed population: Association with progression and prognosis. Futur. Oncol. (London, England) 17, 1311–1323. https://doi.org/10.2217/fon-2020-0154 (2021).
Larsson, S. C. & Wolk, A. Urinary cadmium and mortality from all causes, cancer and cardiovascular disease in the general population: Systematic review and meta-analysis of cohort studies. Int. J. Epidemiol. 45, 782–791. https://doi.org/10.1093/ije/dyv086 (2016).
Parviainen, A., Vázquez-Arias, A., Arrebola, J. P. & Martín-Peinado, F. J. Human health risks associated with urban soils in mining areas. Environ. Res. 206, 112514. https://doi.org/10.1016/j.envres.2021.112514 (2022).
Zhang, Q. et al. Study on the relationship between manganese concentrations in rural drinking water and incidence and mortality caused by cancer in Huai’an city. Biomed. Res. Int. 2014, 645056. https://doi.org/10.1155/2014/645056 (2014).
Chen, F., Cole, P., Mi, Z. & Xing, L. Dietary trace elements and esophageal cancer mortality in Shanxi, China. Epidemiology 3, 402–406. https://doi.org/10.1097/00001648-199209000-00004 (1992).
Fong, L. Y. et al. Zinc treatment reverses and anti-Zn-regulated miRs suppress esophageal carcinomas in vivo. Proc. Natl. Acad. Sci. U S A 120, e2220334120. https://doi.org/10.1073/pnas.2220334120 (2023).
Archanjo, A. B. et al. Elemental characterization of oral cavity squamous cell carcinoma and its relationship with smoking, prognosis and survival. Sci. Rep. 10, 10382. https://doi.org/10.1038/s41598-020-67270-5 (2020).
Cihan, Y. B. Do trace element levels have prognostic value in non-small cell lung cancer patients treated with chemoradiotherapy?. J. BUON 19, 749–756 (2014).
Zhong, L. et al. TP53 mutations in esophageal squamous cell carcinoma. Front. Biosci. (Landmark Ed) 28, 219. https://doi.org/10.31083/j.fbl2809219 (2023).
Acknowledgements
We are grateful to all the volunteers for participating in the present study. We thank Dr Stanley Lin for his constructive comments and language editing. In addition, we would like to thank Dr Yuan Zhang and Zhexuan Lin for their technical guidance for ICP-MS analysis.
Funding
This work was supported by the National Natural Science Foundation of China [81602886]; the Natural Science Foundation of Guangdong Province, China [2020A1515010945]; the Innovation Strong School Project of Department of Education of Guangdong Province (2019KTSCX037); and the Science and Technology Plan Projects in Shantou Guangdong Province ([2022] 88-6).
Author information
Authors and Affiliations
Contributions
Shuyi Qiu: Writing – original draft, visualization, formal analysis, and data curation. Bingmeng Xie: Investigation, formal analysis. Jiahui Liao: Investigation. Jianan Luo: Formal analysis. Xi Liu: Formal analysis. Lihua He: Formal analysis. Yiteng Huang: Conceptualization and funding acquisition. Lin Peng: Project administration, funding acquisition and writing—review & editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Qiu, S., Xie, B., Liao, J. et al. Blood trace elements in association with esophageal squamous cell carcinoma risk, aggressiveness and prognosis in a high incidence region of China. Sci Rep 15, 5208 (2025). https://doi.org/10.1038/s41598-025-89060-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-89060-7