Abstract
Sarcopenia, which leads to reduced quality of life and increased medical burden, is challenging to diagnose in a timely manner. Lipid metabolism plays a role in sarcopenia, and this study explored the associations between blood lipid profile parameters and sarcopenia. Using data from the National Health and Nutrition Examination Survey 2011–2018, we conducted weighted multivariate logistic regression to investigate the associations between lipid ratios and sarcopenia, including non-high-density lipoprotein cholesterol (non-HDL-C) to HDL-C, triglyceride (TG) to HDL-C, low-density lipoprotein cholesterol (LDL-C) to HDL-C, and remnant cholesterol (RC) to HDL-C ratios. We performed subgroup analyses to assess interactions with other covariates and used mediation models to evaluate the mediating roles of inflammatory biomarkers. We included a total of 9500 non-sarcopenic and 849 sarcopenic participants aged 18–59 years. While we observed modest correlations between individual lipid components and sarcopenia, we obtained significant positive associations for lipid ratios. Specifically, non-HDL/HDL-C (OR = 1.09; 95% CI 1.03–1.15; P = 0.003), TG/HDL-C (OR = 1.02; 95% CI 1.02–1.04; P = 0.014), LDL/HDL-C (OR = 1.27; 95% CI 1.11–1.45; P < 0.001), and RC/HDL-C ratios (OR = 1.55; 95% CI 1.16–2.07; P = 0.004) showed strong associations with sarcopenia. These associations were more pronounced in younger participants, those with lower family economic status, and those without self-reported diabetes. Furthermore, there were significant mediation effects of inflammatory biomarkers on the association between non-HDL/HDL-C, LDL/HDL-C, and RC/HDL-C and sarcopenia risk, with proportions ranging from 2.90 to 6.36%. In conclusion, our study demonstrated the positive associations between lipid ratios and sarcopenia in middle-aged adults, suggesting the potential of these lipid ratios for improving sarcopenia case identification. Further research is required to explore the underlying mechanisms.
Similar content being viewed by others
Introduction
Sarcopenia, a progressive and generalized musculoskeletal disorder characterized by the loss of muscle mass and function1, is a major public health concern, particularly among middle-aged and older adults. This condition is associated with a range of adverse health outcomes, including diminished quality of life, increased healthcare utilization, higher medical costs, and elevated mortality risk2,3. While sarcopenia was once considered an inevitable consequence of aging, recent research has identified it as a multifactorial syndrome. Its development is influenced by both intrinsic and extrinsic factors4, including prolonged inactivity, inadequate nutritional intake, and chronic diseases such as diabetes and cancer1,5,6. These findings have heightened considerable interest in sarcopenia among researchers and clinicians.
Increasing evidence suggests that lipid metabolism may play a critical role in sarcopenia7,8. A typical cellular change in sarcopenic is the reduction in muscle fiber quality with age, replaced by intramuscular and intermuscular fat accumulation or myosteatosis9,10. Excessive myosteatosis not only disrupts muscle architecture and decreases mechanical function, but also negatively affects the metabolic mechanisms of muscle tissues. Changes in lipid metabolism in muscle tissues impair the muscle tissue microenvironment by promoting local or systemic inflammation and increasing insulin resistance (IR)10. Conventional blood lipid profiles typically measure individual lipid components, including total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), non-HDL-C, and remnant cholesterol (RC). A cross-sectional study observed negative associations between TC, TG, LDL-C, HDL-C levels and sarcopenia8, and Jang et al.11 reported a positive association between RC and the risk of low muscle mass in a Korean population. However, lipid ratios may provide additional insights into metabolic alternations and better reflect interactions among lipid components12,13. These ratios are increasingly used to investigate associations with lipid-metabolism-related diseases. In particular, the TG/HDL-C ratio is closely related to IR14, suggesting a potential association with sarcopenia. However, few studies have investigated the association between lipid ratios and sarcopenia.
Currently, timely diagnosis of sarcopenia remains challenging, as case identification typically begins with self-reported symptoms or clinical suspicion2. The SARC-F questionnaire, a recommended screening tool for sarcopenia, assesses the patients’ perception of the daily performance in areas such as strength, assistance walking, rising from a chair, stair climbing, and falls2,15. While SARC-F has high specificity, its sensitivity is limited16. Exploring the role of blood lipid profile in sarcopenia may offer quantitative indicators for early case identification, helping to refine the selection of high-risk individuals and improve the efficiency of SARC-F or other diagnostic tools. In this study, we aimed to comprehensively investigate the associations between serum lipid profile and sarcopenia risk using data from the 2011–2018 National Health and Nutrition Examination Survey (NHANES) in the US following a cross-sectional design.
Results
Characteristics of participants
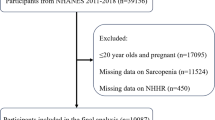
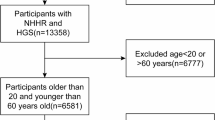
A total of 10,349 participants were included in the study, aged from 18 to 59 years (Fig. 1), whose characteristics were shown in Table 1. While the diagnosis of sarcopenia based on cross-sectional single-point data of body condition has some limitations, the classification of sarcopenic and non-sarcopenic groups in this study followed guidelines established and recommended by the National Institutes of Health. The median age was 37.00 years for non-sarcopenic participants, and 45.00 years for sarcopenic cases (P < 0.001). Sex (P = 0.334) between the two groups showed no significant difference. Compared to non-sarcopenic participants, sarcopenic cases were more likely to be Mexican American (P < 0.001), married (P < 0.001), non- or former- smokers (P = 0.002), had a lower education level (P < 0.001), a lower ratio of family income to poverty (PIR, P < 0.001), higher alcohol consumption (P = 0.030), shorter sedentary time (P = 0.002), higher body mass index (BMI, P < 0.001), and more self-reported diabetes, hypertension, cardiovascular diseases (CVDs, P < 0.001) and cancer (P = 0.028). Notably, there were significant differences between the two groups in lipid indicators (all P < 0.001, Table 1) and five inflammatory biomarkers (Table S1): neutrophil-to-lymphocyte ratio (NLR), derived neutrophil-to-lymphocyte ratio (dNLR), neutrophil-monocyte to lymphocyte ratio (NMLR), systemic inflammatory response index (SIRI), and systemic immune-inflammation index (SII).
Lipid parameters and sarcopenia risk
We first examined the associations between conventional lipid parameters and sarcopenia using weighted logistic models (Table S2). The number of participants included in each model varied depending on the missing values for the outcomes and adjusted covariates. With each 1 mg/dL increase in LDL-C and remnant cholesterol (RC), the estimated odds for sarcopenia increased by 0.5% (95% confidence interval [CI], 1.001–1.009) and 1.4% (95% CI 1.005–1.023), respectively, after adjusting for age, sex, race, education level, marital status, PIR, smoking status, sedentary time, self-reported diabetes, self-reported hypertension, self-reported CVDs, self-reported cancer, and glycohemoglobin. Non-HDL-C (odds ratio [OR] = 1.002; 95% CI 1.000–1.005; P = 0.049) showed a borderline significance. However, HDL-C (OR = 0.986; 95% CI 0.976–0.996; P = 0.008) was negatively associated with the risk of sarcopenia.
Four lipid ratios were analyzed (Table 2). Results across the three logistic models were generally consistent. In model 3, the estimated odds for sarcopenia were 9% greater for each unit increase in non-HDL/HDL-C (95% CI 1.03–1.15; P = 0.003), 2% greater for each unit increase in TG/HDL-C (95% CI 1.02–1.04; P = 0.014), 27% greater for each unit increase in LDL/HDL-C (95% CI 1.11–1.45; P < 0.001), and 55% greater for each unit increase in RC/HDL-C (95% CI 1.16–2.07; P = 0.004). Similarly, compared to the first quartile, a positive association with sarcopenia was observed for non-HDL/HDL-C (Ptrend = 0.001), TG/HDL-C (Ptrend < 0.001), LDL/HDL-C (Ptrend = 0.004), and RC/HDL-C ratio (Ptrend < 0.001) in the higher quartiles when treated as categorical variables.
To assess potential non-linear associations between lipid ratios (non-HDL/HDL-C, TG/HDL-C, LDL/HDL-C, and RC/HDL-C) and sarcopenia risk, we used restricted cubic spline (RCS) logistic regression (Fig. 2). After adjusting for all covariates, a linear association was observed for LDL/HDL-C (Poverall < 0.001, Pnon-linearity = 0.067), while non-linear associations were observed for non-HDL/HDL-C, TG/HDL-C, and RC/HDL-C ratios (Poverall < 0.001, Pnon-linearity < 0.001) with sarcopenia risk.
Associations between the non-HDL/HDL-C (A), TG/HDL-C (B), LDL/HDL-C (C), RC/HDL-C (D) ratios, and sarcopenia among the US population, NHANES, 2011–2018. Restricted cubic spline logistic regression was used after adjusting for age, sex, race/ethnicity, education level, marital status, PIR, smoking status, sedentary time, self-reported diabetes, self-reported hypertension, self-reported CVDs, self-reported cancer, and glycohemoglobin. The solid blue lines represented the central estimates, and the light blue-shaded regions represented the 95% CIs. CI confidence interval, CVDs cardiovascular diseases, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, OR odd ratio, PIR family poverty income ratio, RC remnant cholesterol, TG triglyceride.
Subgroup analysis
The subgroup analysis (Fig. 3) identified that significant associations between non-HDL/HDL-C, TG/HDL-C ratios and sarcopenia risk were more likely to occur in those with PIR < 1.3 (Pinteraction, 0.043 and 0.034, respectively), and no self-reported diabetes (Pinteraction, 0.023 and 0.008, respectively). Significant associations between LDL/HDL-C ratio, RC/HDL-C ratio, and sarcopenia risk were more likely to occur in those with age between 18 and 39 (Pinteraction, 0.042 and 0.041, respectively). Besides, an interaction between the RC/HDL-C ratio and self-reported diabetes was also observed (Pinteraction = 0.041).
Subgroup analysis of associations between lipid ratios and sarcopenia among the US population, NHANES, 2011–2018. CI confidence interval, CVDs cardiovascular diseases, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, OR odd ratio, PIR family poverty income ratio, RC remnant cholesterol, TG triglyceride.
Mediation and sensitivity analysis
We further explored the mediation roles of NLR, dNLR, monocyte-to-lymphocyte ratio (MLR), NMLR, SIRI, and SII in the association between lipid parameters and sarcopenia (Figure S1). NLR, dNLR, NMLR and SII significantly mediated the association between non-HDL/HDL-C and sarcopenia after adjusting for all covariates, with the mediation proportion of 2.90%, 4.90%, 3.42% and 3.74%, respectively (Fig. 4). Furthermore, dNLR and SII explained 5.75% and 4.59% of the association between LDL/HDL-C and sarcopenia, while dNLR and SIRI explained 6.36% and 4.14% of the association between RC/HDL-C and sarcopenia, respectively. Although the direct effects in other mediation models were all significant, no mediation effects were detected (Fig. 4). Finally, the sensitivity analysis after further adjusting for alcohol drinking and propensity score matching (Figure S2) both showed no substantive changes (Table S3, S4).
Mediation analysis results of inflammatory indices on the association between lipid ratios and sarcopenia among the US population, NHANES, 2011–2018. DE direct effects, dNLR derived neutrophil-to-lymphocyte ratio, HDL-C high-density lipoprotein cholesterol, IE indirect effects, LDL-C low-density lipoprotein cholesterol, MLR monocyte-to-lymphocyte ratio, NLR neutrophil-to-lymphocyte ratio, NMLR neutrophil-monocyte to lymphocyte ratio, RC remnant cholesterol, SII systemic immune-inflammation index, SIRI systemic inflammatory response index, TE total effects, TG triglyceride.
Discussion
We investigated the associations between blood lipid profile and sarcopenia risk using data from the 2011–2018 NHANES. We obtained modest correlations between conventional parameters and sarcopenia risk in US adults aged 18–59 years. However, we obtained stronger positive associations between all four lipid ratios, i.e., non-HDL/HDL-C, TG/HDL-C, LDL/HDL-C, and RC/HDL-C ratios, and sarcopenia risk. These findings support the potential use of these lipid ratios for the identification of sarcopenia cases.
The blood lipid profile is a widely used test, and its relationship with sarcopenia has garnered increasing attention from researchers. In terms of conventional lipid parameters, a hospital-based study reported that TG was negatively associated with sarcopenia risk, while HDL-C was positively associated17. Recently, a bidirectional Mendelian randomization analysis highlighted the genetic risks of HDL-C, LDL-D, TC, and TG for sarcopenia18. However, a meta-analysis reported that TG, TC, and LDL-C increased sarcopenia risk, while HDL-C had protective effects19. Our findings align with this meta-analysis for HDL-C and LDL-C, but we did not observe significant associations between TC, TG, and sarcopenia. Additionally, we observed a stronger positive association between RC and sarcopenia, consistent with a Korean study11. These individual lipid parameters may have limitations for screening sarcopenia, as they might overlook the combined effects of different lipids.
Compared to the traditional lipid profile, lipid ratios may exhibit more robust relationship with sarcopenia. The TG/HDL-C ratio, an indicator for IR14,20, suggests a potential link to sarcopenia. Several studies have explored these associations, but their findings have been inconsistent. Three studies involving Chinese participants reported a negative association between the TG/HDL-C ratio and sarcopenia risk17,21,22, whereas a study based on the Korean NHANES reported the opposite result23. One possible reason for these conflicting results is differences in study populations. Genetic variants and lifestyle factors influencing lipid traits may vary across racial groups24,25, resulting in differences in blood lipid levels. Although previous studies have explored the relationships between other lipid ratios and sarcopenia in the U.S. population, with baseline imbalances of race, marital status, smoking status, and some other characteristics26,27, there has been no research examining the association between the TG/HDL-C ratio and sarcopenia. Only Huang et al.28, using NHANES data, identified a negative correlation in older adults between the TG/HDL-C ratio and relative grip strength, a robust measure of sarcopenia, even after adjusting for race and other covariates.
Moreover, we conducted an investigation into the roles of the non-HDL/HDL-C, LDL/HDL-C, and RC/HDL-C ratios, finding that higher levels were associated with an increased risk of sarcopenia. These findings support the hypothesis that imbalances in lipid metabolism are closely linked to muscle health. The non-HDL/HDL-C ratio is a common and reliable indicator of lipid metabolism, which has been reported to be positively associated with sarcopenia26,27, while RC/HDL-C ratio demonstrated the strongest association in our study. Emerging evidence suggests that elevated RC increases the risk of cardiovascular disease29, which is bidirectionally associated with sarcopenia4. RC may impact human health by promoting chronic inflammation and lipid infiltration30,31. Our mediation analysis supported this, revealing the mediating roles of dNLR and SIRI in the RC/HDL-C–sarcopenia relationship. Both dNLR and SIRI are recognized risk factors for sarcopenia incidence and mortality32. Even though HDL-C is generally considered beneficial due to its role in reverse cholesterol transport33, the RC/HDL-C ratio provides a more comprehensive measure of blood cholesterol balance, highlighting its potential to reveal significant associations with sarcopenia.
Sarcopenia is commonly considered an age-related disorder1, underscoring the importance of investigating the interaction between age and lipid ratios. This study showed that the association between lipid ratios and sarcopenia is stronger in younger participants compared to older adults. Consequently, young populations may benefit more from the application of lipid ratios in sarcopenia case-finding. Our subgroup analysis revealed that these ratios perform better in individuals with higher economic status or those without diabetes. Socioeconomic status has been linked to blood lipid levels, likely through behavioral and psychological factors34,35,36. Additionally, diabetes is often associated with abnormal blood lipid metabolism12, which may influence the utility of these ratios. When using lipid ratios, it is essential to consider the target population carefully. Further studies are required to refine and validate the specific populations where these ratios are most effective.
This study had several strengths. First, we investigated four lipid ratios rather than focusing on the individual, conventional components. Second, the data were derived from the large-scale and accurate NHANES database, allowing for adjustment of numerous confounding variables to enhance the reliability and stability of our results. However, the study had some limitations. First, the lipid profile measurements and sarcopenia diagnoses were based on cross-sectional data, which restricts the causal inference. Sarcopenia is a progressive disorder characterized by the loss of muscle mass and function, which cannot be fully captured in a single cross-sectional snapshot. Therefore, we cautiously interpreted our findings, focusing only on the observed associations. Nonetheless, these associations suggests that lipids ratios could be valuable in identifying potential sarcopenia cases. Furthermore, blood lipid levels may vary across different ethnic groups, and there are potential limitations using calculated estimates of lipid parameters. These factors may reduce the generalizability of our findings to other populations.
Methods
Study design and population
Our cross-sectional study utilized data from the 2011–2018 NHANES database. NHANES employs a complex, multi-stage probability sampling process to collect information from a representative sample of Americans37. Its primary goal is to evaluate the health and nutritional status of adults and children in the US. Further details about the NHANES are available on the official website (https://www.cdc.gov/nchs/nhanes/about_nhanes.htm). The NHANES protocol was vigorously reviewed and approved by the National Center for Health Statistics Ethics Review Board to ensure ethical compliance. All participants provided written informed consent before their participation.
The selection criteria for participants in this study from the 2011–2018 NHANES database were as follows: (1) availability of the dual-energy X-ray absorptiometry (DXA) examination results with corresponding voluntary consents; (2) availability of relevant data on TC, HDL-C, and BMI; (3) daily energy intake between 800 and 5,000 kcal; and (4) age of 18 years or older38,39.
Detection and definitions of lipid profile and sarcopenia
Sarcopenia was identified based on the criteria established by the National Institutes of Health, specifically tailored to the characteristics of our research population39,40. Using results from DXA, appendicular skeletal muscle mass (ASM) was calculated by adding the bone-free lean mass measurements of the limbs. Sarcopenia was diagnosed if the ASM adjusted for BMI was below 0.512 for females or 0.789 for males39,40.
The lipid profile includes both conventional parameters and lipid ratios, providing a comprehensive assessment of an individual’s lipid status. The conventional lipid profile focuses on six key parameters (mg/dL): TC, TG, HDL-C, LDL-C, non-HDL-C, and RC. TC, HDL-C, and TG were measured using the Roche Modular P Chemistry Analyzer and the Roche Cobas 6000 Chemistry Analyzer41. LDL-C was calculated using the Friedewald formula: TC–HDL-C–TG/5 (applicable when TG ≤ 400 mg/dL)41,42. Non-HDL-C was defined as TC–HDL-C43,44, and RC was calculated as TC – HDL-C–LDL-C11,43,45. Lipid ratios were determined using the following formulas: non-HDL/HDL-C ratio = non-HDL-C/HDL-C43,46, TG/HDL-C ratio = TG/HDL-C43,47, LDL/HDL-C ratio = LDL-C/HDL-C43,48, and RC/HDL-C ratio = RC/HDL-C43,49.
Definition of covariates and mediators
Our analysis included the following demographic variables: age at screening (continuous), sex (male or female), race/ethnicity (Mexican American, Non-Hispanic White, Non-Hispanic Black, or other races), education level (middle school or below, high school graduation, and college degree or higher), marital status (never married, married, and other statuses such as being widowed, divorced, separated, or living with a partner), and PIR (continuous). Behavioral habits were captured using variables for smoking status, alcohol consumption, and sedentary time behavior duration (continuous). Smoking status was categorized into three groups: current smokers (≥ 100 lifetime cigarettes, currently smoking daily/occasionally), past smokers (≥ 100 lifetime cigarettes, not currently smoking), and never-smokers (< 100 lifetime cigarettes). Alcohol consumption was dichotomized based on the average daily intake over the past 12 months: < 3 drinks per drinking day and ≥ 3 drinks per drinking day. Chronic health conditions, including diabetes, hypertension, CVD, and cancer, were ascertained through self-reported physician diagnoses. For CVD specifically, participants were interviewed using a standardized questionnaire that inquired about diagnoses of congestive heart failure, coronary heart disease, angina pectoris, myocardial infarction, and stroke by a doctor or health professional. Confirmation of any of these conditions indicated the presence of CVD. Additionally, laboratory data on glycohemoglobin (continuous) were included in the analysis.
Based on the complete blood count results from the NHANES database (measured in 1000 cells/μL), we used indices of chronic inflammation to evaluate potential mediation effects. These indices were calculated using the following formulas: NLR = neutrophil/lymphocyte; dNLR = neutrophil/(white blood cell − lymphocyte); MLR = monocyte/lymphocyte; NMLR = (monocyte + neutrophil)/lymphocyte counts; SIRI = neutrophil × monocyte/lymphocyte; and SII = platelet × neutrophil/lymphocyte32.
Statistical analyses
The study participants were categorized into two groups: non-sarcopenia and sarcopenia. To ensure population representativeness, the analysis incorporated survey sampling weights in accordance with NHANES guidelines. The Kolmogorov–Smirnov tests were applied to test the normality of continuous variables. Subsequently, we reported the median and interquartile range (IQR) for these variables. Categorical variables were summarized as frequencies and percentages. Comparisons between the two groups were conducted using the Wilcoxon Rank Sum tests for continuous variables and the Chi-squared tests for categorical variables.
ORs and 95% CIs for sarcopenia risk associated with lipid ratios were determined using weighted multivariable logistic regression models. These lipid ratios were analyzed as both continuous variables and categorical variables, with quartiles based on the distribution in the non-sarcopenic group. We used three models to ensure the stability of the results and accounted for baseline discrepancies through statistical methods: unadjusted model, minimally-adjusted model (adjusted for age and sex), and fully-adjusted model (adjusted for age, sex, race/ethnicity, education level, marital status, PIR, smoking status, sedentary behavior duration, glycohemoglobin, and self-reported diabetes, hypertension, CVD, and cancer). Multicollinearity was tested for the fully-adjusted model (Table S5). To further explore the associations between lipid ratios and sarcopenia, we adopted a multivariate-adjusted RCS logistic regression approach. Specifically, we employed restricted triple spline analysis for a comprehensive examination of these relationships.
Subgroup analyses were conducted to examine the consistency of the findings. Subgroup factors included age (18–39 years old or 40–59 years old), sex, race/ethnicity, education level, marital status, PIR (< 1.3, 1.3–3.49, or ≥ 3.5)46, smoking status, sedentary behavior duration (≤ 360 min or > 360 min, based on the median), self-reported diabetes, hypertension, CVD, cancer, and glycohemoglobin (≤ 5.4 or > 5.4, based on the median). Likelihood ratio tests were conducted between the models with and without interaction terms, producing P-values to assess the statistical significance of interactions. Furthermore, we conducted non-parametric bootstrapping (n = 1000) to evaluate the direct effects, indirect effects, total effects, and the proportion of mediation by inflammatory indices in the relationship between lipid ratios and sarcopenia. The mediation models were adjusted for the same covariates included in the fully-adjusted model.
For sensitivity analysis, we initially integrated alcohol consumption (< 3 or ≥ 3 drinks per day) into the fully adjusted model to address its high missing rate (28.74%). Subsequently, we used propensity score methods to balance covariates between the non-sarcopenia and sarcopenia groups. The propensity scores were calculated from a logistic regression, including all variables in the adjusted model. A 1:2 propensity score-matched subset was generated with a caliper of 0.02, ensuring acceptable balance (standardized mean difference, SMD ≤ 0.1). Finally, conditional logistic regression was applied to assess the associations within the matched subset.
We conducted the statistical analyses using R software (version 4.3.2, R Foundation for Statistical Computing, Vienna, Austria), with statistical significance set as P < 0.05 (two-tailed).
Conclusions
Our study revealed that non-HDL/HDL-C, TG/HDL-C, LDL/HDL-C, and RC/HDL-C ratios were positively associated with sarcopenia risk, with RC/HDL-C ratio demonstrating the strongest association in middle-aged American adults. Our findings underscore the potential of these indicators for the identification of sarcopenia cases. It is important to acknowledge the applicability of these indicators in populations with different family economic status, ages, and diabetes history. Further multi-center population or clinical experimental studies are warranted to examine these associations.
Data availability
Data can be obtained from the NHANES Questionnaires, Datasets, and Related Documentation website: https://wwwn.cdc.gov/nchs/nhanes/Default.aspx.
Abbreviations
- ASM:
-
Appendicular skeletal muscle mass
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- CVD:
-
Cardiovascular disease
- dNLR:
-
Derived neutrophil-to-lymphocyte ratio
- DXA:
-
Dual-energy X-ray absorptiometry
- HDL-C:
-
High-density lipoprotein cholesterol
- IR:
-
Insulin resistance
- LDL-C:
-
Low-density lipoprotein cholesterol
- MLR:
-
Monocyte-to-lymphocyte ratio
- NHANES:
-
National Health and Nutrition Examination Survey
- NLR:
-
Neutrophil-to-lymphocyte ratio
- NMLR:
-
Neutrophil-monocyte to lymphocyte ratio
- OR:
-
Odds ratio
- PIR:
-
Ratio of family income to poverty
- RC:
-
Remnant cholesterol
- RCS:
-
Restricted cubic spline
- SII:
-
Systemic immune-inflammation index
- SIRI:
-
Systemic inflammatory response index
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
References
Cruz-Jentoft, A. J. & Sayer, A. A. Sarcopenia. Lancet 393, 2636–2646 (2019).
Cruz-Jentoft, A. J. et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 48, 16–31 (2019).
Petermann-Rocha, F. et al. Physical capability markers used to define sarcopenia and their association with cardiovascular and respiratory outcomes and all-cause mortality: A prospective study from UK Biobank. Maturitas 138, 69–75 (2020).
Damluji, A. A. et al. Sarcopenia and cardiovascular diseases. Circulation 147, 1534–1553 (2023).
Kerr, N. R. & Booth, F. W. Contributions of physical inactivity and sedentary behavior to metabolic and endocrine diseases. Trends Endocrinol. Metab. 33, 817–827 (2022).
Papadopoulou, S. K. et al. Exercise and nutrition impact on osteoporosis and sarcopenia-The incidence of osteosarcopenia: A narrative review. Nutrients 13, 4499 (2021).
Eshima, H. et al. Lipid hydroperoxides promote sarcopenia through carbonyl stress. Elife 12, e85289 (2023).
Jiang, Y. et al. The association of lipid metabolism and sarcopenia among older patients: a cross-sectional study. Sci. Rep. 13, 17538 (2023).
Frontera, W. R., Zayas, A. R. & Rodriguez, N. Aging of human muscle: understanding sarcopenia at the single muscle cell level. Phys. Med. Rehabil. Clin. N. Am. 23, 201–207 (2012).
Correa-de-Araujo, R. et al. The need for standardized assessment of muscle quality in skeletal muscle function deficit and other aging-related muscle dysfunctions: A symposium report. Front. Physiol. 8, 87 (2017).
Jang, S. Y. et al. Association of remnant cholesterol with sarcopenia in Korean adults: a nationwide population-based study using data from the KNHANES. Front. Endocrinol. 15, 1391733 (2024).
Yang, T. et al. Correlation between the triglyceride-to-high-density lipoprotein cholesterol ratio and other unconventional lipid parameters with the risk of prediabetes and type 2 diabetes in patients with coronary heart disease: a RCSCD-TCM study in China. Cardiovasc. Diabetol. 21, 93 (2022).
Guerrero-Romero, F. et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J. Clin. Endocrinol. Metab. 95, 3347–3351 (2010).
Giannini, C. et al. The triglyceride-to-HDL cholesterol ratio: association with insulin resistance in obese youths of different ethnic backgrounds. Diabetes Care 34, 1869–1874 (2011).
Malmstrom, T. K., Miller, D. K., Simonsick, E. M., Ferrucci, L. & Morley, J. E. SARC-F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J. Cach. Sarcopenia Muscle 7, 28–36 (2016).
Ida, S., Kaneko, R. & Murata, K. SARC-F for screening of sarcopenia among older adults: A meta-analysis of screening test accuracy. J. Am. Med. Dir. Assoc. 19, 685–689 (2018).
Wang, N., Chen, M. & Fang, D. Relationship between serum triglyceride to high-density lipoprotein cholesterol ratio and sarcopenia occurrence rate in community-dwelling Chinese adults. Lipids Health Dis. 19, 248 (2020).
Huang, H. et al. The relationship between serum lipid with sarcopenia: Results from the NHANES 2011–2018 and bidirectional Mendelian randomization study. Exp. Gerontol. 196, 112560 (2024).
Du, Y., Oh, C. & No, J. Associations between sarcopenia and metabolic risk factors: A systematic review and meta-analysis. J. Obes. Metab. Syndr. 27, 175–185 (2018).
Gong, R. et al. Associations between TG/HDL ratio and insulin resistance in the US population: a cross-sectional study. Endocr. Connect. 10, 1502–1512 (2021).
Fu, Q., Zhang, Z., Hu, W. & Yang, Y. The correlation of triglyceride/high-density lipoprotein cholesterol ratio with muscle mass in type 2 diabetes patients. BMC Endocr. Disord. 23, 93 (2023).
Lin, Y., Zhong, S. & Sun, Z. Association between serum triglyceride to high-density lipoprotein cholesterol ratio and sarcopenia among elderly patients with diabetes: a secondary data analysis of the China health and retirement longitudinal study. BMJ Open 13, e075311 (2023).
Chung, T., Kwon, Y., Shim, J. & Lee, Y. Association between serum triglyceride to high-density lipoprotein cholesterol ratio and sarcopenia in elderly Korean males: The Korean National Health and Nutrition Examination Survey. Clin. Chim. Acta 463, 165–168 (2016).
Tang, C. S. et al. Exome-wide association analysis reveals novel coding sequence variants associated with lipid traits in Chinese. Nat. Commun. 6, 10206 (2015).
Kim, Y.-S. et al. Lifestage sex-specific genetic effects on metabolic disorders in an adult population in Korea: The Korean Genome and Epidemiology Study. Int. J. Mol. Sci. 23, 11889 (2022).
Ma, Y. et al. Association between non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio and sarcopenia based on NHANES. Sci. Rep. 4, 30166 (2024).
He, R., Ye, Y., Zhu, Q. & Xie, C. Association between non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio and sarcopenia in individuals with cancer: a cross-sectional study. Lipids Health Dis. 23, 217 (2024).
Huang, Y., Liao, J. & Liu, Y. Triglyceride to high-density lipoprotein cholesterol ratio was negatively associated with relative grip strength in older adults: a cross-sectional study of the NHANES database. Front. Public Health 11, 1222636 (2023).
Quispe, R. et al. Remnant cholesterol predicts cardiovascular disease beyond LDL and ApoB: a primary prevention study. Eur. Heart J. 42, 4324–4332 (2021).
Tall, A. R. & Yvan-Charvet, L. Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol. 15, 104–116 (2015).
White, Z. et al. Cholesterol absorption blocker ezetimibe prevents muscle wasting in severe dysferlin-deficient and mdx mice. J. Cach. Sarcopenia Muscle 13, 544–560 (2022).
Guo, B. et al. Associations of CBC-Derived inflammatory indicators with sarcopenia and mortality in adults: evidence from Nhanes 1999–2006. BMC Geriatr. 24, 432 (2024).
Rosenson, R. S. et al. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation 125, 1905–1919 (2012).
Santo, L. R. E. et al. Socioeconomic status and education level are associated with dyslipidemia in adults not taking lipid-lowering medication: a population-based study. Int. Health 14, 346–353 (2022).
Shohaimi, S., Boekholdt, M. S., Luben, R., Wareham, N. J. & Khaw, K.-T. Distribution of lipid parameters according to different socio-economic indicators- the EPIC-Norfolk prospective population study. BMC Public Health 14, 782 (2014).
Yu, Z. et al. Socio-economic status and serum lipids: a cross-sectional study in a Chinese urban population. J. Clin. Epidemiol. 55, 143–149 (2002).
Paulose-Ram, R., Graber, J. E., Woodwell, D. & Ahluwalia, N. The National Health and Nutrition Examination Survey (NHANES), 2021–2022: Adapting data collection in a COVID-19 environment. Am. J. Public Health 111, 2149–2156 (2021).
Xu, W. et al. Association between oxidative balance score and sarcopenia in US adults: NHANES 2011–2018. Front. Nutr. 11, 1342113 (2024).
Huang, Q. et al. Association between manganese exposure in heavy metals mixtures and the prevalence of sarcopenia in US adults from NHANES 2011–2018. J. Hazard Mater. 464, 133005 (2024).
Studenski, S. A. et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J. Gerontol. A Biol. Sci. Med. Sci. 69, 547–558 (2014).
Abasilim, C., Persky, V. & Turyk, M. E. Association of blood total mercury with dyslipidemia in a sample of U.S. adolescents: Results from the National Health and Nutrition Examination Survey database, 2011–2018. Hyg. Environ. Health Adv. 6, 100047 (2023).
Friedewald, W. T., Levy, R. I. & Fredrickson, D. S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 18, 499–502 (1972).
Sheng, G. et al. Evaluation of the value of conventional and unconventional lipid parameters for predicting the risk of diabetes in a non-diabetic population. J. Transl. Med. 20, 266 (2022).
Zhang, N. et al. Non-high-density lipoprotein cholesterol: High-density lipoprotein cholesterol ratio is an independent risk factor for diabetes mellitus: Results from a population-based cohort study. J. Diabetes 10, 708–714 (2018).
Nordestgaard, B. G. & Varbo, A. Triglycerides and cardiovascular disease. Lancet 384, 626–635 (2014).
Qi, X. et al. The association between non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) and risk of depression among US adults: A cross-sectional NHANES study. J. Affect. Disord. 344, 451–457 (2024).
Khaloo, P. et al. Impact of 3-year changes in lipid parameters and their ratios on incident type 2 diabetes: Tehran lipid and glucose study. Nutr. Metab. (Lond). 15, 50 (2018).
Hong, M. et al. Contribution and interaction of the low-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio and triglyceride to diabetes in hypertensive patients: A cross-sectional study. J. Diabetes Investig. 10, 131–138 (2019).
Zou, Y., Hu, C., Kuang, M. & Chai, Y. Remnant cholesterol/high-density lipoprotein cholesterol ratio is a new powerful tool for identifying non-alcoholic fatty liver disease. BMC Gastroenterol. 22, 134 (2022).
Acknowledgements
We appreciate all participants, investigators, and academics for their valuable contribution to the National Health and Nutrition Examination Survey. This study was supported by the Shandong Provincial Natural Science Foundation (ZR2022QH162), the National Natural Science Foundation of China (82204129 and 82103912), the Taishan Scholars Program of Shandong Province (tsqn202312328), and the Excellent Youth Innovation Team of Shandong Provincial Higher Education Institutions (2022KJ012). The funders were not involved in the collection, analysis, or interpretation of data or the writing or submitting of this report.
Author information
Authors and Affiliations
Contributions
Study concept and design: X.L.Y., H.H.S., H.C., X.R.Y. and T.C.Z.; Acquisition of data: X.L.Y., H.H.S. and T.C.Z.; Analysis and interpretation of data: T.C.Z.; Visualization: X.L.Y. and T.C.Z.; Drafting of the manuscript: X.L.Y.; Critical revision of the manuscript for important intellectual content: X.L.Y., H.C., X.R.Y. and T.C.Z.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics declarations
The NHANES protocol underwent rigorous approval by the National Center for Health Statistics Ethics Review Board to ensure ethical compliance, and all participants in the project provided written informed consent before their involvement.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yin, X., Song, H., Chen, H. et al. Association between lipid ratios and sarcopenia and the mediating roles of inflammatory biomarkers in a cross-sectional study from NHANES 2011–2018. Sci Rep 15, 6617 (2025). https://doi.org/10.1038/s41598-025-90131-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-90131-y