Abstract
Apical periodontitis (AP) results from bacterial contamination of the pulp tissue, with its progression highly influenced by the host’s immune response. This study aimed to evaluate the impact of moderate physical exercise, alone or combined with omega-3 supplementation, on AP induced in rats. The analysis focused on the immuno-inflammatory profile, bacterial presence in the root canal and apical region, bone loss, and collagen fiber production. Thirty Wistar rats were divided into three groups: Control, Physical Exercise (PE), and Physical Exercise + Omega-3 (PEO). Omega-3 supplementation was administered by gavage for 60 days. The swimming protocol included two stages: acclimatization to the aquatic environment and swimming training. AP was induced on the 30th day, and the rats were euthanized on the 60th day. Upper molars were processed and stained using Hematoxylin and Eosin (H&E), Brown and Brenn (BB), Picrosirius Red (PSR), and immunohistochemistry for IL-17, TNF-α, and tartrate-resistant acid phosphatase (TRAP). Microtomographic analysis was also performed. Scores from the analyses were evaluated using Kruskal-Wallis, Tukey, Shapiro-Wilk, Mann-Whitney, and One-Way ANOVA tests, with a significance level of 5% (p < 0.05). The control group exhibited the highest intensity of inflammatory infiltrate (p < 0.05). PE alone reduced TNF-α immunostaining and limited bacterial spread (p < 0.05). Combined with omega-3 supplementation, PE further reduced IL-17 immunostaining and increased the percentage of birefringent immature collagen fibers (p < 0.05). Microtomographic analysis revealed smaller areas of alveolar bone loss in animals subjected to PE (p < 0.05). The control group showed a significantly higher number of TRAP-positive cells (p < 0.05). In conclusion, PE alone enhanced defense mechanisms by reducing inflammation through TNF-α modulation and controlling bacterial contamination. Combined with omega-3 supplementation, PE further improved inflammatory regulation by modulating IL-17 levels, reducing bone loss, and stimulating collagen production, thereby limiting inflammation and decreasing osteoclastic activity.
Similar content being viewed by others
Introduction
Apical periodontitis (AP) results from the infiltration of microorganisms into the root canal system1. When the infectious process and the associated inflammatory response reach the tooth apex, a periapical lesion develops. In an attempt to control the invading microorganisms, the host’s inflammatory response intensifies, leading to the recruitment of defense cells, the release of inflammatory mediators, and the resorption of bone tissue2,3,4. The progression of this process largely depends on the host’s immune response2,5.
If the immune response fails to suppress the bacteria, an overactive reaction from defense cells may occur6. Macrophages, T lymphocytes, and other immune cells release chemical mediators such as tumor necrosis factor-alpha (TNF-α), interleukin-17 (IL-17), prostaglandins, and bradykinins. At elevated levels, these mediators can indirectly trigger osteoclastogenesis7. IL-17 plays a dual role, either mediating host defense against bacterial infections or contributing to chronic inflammation, while TNF-α is linked to bone loss and stimulates the synthesis of prostaglandins and proteases8,9.
Pro-inflammatory cytokines, such as IL-17 and TNF-α, play pivotal roles in pathological bone resorption by promoting osteoclastogenesis and amplifying inflammatory responses10. In addition to these cytokines, tartrate-resistant acid phosphatase (TRAP) is a central enzyme in bone resorption. Secreted by activated osteoclasts, TRAP degrades the bone matrix and acts as a critical biomarker of osteoclast functionality11. Its role extends beyond osteoclastic activity, as TRAP also modulates inflammatory processes, linking it closely to inflammation-driven bone loss and the pathogenesis of bone-related diseases.
Physical exercise (PE) is a well-established strategy for promoting immunomodulation and enhancing the host’s immune response12. It is one of the most influential factors in regulating the defense system, sensitizing the immune response, and reducing susceptibility to inflammation and infections12,13. Regular, moderate-intensity exercise can strengthen the immune system throughout life by suppressing abnormal inflammatory cytokine production, fostering anti-infective and antioxidant agents, increasing immune cell counts, and reducing tissue inflammation14,15,16 .
Omega-3 polyunsaturated fatty acids (ω-3 PUFAs) are widely recognized as dietary supplements with therapeutic effects against chronic inflammatory diseases17,18. Eicosapentaenoic acid and docosahexaenoic acid, the primary ω-3 PUFAs, have anti-inflammatory properties that inhibit bone resorption, suppress lipid mediator synthesis, lower arachidonic acid levels, alter polymorphonuclear leukocyte functions, modulate lymphocyte proliferation, and reduce pro-inflammatory cytokine production. Additionally, they stimulate bone regeneration and enhance the host’s antioxidant capacity3,19,20. ω-3 PUFAs also prevent bone tissue resorption21 and are used in dentistry for treating gingivitis, periodontal disease, AP, and stomatitis22.
Given the lack of studies analyzing the effects of physical activity on AP, this study aimed to evaluate, in Wistar rats, the impact of moderate-intensity physical activity, alone or in combination with ω-3 PUFAs, on infectious and inflammatory oral diseases of endodontic origin. The null hypothesis tested was that physical activity alone or combined with ω-3 PUFAs would not influence bone loss and inflammation caused by AP.
Results
The histological analysis of the teeth of the rats in all groups is shown in Fig. 1. Specimens from all groups exhibited pulp necrosis and periapical lesion formation 30 days after pulp exposure. The intensity of the inflammatory infiltrate was significantly higher in group C (3/10 scored 2, 4/10 scored 3, 3/10 scored 4) compared to the PE (7/10 scored 2, 3/10 scored 3) and PEO (10/10 scored 2) groups (p < 0.05). No statistical difference was observed between the PE and PEO groups (p > 0.05).
Representative images of histological analysis of the periapical region after 30 days of pulp exposure. Group C (A, a1, a2) presented a moderate to intense inflammatory infiltrate, in addition to an extensive area of periapical bone resorption; group PE (B, b1, b2) exhibited a mild to moderate inflammatory infiltrate and a smaller area of bone resorption. The PEO group (C, c1, c2) exhibited a mild inflammatory infiltrate and a small area of apical bone resorption. ab, alveolar bone; ce, cementum; pn, pulp necrosis; (hematoxylin-eosin stain, original magnification ×100 and ×400).
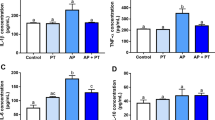
Immunohistochemical analysis revealed moderate levels of the cytokines IL-17 and TNF-α in group C (p < 0.05). The PE group exhibited few immunoreactive cells for TNF-α and moderate immunostaining for IL-17 (p < 0.05). In the PEO group, low immunoreactivity for both IL-17 and TNF-α was observed, with two rats showing extremely low immunoreactivity for TNF-α (p < 0.05) (Table 1; Fig. 2).
Table 2 presents data on bacteriological findings in the thirds of the root and statistical differences among the groups. Figure 3 illustrates the scores assigned based on the presence of bacteria in the root canal and periapical region.
Representative images of the scores attributed to the presence of bacteria in the thirds of the root canal and periapical region. (A) score 0; (B) score 1; (C) score 2; (D) score 3; (E) score 4; (F) score 5. d, dentin; cs, root canal space; p.a., periapical area; (BB stain, original magnification×1000).
When evaluating the predominance of collagen fibers, statistical analysis of immature fibers revealed that the PEO group exhibited a higher percentage of birefringent structures, which was significantly different from groups C and PE (p < 0.05) (Table 3). Groups C (a) and PE (b) displayed a higher percentage of red fibers, whereas the PEO group (c) demonstrated a higher percentage of green fibers.
The microtomographic analysis showed that the animals that performed the physical activity of swimming had less loss of alveolar bone volume compared (165.5 ± 2.8 mm3) to group C (194.5 ± 6.6 mm3) (p < 0.05). In animals that received omega-3 supplementation, the volume of bone structure loss was even smaller (143.7 ± 6.0 mm3) (p < 0.05).
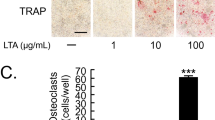
Figure 4 represents the immunohistochemical analysis of each group. It is noted that the groups that performed physical activity had fewer cells immunolabeled for TRAP (osteoclasts) (31.9 ± 6.17), with even better results when omega 3 was associated (20.8 ± 1.47), when compared to the control (42.1 ± 15.34), with statistically significant differences between each group (p < 0.05.)
Discussion
This study evaluated the impact of moderate-intensity physical exercise, alone or in combination with omega-3 supplementation, on the inflammatory and immunohistochemical profile, bacterial ___location and distribution, and collagen fiber production following AP induction. Physical exercise alone influenced the progression of periapical lesions, TNF-α and TRAP immunostaining, and apical bone loss. Notably, this is the first study to demonstrate that the combination of moderate physical exercise with omega-3 supplementation significantly improved the inflammatory condition induced by AP by limiting bacterial progression, reducing bone tissue loss, modulating pro-inflammatory cytokine immunostaining, and stimulating fibroblast activity. Based on these results, the null hypothesis was rejected, as both physical activity alone and in combination with ω-3 PUFAs demonstrated significant effects on bone loss and inflammation caused by AP.
Exercise reduces oxidative stress and regulates the immune system, thereby strengthening the body’s defense against infections23,24. Previous studies have demonstrated its role in mitigating periodontal disease by reducing prevalence, lesion progression, inflammation, and alveolar bone loss12,25,26,27. This study employed a gradually increased moderate swimming regimen without weight loading, consistent with prior research28,29. Swimming was selected for its natural compatibility with rats and its low-impact nature, which minimizes musculoskeletal stress28. Additionally, swimming in rats has been shown to mimic exercise adaptations observed in humans, reinforcing its translational relevance21,30. Our findings indicate that swimming effectively modulates the immune system, thereby enhancing resistance to microbial infections.
The inflammatory profile revealed that moderate PE significantly reduced AP-associated inflammation. Rats without PE exhibited more intense inflammation and greater apical bone resorption, aligning with the findings of Andrade et al. (2018)31, where swimming reduced inflammation caused by periodontal disease in diabetic rats. Additionally, Ferreira et al. (2024)32 assessed the local and systemic effects of physical activity on AP, demonstrating that AP associated with physical activity resulted in smaller lesion volumes and better preservation of bone trabeculae in the remaining alveolar bone surrounding the lesion. These findings are corroborated by the present study, which offers a more detailed explanation of the positive effects of physical activity on endodontic disease by incorporating additional analyses, such as BB, PSR, and immunohistochemistry for IL-17, TNF-α, and TRAP. Furthermore, this study uniquely explores the combined effects of moderate physical exercise and omega-3 supplementation on AP development, providing novel insights into their synergistic benefits.
The role of omega-3 in AP management has been extensively discussed3,18,33. Omega-3 PUFAs function as anti-inflammatory and antioxidant agents while promoting the resolution of inflammation34. This study combined systemic omega-3 supplementation with physical exercise, yielding significant results.
IL-17 exacerbates inflammation in periapical lesions by stimulating the release of pro-inflammatory cytokines35. Rats subjected to swimming and omega-3 supplementation exhibited low IL-17 immunoreactivity, indicating reduced AP-associated inflammation. In the PE group, moderate IL-17 immunostaining may have resulted from increased bacterial presence in the apical and periapical regions, consistent with findings linking bacterial presence to the release of inflammatory2.
TNF-α, associated with bone loss and inflammation9, showed reduced immunoreactivity in rats subjected to physical exercise, indicating decreased bone resorption and inflammation. This finding supports previous research suggesting that exercise improves serum levels of inflammatory cytokines, including TNF-α36. Su et al. (2022)37 reported that combining exercise with resistance training reduced serum inflammatory factors in diabetic patients.
Our results highlight the impact of physical exercise on bacterial control. The PEO group exhibited bacterial colonies primarily confined to the canal’s middle third, with no bacteria detected in the apical foramen or periapical tissues. This finding suggests enhanced systemic conditions and bacterial containment due to omega-3’s antimicrobial properties38.
PSR staining revealed collagen fiber deposition patterns, with greenish-yellow fibers indicating immature collagen and yellowish-red fibers representing mature collagen39. The PEO group showed a higher prevalence of immature fibers, likely due to active tissue repair driven by omega-3-induced inflammation resolution34. While the PE group also showed more immature fibers than the control group, the differences were not statistically significant.
TRAP, a marker for osteoclast activity40, was more prevalent in the control group, indicating greater bone resorption. Consistent with prior studies41, the PE and PEO groups exhibited smaller periapical lesions, less bone loss, and reduced osteoclast activity. Omega-3’s role in promoting bone formation while inhibiting bone resorption further supports these findings18.
There are inherent limitations to this study due to the use of an animal experimental model. Well-standardized rat models are commonly used in AP and exercise studies28,42. A limitation of this study was the use of male rats, as sex-based differences in swimming behavior and cytokine expression have been documented43,44. Future research should explore female rats’ responses under similar conditions. The use of rats offers the advantage of a homogeneous sample, enabling greater control of external interferences and reducing variability in the results. This model also minimizes bias and provides a basis for future clinical studies. However, while our findings provide valuable insights, extrapolation to humans should be approached with caution. Another limitation of this study is the absence of blood serum analysis. Our primary focus was on the local aspects of AP. However, a more comprehensive evaluation of the systemic effects of physical exercise—such as assessing blood serum markers—should be considered in future research. Addressing these systemic aspects could provide a broader understanding of the interplay between physical exercise, omega-3 supplementation, and AP.
In conclusion, physical activity alone enhanced immune defense, reducing TNF-α-mediated inflammation and bacterial contamination. Combining exercise with omega-3 supplementation further improved inflammatory regulation by controlling IL-17 levels, reducing bone loss, and stimulating collagen production, thereby mitigating osteoclastic activity.
Methods
Experimental design
The experimental protocol was approved by the Animal Ethics Committee (CEUA 0062-2021) of the São Paulo State University (UNESP) School of Dentistry, Araçatuba, São Paulo, Brazil. All experiments were conducted in accordance with relevant guidelines and regulations, and the study adhered to the ARRIVE guidelines (PLoS Bio 8(6), e1000412, 2010).
Thirty male Wistar rats, aged 6 weeks and weighing approximately 200 g, were obtained from the central vivarium of the São Paulo State University (UNESP), School of Dentistry, Araçatuba, and included in the study. The rats were housed in a temperature-controlled room (25ºC) with a 12-hour light/dark cycle and received water and rodent food ad libitum. The exclusion criterion was the inability of rats to perform 60 min of swimming by the end of the acclimatization week to the aquatic environment.
The sample size was determined based on previous studies45. A difference of 1 in the scores was considered significant, with an alpha error of 5% and a power of 95%. The minimum sample size calculated was 7 rats per group. To account for potential losses during the experiments, 3 additional rats were included per group. The rats were randomly distributed into 3 groups (n = 10):
-
Control (C): Rats with AP, without swimming training, and without omega-3 supplementation.
-
Physical Exercise (PE): Rats with AP, subjected to moderate physical exercise (swimming) but not receiving omega-3 supplementation.
-
Physical Exercise + Omega-3 (PEO): Rats with AP, subjected to moderate physical exercise (swimming) and receiving omega-3 supplementation.
A flowchart representing the study design is shown in Fig. 5.
Omega-3 supplementation
Rats in the PEO group received omega-3 (Ômega-3 Catarinense; Laboratório Catarinense SA, Joinville, SC, Brazil) once daily by gavage, at a dosage of 40 mg/kg (60% EPA and 40% DHA), diluted in 1 mL of distilled water, for 60 days3. Rats were weighed twice a week to adjust the omega-3 dosage. Control and PE groups received 1 mL of distilled water daily as a placebo for the same period.
Training protocol
Swimming training was conducted in polypropylene tanks [100 cm (L) × 80 cm (W) × 90 cm (H)] with water maintained at 32–34ºC. PVC pipes were placed inside the tanks to serve as lanes, ensuring separation between rats during swimming sessions, with 5 rats per tank. Training sessions were consistently conducted at the same time each day.
The training protocol included two stages: acclimatization and swimming training28,29.
-
Acclimatization Period: Over 6 days, the rats in the PE and PEO groups began with a 10-minute swim on the first day, with the duration increasing by 10 min each day until they reached 60 min by the sixth day. The seventh day was designated as a rest day. All rats successfully completed 60 min of swimming by the end of the acclimatization week.
-
Swimming Training: For 6 weeks, rats swam for 60 min/day, 5 days a week, with 2 rest days per week. Training was conducted without adding any overload. Rats in the Control group were placed in shallow water for 1 min/day, 5 days a week, for 6 weeks to simulate the same water stimulus as the trained rats. After each session, all rats were dried with cloth towels.
Induction of apical periodontitis
Rats were anesthetized with an intramuscular injection of 87 mg/kg ketamine (Vetaset; Fort Dodge Animal Health Ltd, São Paulo, Brazil) and 13 mg/kg xylazine (Coopazine; Coopers Ltd Brasil, São Paulo, Brazil). AP was induced by exposing the pulp on the occlusal surface of the upper and lower right first and second molars using round surgical burs (LN Long Neck Drill; Maillefer, Dentsply Ind e Com Ltda, Petrópolis, RJ, Brazil). The pulp was left exposed for 30 days, leading to the formation of periapical lesions42.
Euthanasia
On the 60th day, rats were euthanized with an overdose of sodium thiopental anesthetic (240 mg/kg – Thiopentax, Cristalia Produtos Químicos Farmacêuticos Ltda.). A flowchart summarizing the experimental design is provided in Fig. 5.
Sample processing
The right maxillae were collected and fixed in a buffered 4% formaldehyde solution at neutral pH for the first 22 h. Subsequently, the samples were washed in running water for 12 h. After washing, the maxillae were decalcified in 10% EDTA (Sigma-Aldrich) for 3 months. Following the demineralization process, the samples were washed again in running water, dehydrated in alcohol, clarified in xylene, and embedded in paraffin. After embedding, 5-µm-thick serial sections of the upper first molar were prepared using a microtome (Leica - RM 2045). The sections were stained with Hematoxylin and Eosin (H&E), Brown and Brenn (BB), and Picrosirius Red (PSR) techniques.
Immunohistochemical analysis was performed using the indirect immunoperoxidase technique to evaluate TNF-α, IL-17, and TRAP markers. Histological sections of the maxillary molars were deparaffinized in xylene and hydrated in ethanol. Antigen retrieval was performed by placing the slides in a citrate buffer solution (Antigen Retrieval Buffer; Spring Bioscience, Pleasanton, CA, USA) in a pressurized chamber (Decloaking Chamber; Biocare Medical, Concord, CA, USA) at 95 °C for 10 min. Slides were washed with phosphate-buffered saline at each phase of the immunohistochemical reaction.
The slides were then placed in a 3% H2O2 solution for 1 h and 20 min and in 1% bovine serum albumin for 12 h to block the activity of endogenous peroxidase and nonspecific binding. Histological slides were incubated with primary antibodies anti–TNF-α and anti–IL-17 (Santa Cruz Biotechnology, Santa Cruz, CA) for 24 h. Following incubation, the slides were treated with a biotinylated secondary antibody for 1 h and 30 min and with streptavidin-horseradish peroxidase for 1 h and 30 min (Dako Labeled Streptavidin-Biotin Universal Kit; Dako Laboratories). The reaction was developed using the chromogen 3,3’-diaminobenzidine tetrahydrochloride (DAB Chromogen kit; Dako Laboratories) and counterstained with Harris hematoxylin46.
Histological and immunohistochemical analysis
The intensity of periapical inflammation in groups C, PE, and PEO was analyzed using H&E staining and scored as follows:
-
Score 1: No inflammation (0–10 cells).
-
Score 2: Mild inflammation (> 10 and < 25 cells).
-
Score 3: Moderate inflammation (25–125 cells).
-
Score 4: Severe inflammation (> 125 cells)42.
The presence of microorganisms was assessed using BB staining under optical microscopy with oil immersion. Gram-positive bacteria appeared dark blue, while Gram-negative bacteria appeared red47. A modified scoring system from Samuel et al. (2019)2 was used to evaluate bacterial penetration:
-
Score 0: Absence of bacteria.
-
Score 1–5: Progressive bacterial presence from the cervical third to the periapical region.
Collagen fiber maturation levels were evaluated using PSR staining under polarized light microscopy. Greenish-yellow fibers were classified as immature and thin, while yellowish-red fibers were considered mature and thick. The software (Leica QWin V3, Leica Microsystems) automatically calculated the marked area for each type of fiber48.
Immunohistochemical analysis for TNF-α and IL-17 was assessed based on the brown staining in the cell cytoplasm and extracellular matrix. Immunoreactivity scores were assigned as follows:
-
Score 0: Absence of immunoreactive cells.
-
Scores 1–5: Increasing levels of immunoreactivity49.
For TRAP analysis, immunoreactivity was defined by brown staining in the cell cytoplasm and extracellular matrix. The perimeter of bone resorption due to AP was analyzed, and TRAP-positive multinucleated cells were counted. The ratio of positive cells to the resorption perimeter was calculated18.
All analyses were performed by a blinded, calibrated researcher to ensure unbiased interpretation of the data.
Micro-CT analysis
The right jaws were scanned using the µCT system (Bruker SkyScan 1272, Aartselaar, Belgium). The specimens were positioned individually, with the incisor facing upwards. The region of interest (ROI) was defined as the empty space of periapical bone resorption. Alveolar bone volume (BV) was measured using CTAn software (SkyScan)50.
Statistical analysis
Data from each group were tabulated. Non-parametric data were analyzed using the Kruskal-Wallis, Tukey, Shapiro-Wilk, and Mann-Whitney tests, along with one-way ANOVA. A significance level of 5% (p < 0.05) was adopted.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Nair, P. N. Pathogenesis of apical periodontitis and the causes of endodontic failures. Crit. Rev. Oral Biol. Med. 15 (6), 348–381. https://doi.org/10.1177/154411130401500604 (2004).
- Samuel, R. O. et al. Th1/Th2/Th17/Treg Balance in apical periodontitis of Normoglycemic and Diabetic rats. J. Endod. 45 (8), 1009–1015. https://doi.org/10.1016/j.joen.2019.05.003 (2019).
Azuma, M. M. et al. Omega 3 fatty acids alter systemic inflammatory mediators caused by Apical Periodontitis. J. Endod. 47 (2), 272–277. https://doi.org/10.1016/j.joen.2020.11.015 (2021).
Barreiros, D. et al. MMP2 and MMP9 are Associated with apical periodontitis progression and might be modulated by TLR2 and MyD88. Braz Dent. J. Jan-Feb. 29 (1), 43–47. https://doi.org/10.1590/0103-6440201801731 (2018).
Cantiga-Silva, C. et al. Inflammatory profile of apical periodontitis associated with liver fibrosis in rats: histological and immunohistochemical analysis. Int. Endod J. 54 (8), 1353–1361. https://doi.org/10.1111/iej.13519 (2021).
Howait, M. et al. Elevated expression of Macrophage Migration Inhibitory factor promotes inflammatory bone Resorption Induced in a mouse model of Periradicular Periodontitis. J. Immunol. 202 (7), 2035–2043. https://doi.org/10.4049/jimmunol.1801161 (2019).
Cintra, L. T. A. et al. Endodontic medicine: interrelationships among apical periodontitis, systemic disorders, and tissue responses of dental materials. Braz Oral Res. 18 (suppl 1), e68. https://doi.org/10.1590/1807-3107bor-2018.vol32.0068 (2018).
Borgo Sarmento, E. et al. Proinflammatory cytokine expression in apical periodontitis from diabetic patients. Int. J. Dent. 10, 4961827. https://doi.org/10.1155/2023/4961827 (2023).
Almeida-Junior, L. A. et al. TNF-α-TNFR1. Signaling mediates inflammation and bone resorption in Apical Periodontitis. J. Endod. 49 (10), 1319–1328e2. https://doi.org/10.1016/j.joen.2023.07.013 (2023).
Hong, C. Y. et al. Metformin reduces bone resorption in apical periodontitis through regulation of osteoblast and osteoclast differentiation. J. Endod. 49 (9), 1129–1137. https://doi.org/10.1016/j.joen.2023.07.005 (2023).
Hayman, A. R. Tartrate-resistant acid phosphatase (TRAP) and the osteoclast/immune cell dichotomy. Autoimmunity 41 (3), 218–223. https://doi.org/10.1080/08916930701694667 (2008).
Andrade, E. F. et al. Exercise attenuates alveolar bone loss and anxiety-like behaviour in rats with periodontitis. J. Clin. Periodontol. 44 (11), 1153–1163. https://doi.org/10.1111/jcpe.12794 (2017).
Shimojo, G. et al. Exercise activates vagal induction of dopamine and attenuates systemic inflammation. Brain Behav. Immun. 75, 181–191. https://doi.org/10.1016/j.bbi.2018.10.005 (2019).
Lombardi, G., Ziemann, E. & Banfi, G. Physical activity and bone health: what is the role of Immune System? A narrative review of the third way. Front. Endocrinol. (Lausanne). 7;10, 60. https://doi.org/10.3389/fendo.2019.00060 (2019).
Guo, S. et al. Impacts of exercise interventions on different diseases and organ functions in mice. J. Sport Health Sci. 9 (1), 53–73. https://doi.org/10.1016/j.jshs.2019.07.004 (2020).
Simpson, R. J., Kunz, H., Agha, N. & Graff, R. Exercise and the regulation of Immune functions. Prog Mol. Biol. Transl Sci. 135, 355–380. https://doi.org/10.1016/bs.pmbts.2015.08.001 (2015).
Deore, G. D. et al. Omega 3 fatty acids as a host modulator in chronic periodontitis patients: a randomised, double-blind, palcebo-controlled, clinical trial. J. Periodontal Implant Sci. 44 (1), 25–32. https://doi.org/10.5051/jpis.2014.44.1.25 (2014).
Azuma, M. M. et al. Omega 3 fatty acids reduce bone resorption while promoting bone generation in rat apical periodontitis. J. Endod. 43 (6), 970–976. https://doi.org/10.1016/j.joen.2017.01.006 (2017).
Kesavalu, L. et al. Omega 3 fatty acid effect on alveolar bone loss in rats. J. Dent. Res. 85 (7), 648–652. https://doi.org/10.1177/154405910608500713 (2006).
Iwasaki, M. et al. Longitudinal relationship between dietary ω-3 fatty acids and periodontal disease. Nutrition 26 (11–12), 1105–1109. https://doi.org/10.1016/j.nut.2009.09.010 (2010).
Azuma, M. M. et al. Omega 3 fatty acids reduce inflammation in rat apical periodontitis. J. Endod. 44 (4), 604–608. https://doi.org/10.1016/j.joen.2017.12.008 (2018).
Azuma, M. M. et al. The use of omega-3 fatty acids in the treatment of oral diseases. Oral Dis. Mar. 28 (2), 264–274. https://doi.org/10.1111/odi.13667 (2022).
Scheffer, D. D. L. & Latini, A. Exercise-induced immune system response: anti-inflammatory status on peripheral and central organs Biochim Biophys. Acta Mol. Basis Dis. 1866 (10), 165823. https://doi.org/10.1016/j.bbadis.2020.165823 (2020).
Forte, P., Branquinho, L. & Ferraz, R. The relationships between Physical Activity, Exercise, and Sport on the Immune System. Int. J. Environ. Res. Public Health. 19 (11), 6777. https://doi.org/10.3390/ijerph19116777 (2022).
Ferreira, R. O. et al. Physical activity reduces the prevalence of Periodontal Disease: systematic review and Meta-analysis. Front. Physiol. 10, 234. https://doi.org/10.3389/fphys.2019.00234 (2019).
Uchida, F. et al. Effects of Exercise on the oral microbiota and saliva of patients with non-alcoholic fatty liver disease. Int. J. Environ. Res. Public. Health. 18 (7), 3470. https://doi.org/10.3390/ijerph18073470 (2021).
Almohamad, M., Krall Kaye, E., Mofleh, D. & Spartano, N. L. The association of sedentary behaviour and physical activity with periodontal disease in NHANES 2011–2012. J. Clin. Periodontol. 49 (8), 758–767. https://doi.org/10.1111/jcpe.13669 (2022).
Cheng, M. et al. Chronic swimming Exercise ameliorates low-soybean-oil Diet-Induced spatial memory impairment by enhancing BDNF-Mediated synaptic potentiation in developing spontaneously hypertensive rats. Neurochem Res. 43 (5), 1047–1057. https://doi.org/10.1007/s11064-018-2515-x (2018).
Plecevic, S. et al. Comparison of short-term and medium-term swimming training on cardiodynamics and coronary flow in high salt-induced hypertensive and normotensive rats. Mol. Cell. Biochem. 447 (1–2), 33–45. https://doi.org/10.1007/s11010-018-3291-2 (2018).
Voltarelli, F. A., Gobatto, C. A. & de Mello, M. A. Determination of anaerobic threshold in rats using the lactate minimum test. Braz J. Med. Biol. Res. 35 (11), 1389–1394. https://doi.org/10.1590/s0100-879x2002001100018 (2002).
Andrade, E. F. et al. Physical Exercise improves Glycemic and Inflammatory Profile and attenuates progression of Periodontitis in Diabetic rats (HFD/STZ). Nutrients 10 (11), 1702. https://doi.org/10.3390/nu10111702 (2018).
Ferreira, R. O. et al. Physical training attenuates systemic cytokine response and tissue damage triggered by apical periodontitis. Sci. Rep. 14 (1), 8030. https://doi.org/10.1038/s41598-024-58384-1 (2024).
Gobatto, C. A. et al. Maximal lactate steady state in rats submitted to swimming exercise. Comp. Biochem. Physiol. Mol. Integr. Physiol. 130 (1), 21–27. https://doi.org/10.1016/s1095-6433(01)00362-2 (2001).
Djuricic, I. & Calder, P. C. Beneficial outcomes of Omega-6 and Omega 3 Polyunsaturated fatty acids on Human Health: an update for 2021. Nutrients 13 (7), 2421. https://doi.org/10.3390/nu13072421 (2021).
Henriques, L. C., de Brito, L. C., Tavares, W. L., Vieira, L. Q. & Sobrinho, R. Cytokine analysis in lesions refractory to endodontic treatment. J. Endod. 37 (12), 1659–1662. https://doi.org/10.1016/j.joen.2011.08.007 (2011).
Balducci, S. et al. Anti-inflammatory effect of exercise training in subjects with type 2 diabetes and the metabolic syndrome is dependent on exercise modalities and independent of weight loss. Nutr. Metab. Cardiovasc. Dis. 20 (8), 608–617. https://doi.org/10.1016/j.numecd.2009.04.015 (2010).
Su, X., He, J., Cui, J., Li, H. & Men, J. The effects of aerobic exercise combined with resistance training on inflammatory factors and heart rate variability in middle-aged and elderly women with type 2 diabetes mellitus. Ann. Noninvasive Electrocardiol. Off. J. Int. Soc. Holter Noninvasive Electrocardiol. Inc. 27 (6). https://doi.org/10.1111/anec.12996 (2022). e12996.
Ribeiro-Vidal, H. et al. Antimicrobial activity of EPA and DHA against oral pathogenic Bacteria using an in vitro multi-species Subgingival Biofilm Model. Nutrients 12 (9), 2812. https://doi.org/10.3390/nu12092812 (2020).
Cintra, L. T. A. et al. The effect of dental bleaching on pulpal tissue response in a diabetic animal model. Int. Endod J. Aug. 50 (8), 790–798. https://doi.org/10.1111/iej.12692 (2017).
Sarıtekin, E., Üreyen Kaya, B., Aşcı, H. & Özmen, Ö. Anti-inflammatory and antiresorptive functions of melatonin on experimentally induced periapical lesions. Int. Endod J. 52 (10), 1466–1478. https://doi.org/10.1111/iej.13138 (2019).
Sanbe, T. et al. Vitamin C intake inhibits serum lipid peroxidation and osteoclast differentiation on alveolar bone in rats fed on a high-cholesterol diet. Arch. Oral Biol. Mar. 54 (3), 235–240. https://doi.org/10.1016/j.archoralbio.2008.11.001 (2009).
Cintra, L. T. et al. Multiple apical periodontitis influences serum levels of cytokines and nitric oxide. J. Endod. 42 (5), 747–751. https://doi.org/10.1016/j.joen.2016.01.022 (2016).
Barros, H. M. & Ferigolo, M. Ethopharmacology of imipramine in the forced-swimming test: gender differences. Neurosci. Biobehav Rev. 23 (2), 279–286. https://doi.org/10.1016/s0149-7634(98)00029-3 (1998).
Subbotina, A. Y. et al. Age and sex characteristics of the blood Cytokine Profile in rats subjected to prenatal stress. Bull. Exp. Biol. Med. 174 (3), 299–303. https://doi.org/10.1007/s10517-023-05695-4 (2023).
Conti, L. C. et al. Cintra, L.T.A. Relationship between apical periodontitis and atherosclerosis in rats: lipid profile and histological study. Int. Endod J. 53 (10), 1387–1397. https://doi.org/10.1111/iej.13350 (2020).
Benetti, F. et al. The presence of osteocalcin, osteopontin and reactive oxygen species-positive cells in pulp tissue after dental bleaching. Int. Endod J. 52 (5), 665–675. https://doi.org/10.1111/iej.13049 (2019).
Brown, J. H. & Brenn, L. A method for the differential staining of Gram-positive and Gram-negative bacteria in tissue sections. Bull. Johns. Hopkins Hosp. 48, 69–73 (1931).
-Benetti, F. et al. Biomineralization, and Maturation of Collagen by RTR®, Bioglass and DM Bone® materials. Braz Dent. J. Sep-Oct. 31 (5), 477–484. https://doi.org/10.1590/0103-6440202003660 (2020).
-Cosme-Silva, L. et al. Systemic administration of probiotics reduces the severity of apical periodontitis. Int. Endod J. 52 (12), 1738–1749. https://doi.org/10.1111/iej.13192 (2019).
Cosme-Silva, L. et al. Reduced bone resorption and inflammation in apical periodontitis evoked by dietary supplementation with probiotics in rats. Int. Endod J. Aug. 53 (8), 1084–1092. https://doi.org/10.1111/iej.13311 (2020).
Acknowledgements
This study was supported by the Coordination for the Improvement of Higher Education Personnel (CAPES), process-88887.644332/2021-00 and São Paulo State Research Support Foundation (FAPESP), process-2020/13089-3 and 2022/04884-0. The authors also thank the Multi-User Laboratory of FOA-UNESP and FINEP (FINEP/CT-INFRA-Agreement FINEP: 01.12.0530.00-PROINFRA 01/2011) for providing the high resolution computed microtomography system (SkyScan Model 1272) for the analysis of this study.
Author information
Authors and Affiliations
Contributions
Ana Paula Fernandes Ribeiro, Nathalia Evelyn da Silva Machado, Cristiane Cantiga-Silva, Pedro Henrique Chaves de Oliveira, Luciano Tavares Angelo Cintra, Rogério Castilho Jacinto: substantial contributions to the conception, data curation, acquisition, formal analysis, interpretation of data for the work. Ana Paula Fernandes Ribeiro, Luciano Tavares Angelo Cintra, Rogério Castilho Jacinto: involved in drafting the manuscript or revising it critically for important intellectual content. Ana Paula Fernandes Ribeiro, Michely de Lima Rodrigues, Caroline Loureiro, Nathalia Evelyn da Silva Machado, Cristiane Cantiga-Silva, Pedro Henrique Chaves de Oliveira, Luciano Tavares Angelo Cintra, Rogério Castilho Jacinto: agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ribeiro, A.P.F., de Lima Rodrigues, M., Loureiro, C. et al. Physical exercise alone or combined with omega-3 modulates apical periodontitis induced in rats. Sci Rep 15, 8760 (2025). https://doi.org/10.1038/s41598-025-90029-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-90029-9