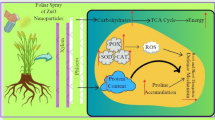
Abstract
Drought-stressed and zinc-deficient soils are major contributors to reduced wheat yields and low-quality grains, especially in semi-arid regions of the world. Zinc-oxide nanoparticles (ZnO-NPs) are adept enough to avoid these losses if applied under the right dose at the right growth stage of many crops including wheat (Triticum aestivum L.). Therefore, a pot experiment was conducted with four levels of ZnO-NPs (0, 50, 100 and 150 ppm), and drought imposed at tillering (D1) and grain filling (D2) stages, considering normal irrigation as control (D0), to explore interactive effects of ZnO-NPs and drought episodes on growth, eco-physiology, yield, and grain quality of wheat. The results depicted dose and growth stage-dependent variations in all recorded parameters. ZnO-NPs (150 ppm) significantly increased the number of grains (12.5%), grain weight (12.4%), total yield (25.5%), and zinc contents (58.6%) when the crop was exposed to drought stress at tillering stage, compared to the control treatment. Likewise, drought at grain filling stage with ZnO-NPs (150 ppm) significantly enhanced plant height, spike length, biomass, zinc contents, and grain protein by 15.5%, 3.2%, 16.7%, 100.0%, and 53.8%, respectively, when compared with control treatment. Thus, ZnO-NPs emerged as a potential drought alleviator and yield-oriented safe nano-fertilizer for wheat in semi-arid regions facing irrigation challenges.
Similar content being viewed by others
Introduction
Drought among all other abiotic stresses is meticulous as one of the top listed environmental issue restricting different plant growth aspects and causing more severe yield losses leading to serious food scarcity1. The population figure across the globe is estimated to rise by about 9–10 billion or even more masses up to the middle of the present century. Contrariwise, exact in the same pace of time, the droughts may lead to serious food insecurity that will probably get worse under anticipated global climate changes2,3,4. Thus, this upsetting global masses’ upsurge, demands increased food production to meet global food requirements. Global extreme weather disasters and increased droughts put global food security at high risk which is getting agitated further by ever-proliferating global residents and their feeding challenges5. Considering all the facts, water for crop irrigation could be branded as the highest curbing natural resource in agroecosystems of arid and semi-arid backgrounds6. Among the prime sources, drought is one of the main sources of insufficient agronomic productions in semi-arid and arid regions across the globe7. For maximum parts of these regions, drought is a spartan restraint of plant growth, development, and productivity8. Pakistan lies in the locale of arid and semi-arid terrain characterized by severe climatic circumstances, with an average annual precipitation of 250–500 mm and temperatures ranging from 20 to 40 °C9. Consequently, drought stress poses a significant challenge to food security in this region10.
Among all cereal crops, wheat is the favorite food for human beings and as feed for animals too11. Wheat and its different byproducts in human diet is basically the inexpensive protein and human calories intake12. Estimatedly, 60% of the daily calorie intake is relied on this small grained crop and that’s why it is envisaged as the most important cereal crop in the world keeping in view its total production, cultivated area, and human consumptions13. So, it can be a very helpful source for efficient and energetic human health globally. In Pakistan, wheat planting is done under wide ranged soil profiles and climatic situations14. To sustain healthy plant life, water serves as a basic unit and its limited supply or unavailability may cause ultimate plant death. Wheat yield is severely affected by drought, particularly at critical growth stages like tillering and anthesis15. Drought directly upsets the grain formation in wheat16,17.
Previously, Hernández-Espinosa et al.18 experimented for two years under six environmental conditions with 54 genotypes of bread wheat and concluded; Grain morphology (specifically grain density and size), as well as Grain quality (specially protein, and flour quality) were severely affected by drought and heat stress. In the past, Ivanova et al.19 observed the effect of drought stress on the seven wheat varieties and reported that drought significantly affected the growth and yield of all tested wheat varieties. Furthermore, the aftermaths of their research results indicated that; 40%, 29%, 15%, and 30% reduction in grain weight, the grains per spike, grain size, and grain yield, respectively were recorded in drought-affected wheat plants as compared to the control treatment. Recently, Ojuederie et al.20 commenced that climate change has a link with global warming. Hence, indispensable innovative technologies are required for food security, especially in the developing world. To cope with drought-induced plant deaths, various strategies are being adopted now a days and among all those, the application of nanomaterials (NMs) has been proven as a promising and effective one. Consequently, various NMs have been reported to enhance crop production which is a go ahead to meet the growing global demands for food, feed, and fuel1,4.
Nanomaterials are natural, incidental, or manufactured materials containing particles, in; an unbound state, an aggregate, or as an agglomerate with size in the range of 1–100 nm21. Nanotech research and development in the agricultural crop sciences is likely the forecast of coming events regarding the next level expansion of genetically modified crops and precision farming techniques22; For the emerging world in agricultural prospects, the drive is to develop drought and pest-resistant crops23. Hence, it has many contributions to crop production as well as to eco-economic stability. The entire value chain of the agricultural production system can be subjugated with the help of nanoparticles24. Nanotechnology in agriculture will grow faster in near future with explanations of profound collaborations between nanoparticles (NPs) and plants25. The presence of NPs inside the plant tissues can restrain oxidative stress enzymes and trigger the plant defense system26. The size and concentration of NPs, their mode of application, plant species, and soil conditions may mold them to be a source of advantageous or adversarial effects on plant efficiency27. Usually, the NPs influence the plants by a two phasic dose-reaction “hormesis” with an up-dose inhibition and low-dose stimulation28.
The distinct physicochemical properties of NPs make them capable to increase plant metabolism29. NPs with dimensional characteristics like lesser physique and outsized surface area are perfect materials for use in plants, offering a significant way to release trace elements step by step in an exact manner, and consequently; NPs have established their place and roles in agriculture30. Zinc (Zn) is an essential constituent of various dynamic enzymes (such as; glutamate dehydrogenase-GDH, catalase-CAT, and superoxide dismutase-SOD), and also take part in chlorophyll and hormone synthesis31. Arough et al.32 indicated the positive effects of Zn application under salt stress. Dimkpa et al.33 reported that nano-ZnO improved grain yield and nutrient translocation in drought-stressed situations. Similarly, nano-ZnO may recruit speedy use mechanisms of seed food reservoirs, zinc uptake, and expression of antioxidants under drought. So, the use of nano-ZnO augmented; seed germination, sprout progression, yield, and WUE in rice, soybean, and sunflower crops34,35,36. Furthermore, Elshayb et al.37 and Abbas et al.38 have reported the beneficial effects of ZnO-NPs on the growth, yield, and grain quality of rice and wheat, respectively, under drought. Therefore, current study aimed; an evaluation of different doses of zinc-oxide nanoparticles (ZnO-NPs) on drought-stressed wheat seedlings at; (1) tillering stage (D1) and, (2) grain-filling stage (D2). We hypothesize that the use of ZnO-NPs will improve the growth, water relations, productivity, physiology and quality of wheat grains under the drought stress.
Materials and methods
Crop husbandry
The current pot experiment was carried out in the wire house of Department of Agronomy, The Islamia University of Bahawalpur, Pakistan (Latitude: 29°23 × 60.00″ N, Longitude: 71°40 × 59.99″ E, and height from sea level approximately 117 m). The experiment was conducted in completely randomized design (CRD) with factorial settings having three replications. The treatments were assigned randomly to experimental units (pots) within each replication to ensure unbiased results and to enhance the reliability of the findings. Four different dose levels of ZnO-NPs (ZnO-NPs0 = 0 ppm, ZnO-NPs1 = 50 ppm, ZnO-NPs2 = 100 ppm, and ZnO-NPs3 = 150 ppm) were evaluated against drought stress at critical growth stages (D0 = no drought, D1 = drought at tillering, D2 = drought at grain filling) of wheat. The seeds of wheat genotype (Galaxy 2013) were purchased from the Regional Agricultural Research Institute, Bahawalpur. The seeds were surface disinfected with 0.5% sodium hypochlorite (NaOCl) solution by dipping for 5 min and subsequently, washed many times by using distilled water. Ten seeds were planted on November 15, 2019, in each pot (30 × 30 cm) filled with 15 kg clay loam dry soil, amended with ZnO-NPs according to the study scheme, under wire house conditions with weather specifications of 70 ± 5% relative humidity and a day/night temperature of 31/20°C, respectively. The NPK was fertilized at a basal dose of 150:100:60 kg ha− 1 at sowing time. Nitrogen was split into 2 doses i.e., half with P, K, and the other half after 3 weeks of plant growth. Periodically, random rotation of pots was kept continuously and manual weeding was done regularly. The results of the physio-chemical analysis of soil samples are presented in Table 1. A transparent plastic sheet was used to cover the wire house to avoid rain, when required. After two weeks of germination, the plants were thinned, leaving only five uniform plants per pot.
Drought imposition and characterization of ZnO-NPs
All the pots were irrigated uniformly until the imposition of drought at tillering and grain filling stages. The 25% water holding capacity (WHC) was maintained in pots under drought at critical growth stages (tillering and grain filling) of wheat and 80% WHC was considered as control. The soil WHC was determined before the experiment by saturating the soil and measuring the water retained after drainage. The required amount of water was calculated based on pot weight, and soil moisture content were monitored regularly using a Theta probe (soil moisture sensor). Adjustments were made as needed by withholding or adding water to ensure that soil moisture content remained at the target WHC throughout the experiment.
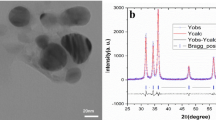
Zinc oxide nanoparticles (ZnO-NPs) used in the current study were sourced as zinc oxide nano powder (Assay ~ 80% zinc basis, MW 81.39 g/mol) from SIGMA ALDRICH®, USA. The ZnO-NPs were characterized to confirm their morphology, size, and purity. The X-ray diffraction spectroscopy (XRD) revealed 99% purity with an average particle size of 70 nm, 10–25 m2g− 1 surface area, and 5.606 gm− 3 density. The morphology and topology of ZnO-NPs were studied using Scanning Electron Microscope (SEM). The SEM analysis represented the spherical-shaped nanoparticles that were agglomerated as previously reported by Ismail et al.39 (Fig. 1).
Plant harvesting and sampling procedures
The wheat plant samples were harvested at maturity stage after 18 weeks of sowing to measure growth, physiology, and yield-related attributes. Similarly, root and shoot samples were also taken to measure the length and dry weights. The harvested root samples were washed with 0.1 M HCl to remove any unwanted contaminations, followed by rewashing with distilled water. Samples were oven dried at 70 °C (Wiseven oven Model Won-155) for 72 h for root and shoot dry weights. Then, samples were crushed into small pieces for other physiological determinations.
Measurements of leaf water relations
Following the techniques considered by, Thomas et al.40 for recording different measurements at different growth stages, the calculation of the Leaf area index (LAI) was done as the ratio of the surface area of a leaf to the ground surface where a plant stands.
From the top of the wheat plants, the 3rd number leaf (fully expanded and the youngest one) of all ten plants in each treatment was taken to determine the excised leaf water loss (ELWL). Then, these leaves were placed in polythene bags and transported all together to the laboratory as quickly as possible to minimize water losses (due to evaporation). These sampled leaves were weighed at three stages, immediately after sampling (fresh weight) (1st stage), then placed the leaves at room temperature for 6 h and wilted weight was obtained (2nd stage), and then dried in an oven for 24 h at 70 °C and the dry weight was taken out (3rd stage). Moreover, excised leaf water loss was calculated by using the following formula as given by Clarke41.
Relative water contents of leaves were calculated as described by Barrs & Weatherley42. Briefly, the fresh weight of fully expended wheat leaves just collected from the field was measured immediately after being carried to the laboratory in plastic bags to avoid loss of water contents. A turgid weight of leaf samples (Wt) was taken by soaking them for 16–18 h under normal conditions (room temperature), and then dried with tissue paper and weighed again. These leaves were oven dried for 72 h at 70 °C and were weighed to record dry weight (Wd).
Wf indicates fresh weight, Wd indicates dry weight, and Wt indicates turgor weight.
Measurement of gas exchange and physiological traits
Leaf gas exchange parameters, such as stomatal conductance (m mol m− 2 s− 1), transpiration rate (µ mol m− 1 s− 2), and net photosynthetic rate (µ mol m− 1 s− 1), were measured using an infrared gas analyzer (Li-COR LI-6250, Li-COR Biosciences, Herts, England). The measurements were taken between 09:00 AM to 10:00 AM on clear, sunny days to lessen the diurnal variability. The topmost fully expanded and healthy young leaves were selected to ensure uniformity in sampling and to avoid bias due to leaf age or damage.
Chlorophyll contents were determined 80 days after seed sowing using fully expanded leaves. A 50 mg leaf sample was extracted in 5 ml of 80% acetone in dark at 4 °C for about 24 h to prevent chlorophyll degradation and the solution was then filtered. The absorbance of the filtered solution was measured with a spectrophotometer (Model; Halo DB-20/DB-20 S, f Dynamica Company, London, UK) at 470, 647 and 664.5 nm wavelengths. Then, the chlorophyll contents were calculated using the equations described by Lichtenthaler43.
Measurement of yield and quality-related grain attributes
Agronomic attributes like tillering count per plant, plant height (cm), spike length (cm), root and shoot lengths (cm), number of grains per spike, test weight (1000 grain weight, g), biomass production per plant (g) and grain yield per plant (g) were calculated and recorded following the standard procedures and protocols. Plant height was measured by selecting five plants from each pot and measuring the height from the soil surface to the spikelet with the help of a measuring tape. A centimeter scale was used to measure spike length (from base to terminal spikelet) from seven random plants and counted the grains from these spikes too. The digital balance was used to quantify 1000-grain weight accurately.
The grain quality traits like protein and zinc concentrations were also tested. Colorimetrically estimated grain N content44 from 0.1 g of grain digested material was converted into protein content45. Additionally, ICP-OES (Model - Optima-2100–DV, Perkin-Elmer, Waltham, MA, USA) was used to determine zinc concentration from digested grain samples. The nutrients uptake by shoots i.e., N, P, and K were measured in consistence with Wolf46.
Statistical analysis
All the recorded parameters were analyzed using a statistical software (Statistix 8.1). The analysis of variance (ANOVA) was performed to check the significance of the treatment effects. When the significant differences were observed, the treatment means were compared using Fisher’s least significant difference (LSD) test at 5% probability level. Then, the data were also analyzed for the principal component analysis (PCA) using R software (2019) to check the association among different growth, yield, and quality attributes. The choice of these methods aligns with the objectives of the study to evaluate the treatment effects and interrelations among measured traits comprehensively (Tables 2 and 3).
Results
Effects of various doses of ZnO-NPs on growth attributes of wheat under drought
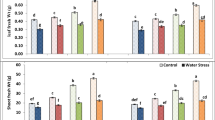
Various parameters like plant height, root length, shoot length, and root-shoot dry weight under drought stress at tillering (D1) and grain filling (D2) stages demonstrated significant improvements upon application of different doses of ZnO-NPs over control (Table 4). During the current study, shoot and root dry weight followed an increasing trend under the application of ZnO-NPs over drought or un-droughted treatments without ZnO-NPs applications. Overall, the application of ZnO-NPs (150 ppm) at D2 produced more shoot dry weight followed by 100 and 50 ppm NPs concentrations. Briefly, supply of 150 ppm (ZnO-NPs3) at the D2 drought level amplified the shoot dry weight by 81.2%, as compared to D0×ZnO-NPs0 treatment. Similarly, the application of ZnO-NPs3 level exhibited the maximum increase in root dry weight (39.9%), followed by ZnO-NPs2 (8.6%), and ZnO-NPs1 (4.2%), as compared with the D0×ZnO-NPs0 treatment (Fig. 2). A significant reduction in shoot length was shown by D1, while drought at both D1 and D2 didn’t affect root length, as compared with control (D0). The data revealed taht application of ZnO-NPs increased plant height, shoot and root length under drought and control treatments. The ZnO-NPs under D1 increased shoot length by 3.1%, 15.6%, and 21.9% at 50, 100, and 150 ppm concentration respectively as compared with shoot length under D1 without ZnO-NPs application (0 ppm). On the other hand, D2 treatment showed a maximum shoot length increment of 11.8% at 150 ppm NPs (ZnO-NPs3) as compared to similar D2 drought level with 0 ppm ZnO-NPs application rate (Fig. 2; Table 3).
Effect of different doses of zinc oxide nanoparticles on morphological traits of wheat under various stage-based drought levels. The data presented in the form of each bar is an average of three replications (n = 3). The different lower-case letters (a, b, c, d) exhibited on the bars represent statistically significant differences among the studied treatments through LSD test at 5% probability index (P ≤ 0.05). The abbreviations are as following: D0 = no drought (control), D1 = drought at tillering, D2 = drought at grain filling, ZnO-NP0 = zinc oxide nanoparticles at 0 ppm, ZnO-NP1 = zinc oxide nanoparticles at 50 ppm, ZnO-NP2 = zinc oxide nanoparticles at 100 ppm, and ZnO-NP3 = zinc oxide nanoparticles at 150 ppm.
Effects of various doses of ZnO-NPs on yield-related traits of wheat under drought
The data regarding different yield-related attributes of drought-stressed wheat under the effect of ZnO-NPs is given in Table 2. It was observed that drought stress on grain filling stage (D2) with no NPs amendment more negatively affected yield related attributes as compared to drought at tillering stage (D1). However, ZnO-NPs application significantly alleviated the toxic impacts of drought at both growth stages (tillering and grain filling) and improved grain yield and its related traits in wheat crop. Particularly, at D0 drought stress, the findings showed that ZnO-NPs3 (150 ppm) application level increased No. of grains per spike (6.8%), 1000 grain weight (7.9%), grain yield per plant (16.0%), no. of tillers (32.2%), and spike length (12.5%) followed by ZnO-NPs2 (100 ppm) and ZnO-NPs1 (50 ppm), as compared with D0×ZnO-NPs0 interaction. While at D2 drought stress, application of ZnO-NPs3 (150 ppm) prominently enhanced the no. of grains per spike, 1000 grain weight, grain yield per plant, no. of tillers per plant, and spike length by 11.2%, 5.1%, 19.8%, 33.3%, and 20.1% correspondingly, as compared with the D2×ZnO-NPs0 treatment (Table 4).
Effects of various doses of ZnO-NPs on physiology of wheat under drought
The findings presented in Fig. 3 describe the adverse impacts of drought on different leaf-related parameters like leaf area index (LAI), excised leaf water loss (ELWL, %), leaf relative water content (LRWC, %), and chlorophyll contents. All the parameters, excluding LAI, followed a declining trend across drought treatments even when ZnO-NPs, when applied at lower doses. Interaction of drought and ZnO-NPs represented the highest ELWL and LRWC at D0×ZnO-NPs0 followed by D0×ZnO-NPs3, D1×ZnO-NPs0, and D1×ZnO-NPs3 respectively. It was noticed that drought stress at tillering stage (D1) showed the lowest LAI after the application of lesser doses of ZnO-NPs (50 and 100 ppm), compared with the LAI at grain filling stage drought treatment (D2). For example, after application of ZnO-NPs3 (150 ppm rate) at D2 drought treatments exhibited an increment of 45.0% in LAI, over relevant ZnO-NPs3 treatment without drought stress (D0). Meanwhile, drought stress at tillering (D1) and grain filling (D2) stages showed a significant decrease in LRWC by 5.1% and 9.6%, while a significant increase was shown in chlorophyll contents by 12.90% and 2.21%, respectively, over un-droughted treatment without any NPs dose (D0×ZnO-NPs0). At the other end, the dose level of 150 ppm NPs (ZnO-NPs3) significantly increased LRWC at tillering stage under drought stress (D1), but no dose level of ZnO-NPs at grain filling could perform any beneficial effect. For example, D1 drought stress, the findings showed that ZnO-NPs3 (150 ppm) application level increased leaf relative water contents (LRWC) by 3.5%, and chlorophyll contents by 139.4% followed by ZnO-NPs2 (100 ppm), as compared with D1×ZnO-NPs0 interactive treatment (Tables 4 and 5).
Effect of different doses of ZnO-NPs on leaf water relations of wheat under various stage-based drought levels. The different lower-case letters (a, b, c, d) exhibited on the bars represent statistically significant differences among the studied treatments through LSD test at 5% probability index (P ≤ 0.05). The data presented in the form of each bar is an average of three replications (n = 3). The abbreviations are as following: D0 = no drought (control), D1 = drought at tillering, D2 = drought at grain filling, ZnO-NP0 = zinc oxide nanoparticles at 0 ppm, ZnO-NP1 = zinc oxide nanoparticles at 50 ppm, ZnO-NP2 = zinc oxide nanoparticles at 100 ppm, ZnO-NP3 = zinc oxide nanoparticles at 150 ppm.
Effects of various doses of ZnO-NPs on leaf gas exchange of wheat under drought
A dissimilar crux in gaseous exchange attributes of wheat leaves i.e., leaf stomatal conductance (SC), transpiration rate (Tr), net photosynthetic rate (Pn) under the interactive effect of ZnO-NPs and drought conditions has been presented in Table 3. A significant difference among gas exchange traits under various drought levels was greatly o assessed. A great reduction was noticed in SC by 3.5% and 5.7%, Tr by 77.5% and 88.5%, and Pn by 62.6% and 75.4%, respectively, after the exposure of wheat seedlings with water stress at tillering (D1) and grain filling (D1) stages than un-droughted (D0) treatment. Nonetheless, the application of ZnO-NPs2 (100 ppm) and ZnO-NPs3 (150 ppm) treatments performed relatively better under drought and non-drought conditions compared with ZnO-NPs1 (50 ppm) treatment. For instance, an increment of 121.2% and 94.1% in Tr, and 67.3% and 51.1% in Pn rate was noticed under D1×ZnO-NPs3 and D2×ZnO-NPs3 treatments, accordingly, when compared with the relevant drought treatments without ZnO-NPs application. However, the effect of all ZnO-NPs doses was not significant on SC rate, in comparison with relevant control treatments (Table 5).
Effect of different doses of ZnO-NPs on wheat grain quality under drought
The results regarding wheat grain quality indices such as grain zinc (Gzn) and protein contents (Gprot.) under the inter-active effect of ZnO-NPs and drought conditions has been presented in Table 3. The gain Zn contents were significantly reduced under the drought conditions at D1 and D2 stages without any dose of ZnO-NPs, and the highest decline of 17.6% in Gzn contents was noticed at D2×ZnO-NPs0 treatment (Table 5). However, supply of ZnO-NPs showed a worth-noticing increase in grain Zn contents under drought and non-drought growth conditions. For example, drought at tillering (D1) boosted Gzn contents by 62.1% and 53.1% under the use of ZnO-NPs3 (150 ppm) and ZnO-NPs2 (100 ppm), respectively, as compared with the same drought level without NPs amendment. On the other hand, significantly lowest grain protein contents were observed under all non-drought conditions. However, the drought stress alone or in combination with ZnO-NPs amendment showed a significantly positive impact on protein contents in grain, as compared to non-drought treatments. It was interesting to observe that grain protein accumulation efficiency was much higher at D2 drought level than the D1 drought level. The accumulation of grain protein contents was increased at both D1 and D2 drought stages under the application of ZnO-NPs3 by 3.5% and 8.1%, and ZnO-NPs2 by 3.1% and 2.1%, in comparison with the corresponding drought stage without ZnO-NPs, respectively.
Effects of various doses of ZnO-NPs on nutrient uptake by wheat under drought
The data regarding the uptake of major nutrients by wheat crop e.g., nitrogen (N), phosphorous (P), and potassium (K) under the inter-active influence of ZnO-NPs and drought conditions has been presented in Fig. 4. In brief, the obtained findings suggested that N, and K showed a significant increase under drought-stressed treatments alone or in combination with ZnO-NPs dose levels. For example, the uptake of N was extensively increased under D1×ZnO-NPs0 by 109.5% and under D2×ZnO-NPs0 by 180.9%, when compared with the non-droughted treatment with no ZnO-NPs application (D0×ZnO-NPs0). Similarly, further increase in N (59.0% and 60.9%) and K (43.3% and 45.1%) uptake by wheat leaves was observed under the supply of ZnO-NPs2 and ZnO-NPs3 at grain filling drought stage (D2), accordingly, as compared with the D0 treatment in combination with the corresponding ZnO-NPs application rate. The response of phosphorous (P) uptake under various levels of ZnO-NPs and drought at tillering and grain filling stages was quite different than N and K uptake. There was no significant difference among the P uptake rates of wheat crop under all application rates of ZnO-NPs at D0 drought level. However, P uptake was significantly reduced by 53.5% and 48.7% under drought at tillering (D1), and by 51.3% and 42.1% under drought at grain filling stage (D1) after the application of ZnO-NPs2 and ZnO-NPs3 dose levels, as compared with same application rates of ZnO-NPs at D0 drought level.
Effect of different doses of ZnO-NPs on nutrients (NPK) uptake by wheat leaves under various stage-based drought levels. The different lower-case letters (a, b, c, d) exhibited on the bars represent statistically significant differences among the studied treatments through LSD test at 5% probability index (P ≤ 0.05). The data presented in the form of each bar is an average of three replications (n = 3). The abbreviations are as following: D0 = no drought (control), D1 = drought at tillering, D2 = drought at grain filling, ZnO-NP0 = zinc oxide nanoparticles at 0 ppm, ZnO-NP1 = zinc oxide nanoparticles at 50 ppm, ZnO-NP2 = zinc oxide nanoparticles at 100 ppm, and ZnO-NP3 = zinc oxide nanoparticles at 150 ppm.
Principal components analysis (PCA)
To study the variations and associations among different growth, physiological, yield, and quality-linked parameters of wheat under the interaction of different; drought-stressed growth stages and dose levels of ZnO-NPs, the results were analyzed by performing a principal component analysis (PCA) to distinguish the beneficial treatments (Fig. 5). The treatments with different impacts on different parameters were scattered into different clades in PCA – Biplot Analysis (Fig. 5). The first two PCs (PC1 62.1% and PC2 23.4%) covered the 85.5% variability of the total variation from the 24 parameters. The first principal component (PC1) was related to all the parameters under study except nitrogen and K uptake (N, K), grain protein (Gprot.), leaf area index (LAI), root length (RL), and shoot dry weight (SDW) which were attributed to PC2. All the treatments of ZnO-NPs (0, 50, 100, and 150 ppm) under various drought intensities (D0 = Control, D1 = Tillering stage drought, and D2 = Grain filling stage drought) in wheat revealed variability among the treatments and stage-based drought episodes. Moreover, the amount of N and K uptake, grain protein, LAI, root length, and shoot dry weights were boosted under drought with negative eigenvalues.
Principal component analysis (PCA) based on growth, physiological, yield, and quality parameters of wheat under different levels of ZnO-NPs and stage-based drought stress. The abbreviations are as following: D0 = no drought (control), D1 = drought at tillering, D2 = drought at grain filling, NP0 = zinc oxide nanoparticles at 0 ppm, NP1 = zinc oxide nanoparticles at 50 ppm, NP2 = zinc oxide nanoparticles at 100 ppm, NP3 = zinc oxide nanoparticles at 150 ppm, PH = plant height, LAI = Leaf area index, SL = spike length, NGS = number of grains per spike, Test Wt. = 1000 grain weight, GY = grain yield per plant (g), BP = Biomass production per plant (g), TLP = Tillers per plant, RL = Root length, SHL = Shoot length, RDW = Root dry weight, SDW = Shoot dry weight, Chl. = Chlorophyll, Pn = Photosynthesis rate, Tr = Transpiration rate, SC = Stomatal conductance, LRWC = Leaf relative water content, ELWL = Excised leaf water loss, N, P, K = Nitrogen, phosphorous and potassium uptakes, Gprot. = Grain protein, and Gzn = Grain zinc content.
Discussion
Drought adversely affects the growth, yield and overall productivity of wheat, especially at critical growth stages47,48. The management of adverse drought impacts is possible by applying various strategies, including the use of nanotechnology49. Zinc oxide nanoparticles (ZnO-NPs) have shown potential in improving crop growth, grain yield and nutritional quality by promoting penological development in cereals50.
The shoots and roots are fundamental plant parts, with roots playing critical role in absorbing minerals and water from the soil, while also serving as sinks for certain nutrients. Our findings indicated that ZnO-NPs application effectively mitigated the ill effects of drought by enhancing both root and shoot lengths. This aligns with the previous studies by Singh et al.51, who reported increase root and shoot growth by the application of ZnO-NPs. Similarly, Sabir et al.52 and Solanki and Laura53 reported the significant increments in shoot and root lengths of Zea mays L. and wheat, respectively. Further, our study found decreased dry weights trends under drought over control but different doses of ZnO-NPs improved the root and shoot dry weights at tillering and grain filling stages under drought. So, ZnO-NPs have the potential to mitigate oxidative stress effects. Rizwan et al.54 also reported similar results by ZnO-NPs application on wheat and noticed a significant increase in root and shoot dry biomass under heavy metal stress might be due to the improved the nutrient uptake facilitated by ZnO-NPs.
Significant reduction in leaf area index (LAI) and leaf chlorophyll contents under drought stress conditions was due to the increased production of reactive oxygen species that triggers lipid peroxidation and subsequently chlorophyll destruction55. Similar outcomes have been reported by Aslam et al.56 in quinoa under drought conditions. ZnO-NPs (150ppm) enhance the cytokinin’s productions that improve metabolic activity and cell growth that significantly increase the LAI and chlorophyll. Likewise, lower concentrations of ZnO-NPs increased the LAI and chlorophyll content, whereas higher concentrations reduced these parameters in wheat subjected to drought conditions, as reported by Abbas et al.38. This reduction at higher concentrations is attributed to the negative effects of nanoparticles, which reduced the photosynthetic performance and antioxidant enzyme activities, ultimately leading to a decline in LAI and chlorophyll content38.
Drought stress significantly reduced the excised leaf water loss (ELWL) and leaf relative water content (LRWC) at tillering and grain filling stages as compared to the droughty control. Similarly, Elshayb et al.37 and Muhammad et al.57 reported a decrease in ELWL and LRWC under drought in rice and wheat, respectively, might be due to decreased cell division, soil moisture availability, and reduced plant ability to absorb nutrients. ZnO-NPs application enhanced the water relations (LRWC, ELWL) under both control and water-deficit conditions. Our findings align with previous studies, which reported that nanoparticles enhance the aforementioned parameters by increasing root formation and facilitating hormonal signaling, thereby increasing water uptake and maintaining better water relations under drought stress33,37.
Stomatal conductance (SC), transpiration rate (Tr), net photosynthetic rate (Pn) are important parameters to lookout the drought resilience in wheat. Drought imposed at tillering and grain filling stages reduced the soil water uptake, which eventually leads to closure of stomata and hence decreased the SC, Tr, and Pn as described in this study58. However, ZnO-NPs (150ppm) improved the SC, Tr, and Pn under both control and drought conditions. Likewise, Ahmed et al.59 and Seleiman et al.60 reported that ZnO-NPs improved the SC, Tr, and Pn in Coriandrum sativum L. and maize under drought and salt stress, respectively. This increase may result from improved water uptake, higher photosynthetic efficiency, enhanced phytohormones production and osmolyte accumulation, offering better resistance to wheat59,61.
The ZnO-NPs were reported to induce drought related tolerance in soybean (Glycine max L.) as reported by Sedghi et al.34. The number of tillers per plant in our study decreased more significantly under drought stress at tillering than at the grain filling stage. However, the highest dose of ZnO-NPs increased their number up to 52% more than the control treatment. A similar increase in the number of tillers in rice under arsenic stress was reported by the silica NPs62. Similarly, it has been reported that ZnO-NPs improved the number of tillers in wheat under drought conditions due to enhanced water and nutrient uptake57 .
Plant height (PH) is correlated with total biomass accumulation in plants. Drought negatively affected PH at tillering stage. Poor nutrient availability under drought stress could affect PH and spike length (SL) of wheat63. ZnO-NPs effectively increased PH and SL at each drought level. Raliya et al.64 reported increased PH in tomato plants by the application of ZnO-NPs (250 ppm). Similarly, an increase in PH and SL of wheat under drought stress has been reported by the application of ZnO-NPs in low concentrations, however, higher concentration (160 ppm) decreased the studied parameters due to toxicity, oxidative stress, and interference with nutrient uptake38.
Drought stress significantly reduced the grain protein and zinc content, uptake of nitrogen (N), phosphorus (P), and potassium (K) as compared to control treatment. Similarly, significant reduction in above mentioned parameters have been reported by Aslam et al.56 and Abbas et al.38 in quinoa and wheat, respectively, as drought stress reduced the uptake and translocation of nutrients from roots to aerials parts of plants due to limited water availability. However, ZnO-NPs (150ppm) improved the nutrient absorption and so increased the protein, zinc, N, P, and K in wheat grains as compared to control treatment. Similar results have been reported in various crops, demonstrating that ZnO-NPs enhance theses quality parameters, thereby supporting our findings.
The number of spikelets per spike (NSPS), number of grains per spike (NGS), 1000 grain weight, biomass and grain yield were significantly affected by drought imposed at tillering and grain filling stages. The reduction in all these parameters have been reported previously by Aslam et al.56 and Muhammad et al.57 in quinoa and wheat under drought conditions, respectively. This reduction might be attributed to decreased plant metabolic functions, poor absorption and distribution of photosynthates under limited water supply58. The decreased NSPS, NGS, 1000 grain weight, biomass and yield under drought conditions was significantly improved by ZnO-NPs (150ppm), which might be due to the increased water and nutrient absorption, osmolytes accumulation and enhanced photosynthetic efficiency. This increase aligns with the findings of Dimkpa et al.33 and Abbas et al.38 in sorghum and wheat, respectively, which supports our results. However, Wang et al. reported a decrease in growth, gas exchange parameters, and biomass production in tomato plants at higher concentrations of ZnO-NPs (400 and 800 mg dm− 3). Furthermore, it has been reported that higher concentrations of ZnO-NPs can have toxic effects on crops leading to reduced growth, chlorophyll contents, stomatal conductance, and photosynthetic rate, ultimately reducing the biomass and yield65,66. The PCA biplot discovered several significant factors, including leaf chlorophyll contents, SC, Tr, and Pn as important descriptive variables enhancing the resilience of wheat to drought, aligning with the results of Raza et al.58 (Fig. 5).
Conclusions
The current study revealed that varying dose levels of ZnO-NPs produced different effects on growth, yield, morpho-physiological traits, water relations, nutrient absorption, and grain quality parameters of wheat at critical growth stages (tillering and grain filling) under drought stress. Notably, the grain-filling stage was found as the most drought sensitive stage. Among the applied treatments, ZnO-NPs at 150 ppm showed the most promising effects in mitigating drought stress. The use of ZnO-NPs claimed an improvement in root shoot dimensions, plant dry biomass, grain yield, yield components, nutrient uptake, leaf relative water contents, gas exchange traits, chlorophyll concentration, grain zinc accumulation, and protein contents in drought subjected wheat at both tillering and grain filling stages. Since very little is known about the field-level application of ZnO-NPs, extensive field studies on various cereal crops under different drought exposures are required to validate their broader applicability.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Baloch, H. et al. Moringa leaf extract enhances the growth and yield characteristics of buckwheat genotypes by modulating the biochemical and physiological activities. Asian J. Agric. Biol. 4, 2023328 (2024).
Glick, B. R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 169, 30–39 (2014).
Etesami, H. & Maheshwari, D. K. Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: Action mechanisms and future prospects. Ecotoxicol. Environ. Saf. 156, 225–246 (2018).
Kah, M., Tufenkji, N. & White, J. C. Nano-enabled strategies to enhance crop nutrition and protection. Nat. Nanotechnol. 14, 532–540 (2019).
Lesk, C., Rowhani, P. & Ramankutty, N. Influence of extreme weather disasters on global crop production. Nature 529, 84–87 (2016).
García-Tejero, I. F., Carbonell, R., Ordoñez, R., Torres, F. P. & Durán Zuazo, V. H. In Soil Health Restoration and Management203–230 (Springer, 2020).
Nezhadahmadi, A., Prodhan, Z. & Faruq, G. Drought tolerance in wheat. Sci. World J. 610721 https://doi.org/10.1155/2013/610721 (2013).
Anwar, H. et al. Zinc-coated urea and zinc-solubilizing microbes: Synergistic strategies for improving zinc bioavailability in dry region soils. Asian J. Agric. Biol. 2024091 (2024).
Zaman, M. et al. Spatiotemporal variability of precipitation concentration in Pakistan. Int. J. Climatol. 43, 7646–7666 (2023).
Jaleel, C. A. et al. Drought stress in plants: A review on morphological characteristics and pigments composition. Int. J. Agric. Biol. 11, 100–105 (2009).
Vaughan, J. G. & Judd, P. A. The Oxford book of Health Foods (Oxford University Press, 2003).
Lev-Yadun, S., Gopher, A. & Abbo, S. The cradle of agriculture. Science 288, 1602–1603 (2000).
Çakmak, İ. et al. Triticum dicoccoides: An important genetic resource for increasing zinc and iron concentration in modern cultivated wheat. Soil. Sci. Plant. Nutr. 50, 1047–1054 (2004).
Khanzada, B. et al. Water relations in different guar (Cyamopsis tetragonoloba (L.) Taub) genotypes under water stress. Pak J. Bot. 33, 279–287 (2001).
Iqbal, R. et al. Foliar applied iron and zinc improves the growth, physiological and yield related traits of wheat under drought. Int. J. Biosci. 14, 376–387 (2019).
Yang, J., Zhang, J., Wang, Z., Xu, G. & Zhu, Q. Activities of key enzymes in sucrose-to-starch conversion in wheat grains subjected to water deficit during grain filling. Plant. Physiol. 135, 1621–1629 (2004).
Haider, I. et al. Potential effects of biochar application on mitigating the drought stress implications on wheat (Triticum aestivum L.) under various growth stages. J. Saudi Chem. Soc. 24, 974–981 (2020).
Hernández-Espinosa, N. et al. Milling, processing and end-use quality traits of CIMMYT spring bread wheat germplasm under drought and heat stress. Field Crops Res. 215, 104–112 (2018).
Ivanova, E., Doronina, N. & Trotsenko, Y. A. Aerobic methylobacteria are capable of synthesizing auxins. Microbiology 70, 392–397 (2001).
Ojuederie, O. B., Olanrewaju, O. S. & Babalola, O. O. Plant growth promoting rhizobacterial mitigation of drought stress in crop plants: implications for sustainable agriculture. Agronomy 9, 712 (2019).
Rai, P. K. et al. Nanoparticle-plant interaction: implications in energy, environment, and agriculture. Environ. Int. 119, 1–19 (2018).
Prasad, T. et al. Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J. Plant. Nutr. 35, 905–927 (2012).
Prasad, R., Kumar, V. & Prasad, K. S. Nanotechnology in sustainable agriculture: Present concerns and future aspects. Afr. J. Biotechnol. 13, 705–713 (2014).
Subramanian, K. & Tarafdar, J. Prospects of nanotechnology in Indian farming. Indian J. Agric. Sci. 81, 887–893 (2011).
Pulizzi, F. Vol. 14 507–507 (Nature Publishing Group, (2019).
Sindesi, O. A., Ncube, B., Lewu, M. N., Mulidzi, A. R. & Lewu, F. B. Cabbage and Swiss chard yield, irrigation requirement and soil chemical responses in zeolite-amended sandy soil. Asian J. Agric. Biol. 1, 202111387. https://doi.org/10.35495/ajab.2021.11.387 (2023).
Du, W. et al. Interaction of metal oxide nanoparticles with higher terrestrial plants: Physiological and biochemical aspects. Plant. Physiol. Biochem. 110, 210–225 (2017).
Agathokleous, E., Feng, Z., Iavicoli, I. & Calabrese, E. J. The two faces of nanomaterials: A quantification of hormesis in algae and plants. Environ. Int. 131, 105044 (2019).
Siddiqui, M. H., Al-Whaibi, M. H. & Mohammad, F. Nanotechnology and plant sciences. Springer International Publishing, Cham., 303p. doi 10, 978 – 973 (2015).
Naderi, M., Danesh Shahraki, A. & Naderi, R. Application of nanotechnology in the optimization of formulation of chemical fertilizers. Iran. J. Nanatechnol. 12, 16–23 (2011).
LI, W. Y. F. et al. Tonoplast-located GmCLC1 and GmNHX1 from soybean enhance NaCl tolerance in transgenic bright yellow (BY)‐2 cells. Plant. Cell. Environ. 29, 1122–1137 (2006).
Arough, Y. K., Sharifi, R. S., Sedghi, M. & Barmaki, M. Effect of zinc and bio fertilizers on antioxidant enzymes activity, chlorophyll content, soluble sugars and proline in triticale under salinity condition. Not Bot. Horti Agrobot Cluj Napoca. 44, 116–124 (2016).
Dimkpa, C. O. et al. Zinc oxide nanoparticles alleviate drought-induced alterations in sorghum performance, nutrient acquisition, and grain fortification. Sci. Total Environ. 688, 926–934 (2019).
Sedghi, M., Hadi, M. & Toluie, S. G. Effect of nano zinc oxide on the germination parameters of soybean seeds under drought stress. Ann. West. Univ. Timisoara Ser. Biology. 16, 73 (2013).
Seghatoleslami, M. & Forutani, R. Yield and water use efficiency of sunflower as affected by nano ZnO and water stress. J. Adv. Agric. Technol. 2 (2015).
Pavithra, G., Rajashekar Reddy, B., Salimath, M., Geetha, K. & Shankar, A. Zinc oxide nano particles increases Zn uptake, translocation in rice with positive effect on growth, yield and moisture stress tolerance. Indian J. Plant. Physiol. 22, 287–294 (2017).
Elshayb, O. M. et al. The integrative effects of biochar and ZnO nanoparticles for enhancing rice productivity and water use efficiency under irrigation deficit conditions. Plants 11, 1416 (2022).
Abbas, S. F. et al. Enhancing drought tolerance in wheat cultivars through nano-ZnO priming by improving leaf pigments and antioxidant activity. Sustainability 15, 5835 (2023).
Ismail, M., Taha, K., Modwi, A. & Khezami, L. ZnO nanoparticles: surface and X-ray profile analysis. J. Ovonic Res. 14, 381–393 (2018).
Thomas, B., Murphy, D. J. & Murray, B. G. Encyclopedia of Applied Plant Sciences (Academic, 2003).
Clarke, J. in Improving Winter Cereals for Moisture-limiting Areas, Capri (Italy), 27–31 1985. (John Wiley and Sons).
Barrs, H. & Weatherley, P. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust J. Biol. Sci. 15, 413–428 (1962).
Lichtenthaler, H. K. [34] chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 148, 350–382 (1987).
Fatemi, R., Yarnia, M., Mohammadi, S., Vand, E. K. & Mirashkari, B. Screening barley genotypes in terms of some quantitative and qualitative characteristics under normal and water deficit stress conditions. Asian J. Agric. Biol. 2, 2022071. https://doi.org/10.35495/ajab.2022.071 (2023).
Jiao, Y., Grant, C. A. & Bailey, L. D. Growth and nutrient response of flax and durum wheat to phosphorus and zinc fertilizers. Can. J. PlantSci. 87, 461–470 (2007).
Wolf, B. A comprehensive system of leaf analyses and its use for diagnosing crop nutrient status. Commun. Soil. Sci. Plant. Anal. 13, 1035–1059. https://doi.org/10.1080/00103628209367332 (1982).
Sarto, M. V. M. et al. Wheat phenology and yield under drought: A review. Aust. J. Crop Sci. 11, 941–946 (2017).
Zhang, J. et al. Effect of drought on agronomic traits of rice and wheat: A meta-analysis. Int. J. Env Res. Public. Health. 15, 839 (2018).
Ashraf, S. A. et al. Innovations in nanoscience for the sustainable development of food and agriculture with implications on health and environment. Sci. Total Environ. 768, 144990 (2021).
Dimkpa, C. O. et al. Facile coating of urea with low-dose ZnO nanoparticles promotes wheat performance and enhances zn uptake under drought stress. Front. Plant. Sci. 11, 168 (2020).
Singh, K., Madhusudanan, M., Verma, A. K., Kumar, C. & Ramawat, N. Engineered zinc oxide nanoparticles: An alternative to conventional zinc sulphate in neutral and alkaline soils for sustainable wheat production. 3 Biotech. 11, 1–17 (2021).
Sabir, S. et al. Biosynthesis of ZnO nanoparticles using bacillus subtilis: Characterization and nutritive significance for promoting plant growth in Zea mays L. Dose-Response 18, 1559325820958911 (2020).
Solanki, P. & Laura, J. Effect of ZnO nanoparticles on seed germination and seedling growth in wheat (Triticum aestivum). J. Pharmacognosy Phytochemistry. 7, 2048–2052 (2018).
Rizwan, M. et al. Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 214, 269–277 (2019).
Ahmed, T. et al. Nanoparticle-based amelioration of drought stress and cadmium toxicity in rice via triggering the stress responsive genetic mechanisms and nutrient acquisition. Ecotoxicol. Environ. Saf. 209, 111829 (2021).
Aslam, M. U. et al. Improving strategic growth stage-based drought tolerance in quinoa by rhizobacterial inoculation. Commun. Soil Sci. Plant Anal. 51, 853–868 (2020).
Muhammad, F. et al. Ameliorating drought effects in wheat using an exclusive or co-applied rhizobacteria and ZnO nanoparticles. Biology 11, 1564 (2022).
Raza, M. A. S. et al. Cu-nanoparticles enhance the sustainable growth and yield of drought-subjected wheat through physiological progress. Sci. Rep. 14, 14254 (2024).
Ahmed, S. et al. Characterizing stomatal attributes and photosynthetic induction in relation to biochemical changes in Coriandrum sativum L. by foliar-applied zinc oxide nanoparticles under drought conditions. Front. Plant Sci. 13, 1079283 (2023).
Seleiman, M. F., Ahmad, A., Alhammad, B. A. & Tola, E. Exogenous application of zinc oxide nanoparticles improved antioxidants, photosynthetic, and yield traits in salt-stressed maize. Agronomy 13, 2645 (2023).
Rasheed, A. et al. The role of nanoparticles in plant biochemical, physiological, and molecular responses under drought stress: A review. Front. Plant Sci. 13, 976179 (2022).
Liu, C., Wei, L., Zhang, S., Xu, X. & Li, F. Effects of Nanoscale silica sol foliar application on arsenic uptake, distribution and oxidative damage defense in rice (Oryza sativa L.) under arsenic stress. RSC Adv. 4, 57227–57234 (2014).
Raza, M. A. S., Saleem, M. F., Ashraf, M. Y., Ali, A. & Asghar, H. N. Glycinebetaine applied under drought improved the physiological efficiency of wheat (Triticum aestivum L). Plant. Soil. Environnment. 31, 67–71 (2012).
Raliya, R., Nair, R., Chavalmane, S., Wang, W. N. & Biswas, P. Mechanistic evaluation of translocation and physiological impact of titanium dioxide and zinc oxide nanoparticles on the tomato (Solanum lycopersicum L.) plant. Metallomics 7, 1584–1594 (2015).
Srivastav, A. et al. Effect of ZnO nanoparticles on growth and biochemical responses of wheat and maize. Plants 10, 2556 (2021).
Liu, L., Nian, H. & Lian, T. Plants and rhizospheric environment: Affected by zinc oxide nanoparticles (ZnO NPs). A review. Plant Physiol. Biochem. 185, 91–100 (2022).
Acknowledgements
The authors extend their appreciation to the Researchers Supporting Project number (RSP2025R637), King Saud University, Riyadh, Saudi Arabia.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Researchers Supporting Project number (RSP2025R637), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
Conceptualization, M.A.S.R., F.M., and R.I.; Data curation, M.F., M.U.A., N.A.; Formal analysis, M.T., M.A.B., M.A.A.; Investigation, M.R., M.U.A., R.I. and M.A.S.R.; Methodology, M.U.A., R.I., M.A.S.R., M.T., M.A.B., M.A.A.; Software, N.A.; Supervision, M.A.S.R.; Writing - original draft, M.A.S.R., F.M., and R.I.; Writing - review & editing, All authors. Visualization, M.A.S.R., F.M., and R.I.; project administration, M.A.S.R. and R.I.; funding acquisition, M.R., R.I. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The wheat seeds (Galaxy 2013) were purchased from the Regional Agricultural Research Institute (RARI) Bahawalpur, Pakistan. All experimental studies and experimental materials involved in this research are in full compliance with relevant institutional, national and international guidelines and legislation.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Clinical trial number
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Raza, M.A.S., Muhammad, F., Farooq, M. et al. ZnO-nanoparticles and stage-based drought tolerance in wheat (Triticum aestivum L.): effect on morpho-physiology, nutrients uptake, grain yield and quality. Sci Rep 15, 5309 (2025). https://doi.org/10.1038/s41598-025-89718-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-89718-2