Abstract
Shanxi Province is the northernmost distribution range of Chinese forest musk deer (Moschus berezovskii), with wild populations scattered across Zhongtiao (ZT), Lüliang (LL), and Taiyue (TY) mountain ranges. Using line transect surveys and local guide assistance, coupled with infrared camera data, this study systematically collected fecal samples from wild forest musk deer from five nature reserves across the three mountain ranges. Genetic diversity was analyzed using the mtDNA control region, which yielded 129 effective sequences of 656 bp and 20 distinct haplotypes. Haplotype and nucleotide diversities were 0.916 and 0.01505, respectively, indicating a relatively high overall genetic diversity in the studied populations. Significant genetic differentiation was observed between the Lüliang–Taiyue (LLTY) and ZT mountain range populations, with most of the genetic variation existing within the populations. No significant correlation was detected between the geographical and genetic distances of the samples, which may have resulted from historical agricultural activities in Shanxi impeding gene flow among populations. Therefore, genetic exchange programs are recommended to improve the genetic diversity and population fitness of forest musk deer in this region to facilitate their recovery and growth.
Similar content being viewed by others
Introduction
The genetic diversity of wild animals reflects their evolutionary potential and the quality of their genetic resources, which are also important genetic parameters for evaluating the status of populations of specific species1,2,3. With the development of molecular scatology techniques, the collection and extraction of fecal DNA have been widely used to analyze the genetic diversity of wild animals4,5,6,7. Compared to nuclear DNA, mitochondrial DNA (mtDNA) has multiple copies, simple structure, and high stability8,9. The mtDNA control region (CR) is a non-coding region of the mitochondrial genome rich in A and T bases and has the fastest evolutionary and highest polymorphism rates10,11. Therefore, it has been widely applied in research on genetic diversity, population genetic structure, subspecies, and population differentiation in wild animals12,13,14,15.
Forest musk deer (Moschus berezovskii) is endemic to East Asian forests. Males possess a musk gland that secretes musk, a valuable raw material for traditional medicine and the perfume industry, making this species a valuable medicinal and economic resource16. Owing to illegal poaching, overexploitation, and habitat fragmentation, wild populations of forest musk deer have declined considerably over the past few decades17. They are listed as an endangered species on the International Union for Conservation of Nature Red List18 and is a first-class state-protected wildlife species in China. Only one previous study has investigated the genetic diversity of wild forest musk deer19, whereas another study has combined wild and captive populations20. Located in the Palearctic biogeographic region, the wild musk deer population in Shanxi Province has historically been classified as the Siberian musk deer (Moschus moschiferus)17,21. However, based on the molecular identification by our research team, these populations should be reclassified as forest musk deer (to be reported elsewhere). The current genetic diversity of these populations in Shanxi Province remains poorly understood. Therefore, the present study conducted fecal sampling across all known distribution areas of forest musk deer in Shanxi Province, analyzed their genetic diversity via mtDNA CR, and investigated the correlation between mtDNA CR genetic distances and geographical distances. Based on these findings, conservation strategies for forest musk deer in Shanxi Province were proposed.
Results
Sample collection and species identification
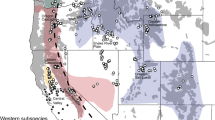
In this study, 243 fecal samples were collected along the sampling lines (Fig. 1) from 5 nature reserves in Shanxi Province. After DNA extraction and polymerase chain reaction (PCR) amplification, 217 fecal samples successfully yielded the target mtDNA CR sequences. Using BLAST searches, sequence assembly, and multiple sequence alignment, 129 forest musk deer mtDNA CR sequences were obtained. The specific sampling numbers and species identification results for the mtDNA CR sequences from each nature reserve are presented in Table 1. The ZT mountain range had the highest number of forest musk deer fecal samples, whereas the TY mountain range had the lowest. Among the individual nature reserves, the number of samples were distributed from highest to lowest as follows: Shanxi Lishan National Nature Reserve (LS) > Yangcheng Mangrove Gibbon National Nature Reserve (MH) > Shanxi Lingkongshan National Nature Reserve (LKS) > Shanxi Luyashan National Nature Reserve (LYS) > Shanxi Pangquangou National Nature Reserve (PQG).
Analysis of mtDNA CR sequences
The effective length of 129 forest musk deer mtDNA CR sequences was 656 bp. The average nucleotide compositions were T (32.5%), C (14.7%), A (30.0%), and G (22.8%). The average A + T content (62.5%) was greater than the average G + C content (37.5%), indicating a typical A + T bias characteristic of vertebrate mitochondrial genomes. There was 1 deletion, 612 conserved, and 43 variable (polymorphic) sites: 8 single-nucleotide variants and 35 parsimony-informative sites. Among the single-nucleotide variant and parsimony-informative sites, there were 8 and 35 two-variant sites, respectively. No three- or four-nucleotide variant sites were observed.
Haplotype analysis
Twenty haplotypes were identified from 129 mtDNA CR sequences. Using the EstimateS 9.1.0 software, the abundance-based coverage estimator (ACE) value was 22.48, suggesting that the haplotype richness of the target forest musk deer population in the surveyed area was approximately 22 haplotypes. Considering sampling effectiveness, the observed 20 haplotypes accounted for approximately 89% of the estimated total haplotype richness, indicating that most haplotypes in the study population were identified. The haplotype accumulation curve (S. Figure 1) exhibited a rapid initial increase, followed by leveling off, suggesting that the sample size was large enough to identify the majority of mtDNA haplotypes and the sampling was sufficient for this kind of data analysis.
As shown in S. Table 1, 15 of the 20 haplotypes were observed exclusively within a single nature reserve in the whole study area, making them unique haplotypes. The ZT mountain range had the highest number of unique haplotypes, whereas the TY mountain range had the lowest. One haplotype (Hap2) was shared between the LL and TY mountain ranges; however, no shared haplotypes were detected between these two mountain ranges and ZT mountain range. At the nature reserve level, LYS and LKS had only a single haplotype each, with the LYS haplotype (Hap1) being endemic, whereas that of LKS haplotype (Hap2) was shared with that of PQG. The five D-loop sequences from the PQG contained three haplotypes (Hap2, Hap3, and Hap4), with Hap3 and Hap4 being endemic to this reserve. LS had the highest number of haplotypes, with 15 haplotypes out of 20, accounting for 75% of the haplotypes identified. Furthermore, 11 of the 15 haplotypes were endemic to LS, accounting for 55% of the total number of identified haplotypes. Among the 24 D-loop sequences from MH, there were 5 haplotypes, with Hap20 endemic to this site. As shown in S. Table 1, Hap6 was the most common haplotype, observed in 23 of 129 samples (17.83%). Hap5 and Hap14 were relatively common, occurring 16 and 15 times (12.40% and 11.62%), respectively. Five haplotypes (Hap8, Hap9, Hap13, Hap17, and Hap19) were found only once, representing rare haplotypes, and all of these were detected in the LS reserve.
As shown in Table 2, the Hd of the 20 haplotypes was 0.916, Pi was 0.01505, and the average number of nucleotide differences (k) was 9.858. Among the native reserves, the populations in LS had the highest genetic diversity parameters. Contrastingly, LYS and LKS had the lowest genetic diversity. The haplotype polymorphism sites are presented in S. Figure 2.
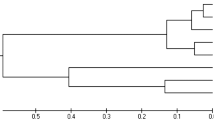
An NJ phylogenetic tree was constructed based on the genetic distances of the mtDNA CR haplotypes generated from Shanxi Province (Fig. 2). The 20 haplotypes were divided into 3 major clades, each containing rare haplotypes. NJ tree clustering (Fig. 2) and median-joining network (Fig. 3) yielded consistent results. Haplotypes from the five nature reserves were interspersed, forming the largest dominant clade, Clade I, which included 11 haplotypes. Clades II and III comprised haplotypes from LS and shared haplotypes from LS and MH, containing four and five haplotypes, respectively.
Neighbor-joining phylogenetic tree of wild forest musk deer mtDNA D-loop haplotypes in Shanxi Province. Shanxi Luyashan National Nature Reserve (LYS), Shanxi Pangquangou National Nature Reserve (PQG), Shanxi Lingkongshan National Nature Reserve (LKS), Shanxi Lishan National Nature Reserve (LS), Yangcheng Mangrove Gibbon National Nature Reserve (MH).
Median-joining network of wild forest musk deer mtDNA D-loop haplotypes in Shanxi Province. Shanxi Luyashan National Nature Reserve (LYS), Shanxi Pangquangou National Nature Reserve (PQG), Shanxi Lingkongshan National Nature Reserve (LKS), Shanxi Lishan National Nature Reserve (LS), Yangcheng Mangrove Gibbon National Nature Reserve (MH). The black nodes represent missing haplotypes, and the number of short lines on the segments between haplotypes indicates the number of mutational steps. The I-III numbers refer to Clad I-III.
The second NJ phylogenetic tree (S. Figure 3) was constructed based on the genetic distances of collected CR haplotype data for four additional populations, including captive and wild forest musk deer from Shaanxi and Sichuan Provinces, and the haplotypes generated from Shanxi Province. This tree included a total of 93 haplotypes, each trimmed to a length of 571 bp. The haplotypes of the Shanxi, Shaanxi and Sichuan populations (both wild and captive) were not completely separated on the phylogenetic tree, with some haplotypes intermingling Additionally, haplotypes of captive populations (from Shaanxi and Sichuan) partially overlapped with those of wild populations. However, the haplotypes of the Shanxi population showed some degree of differentiation from those of the Shaanxi and Sichuan populations. Although haplotypes from different populations were not completely separated on the phylogenetic tree, the majority of haplotypes were geographically distinct, with only 8 out of 93 haplotypes shared between different Provinces.
Population genetic structure
Based on the genetic distances between the haplotypes and the mountain ranges where the nature reserves were located, the five nature reserves were divided into two geographic groups: the Lüliang and Taiyue (LLTY) mountain range group and the Zhongtiao (ZT) mountain range group.
As illustrated in Table 3, the average genetic distance between LS and MH was the largest, whereas that between LYS and LKS was the least. The internal genetic distance within LS was the greatest, whereas LYS and LKS, with only one haplotype each, had the least internal genetic distance of 0. The genetic distance between the LLTY and ZT mountain range groups was greater than that within the LLTY group but smaller than the distance within the ZT group.
As illustrated in Table 4, there were significant genetic differences (P < 0.05) between the LKS and the other four nature reserves, LS and LYS, and LLTY and the ZT mountain range groups. No significant genetic differences (P > 0.05) were found among the other populations. Gene flow was greatest between the LS and MH populations (12.20) and the least between the LYS and LKS populations (0).
AMOVA (Table 5) revealed that much of the genetic variation in the wild forest musk deer populations from the five nature reserves in Shanxi Province was within the populations (88.58%), whereas genetic differentiation among the populations was relatively low (11.42%). The highly significant Fixation Index of 0.1142 indicated that genetic differences among populations were unlikely caused by random factors, indicating that more genetic variation occurred within the reserve populations than among the five reserves in Shanxi Province.
Population historical dynamics
The results of neutrality tests (Tajima’s D and Fu’s Fs) revealed positive values that were not statistically significant (Table 6), indicating that the wild forest musk deer populations in the five nature reserves of Shanxi Province were consistent with that of the neutral selection model and the populations had not expanded. Population dynamics analyses were not conducted for the LYS and LKS nature reserves, each containing only one haplotype. The mismatch distribution curve exhibits a multimodal pattern (Fig. 4) that does not fit the population expansion model. Collectively, these results suggest that wild forest musk deer populations in the five nature reserves of Shanxi Province may not have undergone rapid expansion events.
Mantel analysis of geographic and genetic distances
A Mantel test was performed using GenAlEx software to analyze the correlation between geographic and genetic distances for wild forest musk deer populations in Shanxi Province (Fig. 5). The results revealed that the correlation between geographic and genetic distances was weak (Rxy = 0.009) and not statistically significant (P = 0.390). Furthermore, regression analysis revealed that geographic distance had an extremely low explanatory power for genetic distance (R2 = 0.0061).
Discussion
Our study was based on 129 mtDNA CR sequences from five nature reserves across three mountain ranges in Shanxi Province, which defined 20 haplotypes. The Hd was 0.916, and the Pi was 0.01505. In contrast to other wild forest musk deer populations, the Hd of the wild population in Shanxi Province was greater than that of the wild population in the Baishui River, Sichuan Province (0.7213)19 but slightly lower than that of the wild and captive populations in Qinling, Shaanxi Province (0.931)20. Compared with captive forest musk deer populations, Hd in Shanxi Province was greater than that in the captive population in the Shaanxi Province (0.908)22 but lower than that in the captive population in the Sichuan Province (0.934)23. However, the Pi was lower than that of the aforementioned four forest musk deer populations 19,20,22,23. Compared with those of other musk deer species, the Hd and Pi of the wild forest musk deer in Shanxi Province were greater than those of the wild Alpine musk deer in the Helan Mountain (Hd = 0.716 and Pi = 0.00156)24 but lower than those of the wild populations of the Siberian musk deer in Northern Russia (Hd = 0.970 ± 0.018 and Pi = 0.026 ± 0.005)25, indicating that the wild forest musk deer population in Shanxi Province maintains a relatively high genetic diversity, making it a valuable genetic resource with important conservation value26.
The LLTY mountain range group (consisting of the LYS, PQG, and LKS nature reserves) contained 4 haplotypes, whereas the ZT mountain range group contained 16 haplotypes, and the 2 groups were distinct, indicating that the LLTY and ZT mountain range groups harbor unique genetic resources. In comparison, the LS population from the ZT mountain range group had the highest genetic diversity, with 75% of the total and 55% of unique haplotype numbers found in the whole study area. Five rare haplotypes were found in this population, demonstrating the important role of the LS population in maintaining the overall genetic diversity of forest musk deer in Shanxi Province. Furthermore, four of the five haplotypes found in the MH population were shared with the LS population, while the fifth haplotype was unique to the MH population. This further corroborated the critical role of the musk deer population inhabiting the ZT mountain range in preserving the genetic diversity of forest musk deer in Shanxi.
Genetic structure analysis revealed that the LS and MH populations within the ZT mountain range presented insignificant genetic differentiation (Fst < 0.05, P > 0.05), with a high gene flow of 12.20, indicating frequent genetic exchange between the two, likely because they belong to the same mountain range and are approximately 43.8 km apart. Contrastingly, a relatively high level of genetic differentiation was detected between the LLTY and ZT mountain ranges (Fst = 0.20305, P < 0.05)27, with weaker gene flow (Nm = 1.96245) than that within the ZT mountain range. The AMOVA results revealed that much of the genetic variation in the wild forest musk deer populations in the five nature reserves in Shanxi Province exhibited within-population variation, whereas genetic differentiation among populations was relatively low. Combining the genetic distance, genetic differentiation values, and gene flow between the populations, it was determined that much of the genetic variation originated from the ZT mountain range group and, more precisely, from the LS population, indicating that the LS population accounts for the vast majority of genetic variation in wild forest musk deer populations across Shanxi Province.
Although the average genetic distance between the LS and MH populations is the largest among the studied populations, the genetic structure analysis (Fst < 0.05, P > 0.05) revealed insignificant genetic differentiation. The apparent inconsistency can be attributed to the high genetic diversity within the LS population. The LS population contains 15 haplotypes, accounting for most of the haplotype diversity in the Shanxi region, while the MH population has only 5 haplotypes, 4 of which are shared with the LS population. This shared genetic background reduces inter-population differentiation, as reflected by the low and insignificant Fst value. Moreover, AMOVA analysis confirmed that most of the genetic variation originates from populations rather than between populations, further supporting this conclusion. Therefore, the large genetic distance reflects the contribution of LS population’s internal diversity, rather than true differentiation between LS and MH.
Neutrality tests and mismatch distribution curves are commonly used to analyze the historical dynamics of populations. Specifically, when Tajima’s D and Fu’s Fs values are negative and statistically significant28 and the mismatch distribution curve exhibited a unimodal pattern29, indicating that the target population had experienced population expansion. However, the neutrality test results for the wild forest musk deer populations in Shanxi Province had positive values that were not statistically significant, and the mismatch distribution curves exhibited a multimodal pattern, suggesting that forest musk deer in Shanxi Province may not have experienced rapid expansion.
Geographic isolation has been widely accepted as a key factor driving population genetic differentiation30,31,32. However, the Mantel test analysis of wild forest musk deer populations in Shanxi revealed no significant correlation between geographical and genetic distances, suggesting that geographic distance may not be the primary driver of the observed genetic differentiation. We postulate that Shanxi Province, characterized by rugged mountainous terrain interspersed with basins and as the historic cradle of Chinese agricultural civilization33,34,35, presents a landscape in which intense human economic activities within the basin areas have likely impeded gene flow among forest musk deer populations. This landscape-level anthropogenic influence may be the principal reason for the relatively pronounced genetic differentiation observed among the geographical populations of forest musk deer in Shanxi and most of the genetic variation was within rather than among populations.
The captive breeding of forest musk deer first began in Shaanxi Province in 1958, which was only 66 years ago. The captive populations in Shaanxi and Sichuan Provinces are derived from local wild populations, which explains the observed overlap between the haplotypes of the captive populations and those of the wild populations. Additionally, there have been exchanges of genetic resources between the captive forest musk deer in Sichuan and Shaanxi. Shaanxi Province is located in central China, with Shanxi Province situated to its northeast and Sichuan Province to its southwest. Shaanxi shares a border with both Shanxi and Sichuan, indicating historical gene flow among these populations. These factors account for why the haplotypes of the different populations (both wild and captive) from Shanxi, Shaanxi, and Sichuan were not completely separated in the phylogenetic tree, with some haplotypes intermingling. However, despite this gene flow, the haplotypes of the Shanxi population exhibited a certain degree of differentiation compared to those from Shaanxi and Sichuan. Moreover, the majority of haplotypes were geographically distinct (only 8 out of 93 haplotypes shared between different Provinces), suggesting that the forest musk deer populations in the different Provinces are to a large extent genetically unique. The haplotypes of the wild populations (particularly those in Shanxi) were not fully represented by the captive populations. Therefore, it is recommended to enhance the protection of the wild populations with high genetic diversity and to incorporate more genetic diversity into the genetic management of captive populations to avoid the risks of genetic drift and inbreeding.
Conclusion
Given that the wild forest musk deer populations in Shanxi Province still maintain relatively high genetic diversity, particularly with the ZT mountain range group occupying a prominent position in genetic variation, we propose the following conservation strategy from an ecological genetics’ perspective. Rather than viewing the genetic diversity of the different mountain range system populations in isolation, we recommend considering them as a metapopulation genetic resource. The implementation of strategic interchanges of genetic material among mountain range groups could be an important management approach for improving and enriching the overall genetic diversity of various local populations. This genetic rescue strategy may be crucial for promoting the recovery and long-term evolutionary resilience of wild forest musk deer populations across Shanxi Province.
Materials and methods
Study area
Shanxi Province is in the mountainous area of the southern Loess Plateau and has a temperate continental monsoon climate with four distinct seasons: hot and rainy summers and cold and dry winters. The average annual temperature is 8.5 °C, with January and July being the coldest and hottest months, with average temperatures of approximately -8.5 °C and 23.5 °C, respectively36. The LL, ZT, and TY mountain ranges have elevations ranging from 1,500 to 3,000 m, and precipitation increases with increasing elevations ranging from 400 to 600 mm. Vegetation varies with altitude, presenting as crops, shrublands, mixed coniferous-deciduous and coniferous forests, and subalpine meadows. The dominant tree species were Picea asperata, Larix gmelinii, Pinus tabuliformis, and Quercus mongolica37,38. These three mountain ranges are home to several national first-class key-protected endangered species, including forest musk deer, leopards, and Chinese Grouse39.
The present study was conducted from February to October 2023 in five national nature reserves across three mountain ranges in Shanxi Province: Shanxi Luyashan National Nature Reserve (LYS) and Shanxi Pangquangou National Nature Reserve (PQG) from the LL mountain range; Shanxi Lishan National Nature Reserve (LS) and Yangcheng Mangrove Gibbon National Nature Reserve (MH) from the ZT mountain range; and Shanxi Lingkongshan National Nature Reserve (LKS) from the TY mountain range (S. Table 2 and Fig. 1). These areas were selected because they are known habitats of wild forest musk deer populations, and systematic surveys and fecal sample collections were carried out at these sites.
Sample collection
We reviewed historical grid-based infrared camera data and interviewed local herb collectors, shepherds, and experienced forest rangers to preliminarily determine the potential locations where forest musk deer may appear. Guided by these local informants, we visited areas where forest musk deer had been previously observed and collected suspected forest musk deer feces along survey routes. The forest musk deer fecal pellets were bluntly elliptical, elongated, and cylindrical, approximately 9–10 mm long and 5–6 mm wide40. Owing to the strong territoriality of musk deer, we collected fecal samples at least 300 m apart (considering the territory range of published literature)41,42,43,44 to minimize the likelihood of duplicate sampling. All fecal samples that fit the described morphological characteristics were collected using disposable polyethylene gloves and placed in 25-mL sterile RNase-free centrifuge tubes, with 30 pellets collected per pile. Sample details, including the collection date, locality, sample ID, and freshness of the feces, were recorded. Upon completion of the daily sampling, all samples were stored at -20 °C until further processing.
DNA extraction and polymerase chain reaction amplification
Fecal samples were preprocessed according to the methods described by Piggott45 and Tang46. Approximately eight fecal pellets were processed into cell suspensions, and genomic DNA was extracted from the fecal samples using a blood/cell/tissue genomic DNA extraction kit (Tiangen Biotech Co., Ltd., Beijing, China). The extracted DNA was stored at -20 °C until subsequent analyses.
The PCR system had a total volume of 25 μL, consisting of 12.5 μL of 2 × Es Taq MasterMix Dye (CoWin Biosciences, Cambridge, MA, USA), 4 μL bovine serum albumin (Thermo Fisher Scientific, Waltham, MA, USA), 30–50 ng of DNA template, 1 μL each of forward and reverse primers (10 μM), and the remaining volume was filled with double distilled H2O.
The mitochondrial CR primers used were from Feng47, with the forward primer F: 5′-CAACTAACCTCCCTAAGACTTCAAG-3′ and the reverse primer R: 5′-CCAAATGTATGACAGCACAGTTATG-3′. The PCR program was as follows: 95 °C predenaturation for 1 min; 94 °C predenaturation for 4 min; 94 °C denaturation for 30 s; 56 °C annealing for 45 s; 68 °C extension for 1 min; 35 cycles; final extension at 68 °C for 10 min; and storage at 4 °C.
After PCR, 5 μL of the amplified product was subjected to 1% agarose gel electrophoresis to check for a clear and single target band. The qualified PCR products were sent to Sangon Biotech Co., Ltd., Shanghai, China, for purification and Sanger sequencing in the forward and reverse directions.
Data analysis
Successfully sequenced mtDNA CR sequences were searched for and compared with existing forest musk deer sequences in the National Center for Biotechnology Information database. Samples with more than 98% similarity to known forest musk deer sequences were considered to belong to the target species. The forward and reverse sequences identified as forest musk deer were assembled and trimmed using SeqMan Pro software in DNASTAR Lasergene 7.1 (DNASTAR, Madison, WI, USA), with manual corrections48. All sequences were aligned and analyzed using Molecular Evolutionary Genetic Analysis (MEGA) 11, and nucleotide composition and computed genetic distances were analyzed based on the Kimura 2-parameter model49. DnaSP v5 (DNASTAR) was used to calculate the number of polymorphic sites, haplotype number (h), nucleotide diversity (Pi), haplotype diversity (Hd), and average number of nucleotide differences (K) for each nature reserve and to generate mismatch distribution plots50.
The haplotype sequences were organized and used to construct a neighbor-joining (NJ) phylogenetic tree using MEGA 11 with 1,000 bootstrap replications49. The tree was then beautified via TVBOT software51. A haplotype network was constructed using POPART 1.7 (University of Otago, Dunedin, New Zealand)52. Further analyses, including analysis of molecular variance (AMOVA), pairwise genetic differentiation (Fst) calculations, and Tajima’s D and Fu’s Fs tests, were conducted using Arlequin 3.5 (Swiss Institute of Bioinformatics, Geneva, Switzerland)53. The total number of haplotypes was estimated using the ACE in EstimateS 9.1.0 (Swiss Institute of Bioinformatics)54 and a haplotype accumulation curve was plotted.
The genetic distances between samples were calculated using GenAlEx 6.51 (Swiss Institute of Bioinformatics)55, and the geographic distances between sampling locations were determined based on the coordinates. The Mantel test was performed to explore the correlation between the genetic and geographic distances of forest musk deer populations.
Finally, collecting CR haplotype data for four additional populations in GenBank from previous studies19,20,22,23, including captive and wild forest musk deer from Shaanxi and Sichuan Provinces. Additionally, the CR haplotype sequence of Bos taurus was included as an outgroup to root the phylogenetic tree. By combining these data with the haplotype data from this research, the second phylogenetic tree was constructed. All the CR haplotype sequences above were trimmed to 571 bp to ensure consistent alignment.
Data availability
The mtDNA CR haplotype sequence data are available in GenBank, accession: PQ534305-PQ534324.
References
Lande, R. Genetics and demography in biological conservation. Science 241, 1455–1460 (1988).
Frankham, R. Conservation genetics. Annu. Rev. Genet. 29, 305–327 (1995).
Frankham, R. Relationship of genetic variation to population size in wildlife. Conserv. Biol. 10, 1500–1508 (1996).
Bergl, R. A. & Vigilant, L. Genetic analysis reveals population structure and recent migration within the highly fragmented range of the Cross River gorilla (Gorilla gorilla diehli). Mol. Ecol. 16, 501–516 (2007).
Reddy, P. A. et al. Genetic evidence of tiger population structure and migration within an isolated and fragmented landscape in Northwest India. PLoS ONE 7, e29827 (2012).
Wultsch, C., Waits, L. P. & Kelly, M. J. Noninvasive individual and species identification of jaguars (Panthera onca), pumas (Puma concolor) and ocelots (Leopardus pardalis) in Belize, Central America using cross-species microsatellites and faecal DNA. Mol. Ecol. Resour. 14, 1171–1182 (2014).
Zemanova, M. A. & Ramp, D. Genetic structure and gene flow in eastern grey kangaroos in an isolated conservation reserve. Australas. I. Min. Met. 13, 570 (2021).
Brown, W. M., George, M. & Wilson, A. C. Rapid evolution of animal mitochondrial DNA. Proc. Natl. Acad. Sci. 76, 1967–1971 (1979).
Avise, J. C. et al. Intraspecific phylogeography: The mitochondrial DNA bridge between population genetics and systematics. Annu. Rev. Ecol. Syst. 18, 489–522 (1987).
Saccone, C., Attimonelli, M. & Sbisà, E. Structural elements highly preserved during the evolution of the D-loop-containing region in vertebrate mitochondrial DNA. J. Mol. Evol. 26, 205–211 (1987).
Sbisà, E., Tanzariello, F., Reyes, A., Pesole, G. & Saccone, C. Mammalian mitochondrial D-loop region structural analysis: Identification of new conserved sequences and their functional and evolutionary implications. Gene 205, 125–140 (1997).
Moum, T. & Árnason, E. Genetic diversity and population history of two related seabird species based on mitochondrial DNA control region sequences. Mol. Ecol. 10, 2463–2478 (2001).
Grunwald, C., Stabile, J., Waldman, J. R., Gross, R. & Wirgin, I. Population genetics of shortnose sturgeon Acipenser brevirostrum based on mitochondrial DNA control region sequences. Mol. Ecol. 11, 1885–1898 (2002).
Nyakaana, S., Arctander, P. & Siegismund, H. R. Population structure of the African savannah elephant inferred from mitochondrial control region sequences and nuclear microsatellite loci. Heredity 89, 90–98 (2002).
Liu, Z. et al. Phylogeography and population structure of the Yunnan snub-nosed monkey (Rhinopithecus bieti) inferred from mitochondrial control region DNA sequence analysis. Mol. Ecol. 16, 3334–3349 (2007).
Green, M. J. B. The distribution, status and conservation of the Himalayan musk deer Moschus chrysogaster. Biol. Conserv. 35, 347–375 (1986).
Yang, Q., Meng, X., Xia, L. & Feng, Z. Conservation status and causes of decline of musk deer (Moschus spp.) in China. Biol. Conserv. 109, 333–342 (2003).
Wang, Y.-X. IUCN red list of threatened species: Moschus berezovskii. https://www.iucnredlist.org/species/13894/103431781 (2015).
Hu, D. M. et al. A genetic diversity study based on microsatellite and mitochondria of forest musk deer in the Baishuihe National nature reserve, Sichuan. Sichuan J. Zool. 40, 641–648 (2021).
Feng, H., Feng, C. L., Huang, Y. & Tang, J. Structure of mitochondrial DNA control region and genetic diversity of Moschus berezovskii populations in Shaanxi Province. Genet. Mol. Res. 15 (2016).
Yang, C. et al. DNA barcoding revises a misidentification on musk deer. Mitochondrial DNA 26, 605–612 (2015).
Wang, Z. et al. mtDNA CR evidence indicates high genetic diversity of captive forest musk deer in Shaanxi province, China. Animals 13, 2191 (2023).
Peng, H., Liu, S., Zou, F., Zeng, B. & Yue, B. Genetic diversity of captive forest musk deer (Moschus berezovskii ) inferred from the mitochondrial DNA control region. Anim. Genet. 40, 65–72 (2009).
Zhang, Z. R. Population Demography and Conservation Genetics of Alpine Musk Deer in Helan Mountains, China (Northeast Forestry University, 2024). https://doi.org/10.27009/d.cnki.gdblu.2023.000073.
Kholodova, M. V. & Prikhodko, V. I. Molecular genetic diversity of musk deer Moschus moschiferus L., 1758 (Ruminantia, Artiodactyla) from the northern subspecies group. Russ. J. Genet. 42, 783–789 (2006).
Grant, W. & Bowen, B. Shallow population histories in deep evolutionary lineages of marine fishes: insights from sardines and anchovies and lessons for conservation. J. Hered. 89, 415–426 (1998).
Wright, S. Evolution and the Genetics of Populations, Volume 4: Variability Within and Among Natural Populations (University of Chicago Press, 1984).
Tajima, F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123, 585–595 (1989).
Márcia Barbosa, A., Real, R., Muñoz, A.-R. & Brown, J. A. New measures for assessing model equilibrium and prediction mismatch in species distribution models. Divers. Distrib. 19, 1333–1338 (2013).
Wright, S. Isolation by distance. Genetics 28, 114–138 (1943).
Barton, N. H. & Hewitt, G. M. Analysis of hybrid zones. Annu. Rev. Ecol. Syst. 16, 113–148 (1985).
Hewitt, G. The genetic legacy of the Quaternary ice ages. Nature 405, 907–913 (2000).
Ho, P.-T. The loess and the origin of Chinese agriculture. Am. Hist. Rev. 75, 1–36 (1969).
Wang, L., Wang, X. Y., Wen, Q. F. & Shi, Y. Shanxi Province is the origin center of primitive farming. Agricult. Archaeol. 21–25 (2010).
He, Y. Characteristics and advantages of traditional agricultural economy. In The Agricultural Civilization of Ancient China (ed. He, Y.) 19–36 (Springer Nature, 2023). https://doi.org/10.1007/978-981-19-8469-3_3.
Editorial Committee of the Great Dictionary of China’s Administrative Divisions. Great Dictionary of China’s Administrative Divisions, 409–410 (Zhejiang People’s Publishing House: Zhejiang Electronic Audio-Visual Publishing House, 1999).
Wu, Z. Y. 172254. Chinese Vegetation, 1357–1375 (Beijing (China) Science Press, 1980).
Qiu, L. C. & Wang, S. Y. Spatial patterns and successions of vegetation in Shanxi. Plant Res. 34, 6 (2014).
Fan, L. S. & Liu, H. J. Mammals of Shanxi 72–75 (China Forestry Publishing House, 1996).
Wu, J. Y. & Wang, W. Musk Deer of China 49–50 (China Forestry Publishing House, 2006).
Zheng, S. W. & Pi, N. L. Ecological studies on the alpine musk deer. Acta Zool. Sin. 25, 176–186 (1979).
Harestad, A. S. & Bunnel, F. L. Home range and body weight–a reevaluation. Ecology 60, 389–402 (1979).
Wu, J. P., Zhang, Y., Fu, D. L. & Lan, W. X. Comparison of home range of Siberian musk deer and goral in winter. J. Northeast For. Univ. 36, 58–60 (2008).
Liu, J. H. et al. Home range utilization and individual dispersal of re-introduced forest musk deer (Moschus berezovskii). Acta Theriologica Sin. 40, 109–119 (2020).
Piggott, M. P. & Taylor, A. C. Extensive evaluation of faecal preservation and DNA extraction methods in Australian native and introduced species. Aust. J. Zool. 51, 341–355 (2003).
Tang, J. et al. Efficiency and analysis of DNA extraction from forest musk deer Feces using two methods. Northwest Agricult. J. 27, 326–330 (2018).
Feng, H., Huang, Y., Ren, Y., Feng, C. L. & Liu, X. N. Structure of the mitochondrial DNA D-Loop region and genetic diversity of Moschus berezovskii in Shaanxi Province. Acta Zool. Sin. 34, 5887–5895 (2014).
Burland, T. G. DNASTAR’s Lasergene sequence analysis software. In Bioinformatics Methods and Protocols (eds Misener, S. & Krawetz, S. A.) 71–91 (Humana Press, 1999). https://doi.org/10.1385/1-59259-192-2:71.
Tamura, K., Stecher, G. & Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evolut. 38, 3022–3027 (2021).
Librado, P. & Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25, 1451–1452 (2009).
Xie, J. et al. Tree Visualization By One Table (tvBOT): a web application for visualizing, modifying and annotating phylogenetic trees. Nucleic Acids Res. 51, W587–W592 (2023).
Leigh, J. W. & Bryant, D. popart: full-feature software for haplotype network construction. Methods Ecol. Evolut. 6, 1110–1116 (2015).
Excoffier, L. & Lischer, H. E. L. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 10, 564–567 (2010).
Colwell, R. K. EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples. Version 9.1.0. Available online: https://www.robertkcolwell.org/pages/1407-estimates (2019).
Peakall, R. & Smouse, P. E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—An update. Bioinformatics 28, 2537–2539 (2012).
Acknowledgements
We are very grateful to the staff and local guides of the Shanxi Luyashan National Nature Reserve, Shanxi Pangquangou National Nature Reserve, Shanxi Lishan National Nature Reserve, Yangcheng Mangrove Gibbon National Nature Reserve, and Shanxi Lingkongshan National Nature Reserve (LKS) for their tremendous support and assistance in sample collection. We thank our sampling team members for their hard work in Shanxi Province.
Funding
This study was funded by the National Key Research and Development Program of China (2023YFC3304000); the Muskdeer Survey Project in Shanxi Province (2024HXFWBH).
Author information
Authors and Affiliations
Contributions
L. H., Y. L., Z. L., C. P., and X. L. collected the samples. L.H. and X.L. conceived and conducted the experiments. L.H. analyzed the results. Y. L. prepared Fig. 1. L.H. wrote the paper, and D.H. reviewed the manuscript. All the authors approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hai, L., Li, Y., Lan, X. et al. Insight into mtDNA diversity of wild forest musk deer (Moschus berezovskii) in Shanxi Province mountains. Sci Rep 15, 5523 (2025). https://doi.org/10.1038/s41598-025-89478-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-89478-z