Abstract
To determine the volatile organic compounds (VOCs) generated by casting resin sand and its binder during the casting process, this paper studied the process of resin pyrolysis and the different types of VOCs generated under different atmospheric conditions, as well as the temperature range of which the main pollutants are produced. Results indicate that resin pyrolysis can affect the types and characteristics of products under different atmospheric conditions. Oxygen in the air can accelerate the decomposition or combustion of organic matter in the sample. Under anaerobic conditions, the main products are benzene and ester products, primarily including dimethyl succinate and 1,2,3,5-tetramethylbenzene, in temperature range of 200–600 ℃. Under oxygen-containing conditions, the main products are ethers, benzene, and hydrocarbons, specifically ethylene oxide and 2-methylbutane, produced at temperatures above 200 ℃. This study provides theoretical guidance for the optimization design and environmental protection of molding sand and its binders in the casting industry, and provides technical support for the industrial application and development of molding sand.
Similar content being viewed by others
Introduction
Metal casting industry, as the foundation of the mechanical manufacturing field, occupies an important position in economic development1,2,3. China is the most important casting production country in the world, with casting production accounting for over 55% of the global total production. Among various casting methods, sand casting is a commonly used technique, which has the advantages of flexible structure, low cost, and simple process4,5,6,7,8. While sand casting promotes socio-economic development, it also brings enormous pressure to the environment, mainly manifested in atmospheric pollution, such as particulate matter, inorganic gaseous pollutants and volatile organic compounds (VOCs)9,10. In particular, VOCs are important precursors of atmospheric photochemical reactions, which not only exacerbate haze formation, but also pose a threat to human health, affecting immune, neurological, digestive and other system functions11,12,13. Researches showed that VOCs released during sand casting can damage the respiratory system of workers and increase the risk of lung cancer14. Therefore, in order to protect the health and safety of workers and the general public, air pollution prevention and control technology in casting has become a key issue of concern and research in various countries.
The pyrolysis of raw materials for making sand and sand molds is the main source of VOCs in sand casting. Several reports in the literature have investigated the pyrolysis products of different adhesives. For example, Kmita et al. studied the decomposition of casting adhesives TPA70 and ESTROFEN, and found that their pyrolysis products mainly included benzene and its derivatives, as well as phenol and its derivatives15. Dungan and Reeves conducted pyrolysis experiments on Novolac, phenolic urethane and furan binders at 750 ℃. Results showed that the main thermal decomposition products were derivatives of phenol, benzene, and furan, respectively4. Holtzer et al. conducted pyrolysis analysis of alkyd resin in the range of 500–1300 ℃. The main compounds detected were furfuryl alcohol, formaldehyde, phenol, BTEX compounds (benzene, toluene, ethylbenzene, xylene), as well as hydrocarbons and polycyclic aromatic hydrocarbons (PAHs)16. However, the types of molding sand and its binders studied so far are limited, and cannot fully reflect the emission of VOCs during the casting process9,17. Among all resin bonded sands, molding sand with phenolic resin and isocyanate as binders is the most widely used in sand casting. Therefore, this article selects phenolic resin and isocyanate cured sand cores, coated sand, phenolic resin and isocyanate as research objects.
When molten metal comes into contact with molding sand, the surface of the mold in direct contact with the melt reacts with air at high temperature and undergoes pyrolysis under aerobic conditions10,14,18,19,20. The temperature below the surface of the core increases through heat conduction and the core area undergoes slow pyrolysis under anaerobic or low oxygen conditions, as shown in Fig. 1. Kmita et al. studied the thermal decomposition of ester cured alkaline resin (PF type A) in different atmospheres, including inert, reducing, and oxidizing atmospheres15. The study showed that the resin had the highest mass loss in inert and reducing atmospheres, while in oxidizing atmospheres, the maximum mass loss was found in the temperature range between 973 and 983 K. Chen et al. conducted pyrolysis of steel casting sand under nitrogen and oxygen free conditions and found that the mass loss of casting sand in air was greater than that in nitrogen21. At present, for the pyrolysis of resins under different atmospheres, the main focus is on mass loss, with limited quantitative research on the types of pollutants and the generation temperature range.
This study, for the first time, explored the effects of different atmospheres and temperatures on the pyrolysis behavior, as well as VOCs generation characteristic, of casting raw materials. The weight loss curves of the raw materials under anaerobic and aerobic conditions were determined by thermogravimetric analysis. The changes in products of molding sand in different temperature ranges were studied using a tube furnace. The composition of pollutants in the main weight loss stage was analyzed using chromatography/mass spectrometry. This study provides theoretical guidance for design optimization and environmental protection of molding sand and its binders in the casting industry, as well as technical support for the industrial application and development of molding sand.
Materials and methods
Materials
The pyrolysis experimental materials used in this study were phenolic resin and isocyanate cured sand cores, coated sand, phenolic resin and isocyanate. The sand cores and coated sand samples were ground into powder form for thermogravimetric and slow pyrolysis experiments.
Methods
Thermal gravimetry method (TGA/DSC 3 + Mettler Toledo Switzerland) was used to observe the thermal decomposition of resin samples (30 mg of solid sample and 5 mg of liquid sample) over a temperature increasing from 30 ℃ to 1200 ℃, with a heating rate of 20 ℃/min. The carrier gases were argon and air, with a volumetric flow rate of 100 mL/min.
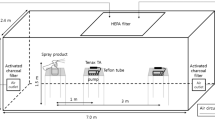
The experimental setup for pyrolysis and detection of four samples was shown in Fig. 2. Solid and liquid samples were pyrolyzed using a tube furnace (TL1700, Nanjing Boyuntong Instrument Technology Co. Ltd). The starting temperature was 30 ℃, the final temperature was 1200 ℃, and the heating rate was 5 ℃/min. In order to provide different atmospheric conditions, argon or air was selected as the carrier gas and a volumetric flow rate of 100 mL/min was maintained. Argon that does not react with the sample provides an anaerobic atmospheric condition, while air provides an aerobic environment. Before the experiment, 30 g of the solid sample and 5 g of the liquid sample were measured out, and placed separately in a quartz boat. At the same time, 10 g of activated carbon was first dried for 12 h in a drying oven, and then placed at the gas outlet of tube furnace to adsorb the gas generated during pyrolysis process. At the beginning of pyrolysis, a quartz boat was pushed into the center position of the corundum tube, heated up to the final temperature, and kept at this temperature for 30 min. The gas components in activated carbon were extracted with 30 ml dichloromethane for 2 h. The extraction solution was filtered with a 0.22 μm needle filter to obtain the sample for analysis.
GC-MS (Agilent 8890 + 5977B GC/MSD, Agilent Technologies Inc.) was used to detect the dichloromethane extracted products using a miniature automatic injector ™. The detailed experimental conditions were as follows: 1 mL of the analytical sample was placed in a sample vial. The carrier gas utilized was high-purity argon (≥ 99.99%), with the injection port temperature set at 25 ℃. The column oven temperature program began at 30 ℃, rised up to 200 ℃ at a rate of 20 ℃/min, and was held at this temperature for 3 min. The carrier gas flow rate was maintained at 1.3 mL/min. The ion source temperature was 300 ℃, while the quadrupole temperature was kept at 150 ℃. Electron bombardment (EI) was selected as the ionization mode. The scanning type employed was selective ion monitoring (SIM). The data collection and processing were carried out using Agilent MassHunter software.
Results and discussion
Analysis of products under anaerobic conditions
Thermogravimetric analysis (TGA) can reveal the weight loss at different temperatures. The TGA curves of phenolic resin and isocyanate cured sand cores, coated sand, phenolic resin and isocyanate are shown in Fig. 3. The weight loss in products during resin pyrolysis can be divided into four stages. The first stage is from room temperature (30 ℃) to 200 ℃. In this stage, the weight loss rate of the cured sand core is about 1%, while the coated sand barely experiences weight loss. Phenolic resin and isocyanate lose 25.52% and 51.69% of their mass, respectively. The weight loss at this stage mainly comes from the evaporation of the moisture content in the sample. The second and third weight loss stages of solidified sand cores and coated sand occur at 200–600 ℃ and 600–1000 ℃, while those of phenolic resin and isocyanate occur at 200–400 ℃ and 400–600 ℃. In both stages, the weight of the samples shows a significant decrease. This indicates that the main thermal decomposition reactions of the resin occur in these two temperature ranges, and the cracking of organic molecules may occur. The fourth stage occurs after the third stage and ends at 1200 ℃, and the TGA curve in this stage tends to flatten, possibly because the product reaches a stable state of carbonization.
The specific pollutant species of VOCs in different raw materials under anaerobic conditions are shown in Fig. 4. The composition characteristics of solid samples show that benzene is the main component, accounting for more than 50%, followed by esters that accounting for about 35%, while the content of hydrocarbons and other products is below 10%. The three major species in the solidified sand core are dimethyl glutarate, 1,2,4,5-tetramethylbenzene and 2-ethyl-1,3-dimethylbenzene, accounting for 16.00%, 13.38% and 8.93%, respectively. In the coated sand, the top three characteristic species are dimethyl succinate, dimethyl glutarate, and 1-methyl-4-(1-methylpropyl) benzene. A few other components exist in the solidified sand core and coated sand, but their quantity is relatively small. The resin in the cold core contains 0.80% ketones, 0.17% phenols, 0.40% alcohols, and 0.36% ethers, while the resin in the hot core contains 1.18% ketones.
The composition characteristics of liquid samples are different from those of solid samples. The proportion of benzene in phenolic resin is 66.36%, while the proportion of other components is relatively low, with esters accounting for 18.65% and hydrocarbons accounting for 13.06%. The pollutants with high contribution in phenolic resins include 1,2,3,5-tetramethylbenzene, 1-ethyl-2,4-dimethylbenzene, and dimethyl succinate. Phenolic resin first undergoes cracking under high temperature and oxygen-free conditions to form compounds containing benzene rings and low molecular weight organic compounds, such as phenol and formaldehyde. Compounds containing benzene rings undergo substitution reactions with low molecular weight organic compounds to form benzene products. The generation of ester products may be attributed to the rearrangement reaction of intermediates during pyrolysis, or may originate from the pyrolysis of other additives in phenolic resins. Esters are the main component of isocyanates, accounting for 46.84%, followed by benzene at 34.75%, while the hydrocarbon content is 13.37%. Isocyanates have a higher ester content at high temperatures, possibly due to the reaction of their carbonyl groups with oxygen-containing functional groups to form esters. The species with higher emission concentrations in isocyanates are dimethyl succinate, dimethyl adipate, and 1,4-diethyl-2-methylbenzene.
To clarify the specific temperature range at which the main products are formed, the products of the four samples at different temperatures were analyzed, and the results are shown in Fig. 5. The pyrolysis products of solidified sand cores are mainly benzene based compounds, accounting for about 65%. The pyrolysis temperature range of benzene based compounds is 30–200℃, 200–600℃, and 600–1000℃. Next are ester products, and their proportion increases with increased temperature from 4.26 to 13.10%. We analyzed the three highest concentrations of pollutants in the solidified sand core. Results show that the pyrolysis ratios of dimethyl glutarate at 30–200 ℃, 200–600 ℃, and 600–1000 ℃ are 2.11%, 7.34%, and 4.86%, respectively. The corresponding pyrolysis ratios of 1,2,4,5-tetramethylbenzene are 6.28%, 7.69%, and 6.06%, while those of 2-ethyl-1,4-dimethylbenzene are 4.95%, 1.42%, and 1.87%.
About 2.08% of benzene is produced in coated sand at 30–200 ℃. At temperatures between 200 and 600 ℃, the highest yields of benzene and esters are 30.26% and 27.43%, respectively, with 1.18% of ketone products. The volume fractions of benzene and esters are 24.36% and 5.42% at 600–1000 ℃, and 7.54% and 1.72% at 1000–1200 ℃, respectively. The contents of dimethyl succinate, dimethyl glutarate, and 1-methyl-4-(1-methylpropyl) benzene in the range of 200–600 ℃ are 15.33%, 9.17%, and 10.51%, respectively.
The products in phenolic resin are mainly concentrated at 30–600 ℃, and the volume fraction of benzene products increases from 10.83% to 41.11% with the increase in temperature. The volume fractions of ester products are 7.54%, 7.35%, and 3.76% at 30–200 ℃, 200–400 ℃, and 400–600 ℃, respectively. In addition, 0.77% aldehyde product at 30–200 ℃, 0.74% ketone product and 5.04% hydrocarbon product at 200–400 ℃, and 1.13% ketone product at 400–600 ℃ are observed. 1,2,3,5-Tetramethylbenzene and 1-ethyl-2,4-dimethylbenzene mainly in the range of 400–600 ℃, with contents of 7.94% and 12.15%, respectively. Dimethyl succinate mainly in the range of 200–400 ℃, with content of 3.99%.
Among the thermal decomposition products of isocyanates, 9.29% ester compounds are observed in the range of 200–400 ℃, while the remaining products are mainly concentrated in the range of 400–600 ℃. The volume fractions of esters, benzene, and hydrocarbons are 37.55%, 34.75%, and 12.93%, respectively. Unlike other samples, 3.61% phenols and 1.44% alcohol compounds are still observed in the range of 400–600 ℃. The proportions of dimethyl succinate, dimethyl adipate, and 1,4-diethyl-2-methylbenzene are 18.00%, 16.97%, and 22.60%, respectively.
From the product distribution of the four samples at different temperatures, it can be seen that in addition to water, benzene and ester products also exist in the cured sand core and phenolic resin at 30–200 ℃. The hot core phenolic resin contains 2.08% of benzene products. The volume fraction and types of products are highest in the solidified sand core at 600–1000 ℃, while the products of coated sand are concentrated at 200–600 ℃. Products of phenolic resin and isocyanate are mainly concentrated at 400–600 ℃.
Analysis of products under oxygen-containing conditions
The weight loss curves of the four samples under oxygen-containing conditions are shown in Fig. 6. Under oxygen-containing conditions, the weight loss processes of the cured sand core and coated sand end at 600 ℃, while under anaerobic conditions it completes at temperature around 1000 ℃. This indicates that oxygen in the air has a significant impact on the weight loss process of the sample, which can accelerate the decomposition or combustion of organic matter in the sample. The weight loss of solidified sand cores and coated sand mainly occurs in three temperature ranges: 30℃–200 ℃, 200℃–400 ℃, and 400℃–600 ℃, corresponding to the evaporation of water and the cracking of organic molecules. Both phenolic resin and isocyanate samples exhibit a weight loss rate of 100% at around 800 ℃, indicating that the samples are completely burned. Compared with anaerobic conditions, there is an additional weight loss stage between 400 and 600 ℃ under oxygen-containing conditions, during which the samples lose about 20% of their mass. This may be due to the chemical reaction between organic residues and oxygen in the air, forming volatile products.
Under oxygen-containing conditions, the compositions of the four sample products are shown in Fig. 7. The solidified sand core is mainly composed of ether products, accounting for 60.71%, followed by benzene products accounting for 12.55%, hydrocarbon products accounting for 11.22%, and ester products accounting for 5.54%. Ethylene oxide is the main ether product, followed by hydrocarbon product 2-methylbutane, accounting for 10.65%. The proportion of ether products in coated sand is 51.85%, followed by benzene products accounting for 35.87%. Hydrocarbon products account for 10.45%, and ester products account for 1.81%. The three main characteristic species of coated sand are ethylene oxide, 1,2,4,5-tetramethylbenzene, and 2-methylbutane. The proportion of ethers in phenolic resin is 35.83%, followed by hydrocarbon products accounting for 30.17%, benzene products accounting for 22.37%, esters and other types accounting for 2.52% and 8.80%, respectively. The pollutants with higher contributions include ethylene oxide, 2-methylbutane, and 1-ethyl-2,4-dimethylbenzene. For isocyanate, ether products account for 34.99%, followed by hydrocarbon and benzene products, accounting for 32.38% and 31.17%, respectively. In addition, there are 1.46% esters and 0.71% other products. The species with higher emission concentrations are ethylene oxide, 3-methoxy-1-propene, and 2-methylbutane.
Compared with anaerobic conditions, there are significant differences in VOCs emission characteristics in oxygen-containing conditions. The addition of oxygen increases the variety of products. Specifically, proportions of ether and hydrocarbon products increase, while those of benzene and ester products decrease. Ethylene oxide and 2-methylbutane are detected in all four products. Ethylene oxide may originate from the epoxidation reaction during the reaction, while 2-methylbutane may originate from the rearrangement or ring opening reaction during pyrolysis. In addition, the content of alcohols, aldehydes, and ketones in other products of the four samples increase compared to anaerobic conditions, possibly due to the promotion of partial oxidation of intermediate products by the addition of oxygen.
The main pyrolysis products of the four samples at different temperature ranges under oxygen-containing conditions are shown in Fig. 8. The main pyrolysis products of the solidified sand core at 30–200 ℃ are esters, benzene and hydrocarbons, accounting for 5.55%, 7.97% and 0.56%, respectively. At 200–400 ℃, ether products account for 30.52%, followed by ketones, hydrocarbons, and benzene products, accounting for 9.97%, 5.61% and 4.04%, respectively. The products at 400–600 ℃ are mainly ether and hydrocarbon products, accounting for 30.19% and 5.59% respectively. Ethylene oxide and 2-methylbutane are mainly concentrated in two temperature ranges of 200–400 ℃ and 400–600 ℃, with volume fractions of 30.52% and 30.19% for ethylene oxide, and 5.61% and 5.05% for 2-methylbutane, respectively.
About 5% of benzene products are generated in the coated sand below 200 ℃, while the highest amount is generated at 400–600 ℃, with ether products accounting for 51.86%, followed by benzene products accounting for 33.37%, hydrocarbon accounting for 8.30% and ester productsaccounting for 1.81%. The volume fractions of ethylene oxide, 1,2,4,5-tetramethylbenzene, and 2-methylbutane at 400–600 ℃ are 51.85%, 15.25% and 8.30%, respectively.
The contribution of phenolic resin products is relatively balanced in different temperature ranges, with hydrocarbon products accounting for 11.81% at 30–200 ℃, followed by 5.71% of alcohol products. At temperatures between 200 and 400 ℃, ether, benzene, and hydrocarbon products are mainly present, accounting for 11.81%, 12.67% and 7.38%, respectively. At 400–600 ℃, ether products are mainly present, accounting for 11.65%. The products at 600–800 ℃ are mainly ether and benzene, accounting for 12.13% and 14.99%, respectively. Ethylene oxide have a certain distribution in temperature ranges of 200–800 ℃, while 2-methylbutane and 1-ethyl-2,4-dimethylbenzene are mainly generated at 200–400 ℃.
Small amounts of ethers, benzene, and hydrocarbons are produced in isocyanates at 30–200 ℃. At temperatures between 200 and 400 ℃, hydrocarbon products are the main component, accounting for 17.35%. At temperatures between 400 and 600 ℃, ether, benzene and hydrocarbon products are mainly present, accounting for 16.33%, 22.98% and 8.44%, respectively. Ethers are the main products at 600–800 ℃, accounting for 16.72%, followed by benzene and hydrocarbons, accounting for 4.91% and 2.63%, respectively. Ethylene oxide and ethyl ether are mainly generated at 400–600 ℃ and 600–800 ℃, 3-methoxy-1-propene are mainly generated at 200–400 ℃, and 2-methylbutane is generated above 200 ℃.
Taking phenolic resin as an example, in the low temperature range (30–200 ℃), the first occurrence is thermogravimetric weight loss. Hydrocarbons originate from partial decomposition of low molecular weight or thermally unstable compounds, while alcohol products may be formed due to partial oxidation reactions of hydrocarbon products. As the temperature increases to the range of 200–400 ℃, the phenolic groups in the resin molecules begin to undergo pyrolysis, producing intermediate products such as ethers, benzene and hydrocarbons. It is worth noting that the ether products generated at this time may originate from the cleavage of phenolic groups in phenolic molecules, while the benzene products may originate from further polymerization and rearrangement of the aromatic ring structure of phenolic molecules. In the higher temperature ranges (400–600 ℃, 600–800 ℃), the phenolic resin undergoes deep cracking, and ether and benzene products are mainly generated at this time. This may be because the phenolic resin molecules undergo thermal cracking under high temperature conditions, and the generated free radicals and ions further recombine, resulting in the formation of various complex chemical products. Due to the ability of oxygen to act as an oxidant, it further promotes the occurrence of some oxidation reactions, such as partial oxidation, epoxidation, etc., resulting in the formation of various ethers and aerobic compounds. The decomposition and oxidation reactions of organic matter occur simultaneously, forming various ethers and oxygen-containing compounds.
Benzene and other aromatic compounds such as 1,2,3,5-tetramethylbenzene have potential toxicity to the human body, which may cause respiratory irritation and health risk of cancer. Esters such as dimethyl succinate, dimethyl glutarate, and ether compounds such as ethylene oxide may cause skin and eye irritation, as well as respiratory problems. In response to such issues, the casting industry should improve ventilation facilities in the workplace, and the exhaust gas should be purified before entering the atmosphere, by using activated carbon or other adsorbents, to reduce the harm to the environment and human health.
Conclusion
Resin pyrolysis can affect the types and properties of products under different atmospheric conditions. Under anaerobic conditions, resin molecules mainly undergo cracking reactions, generating benzene and ester products, represented by dimethyl succinate and 1,2,3,5-tetramethylbenzene. The generation of benzene pollutants is due to the substitution reaction between compounds containing benzene rings and low molecular weight organic compounds during pyrolysis. The generation of ester products may be related to the rearrangement reaction of intermediates during pyrolysis, or may originate from the pyrolysis of other additives in the resin. Taking phenolic resin as an example, under high temperature and anaerobic conditions, phenolic resin first undergoes cracking reaction to generate C6H5·, CH3, oxyradical, etc. (R1), C6H5· undergoes substitution reaction with CH3· to generate 1,2,3,5-tetramethylbenzene (R2), and CH3· undergoes rearrangement reaction with oxygen-containing radicals to generate dimethyl succinate (R3).
Under oxygen-containing conditions, oxygen in the air can accelerate the decomposition or combustion of organic matter in the sample. The products are mainly ether, benzene, and hydrocarbon, with the key products being ethylene oxide and 2-methylbutane, which undergo epoxidation, rearrangement, and substitution reactions(R4, R5).
Moreover, the introduction of oxygen promotes partial oxidation of intermediate products and increases the production of alcohols, aldehydes, and ketones.
The pyrolysis temperature range for different samples varies. Under anaerobic conditions, the main pyrolysis temperature ranges for cured sand cores, coated phenolic resins, and isocyanates are 200–600 ℃, 600–1000 ℃, 200–400 ℃ and 400–600 ℃. All four sample products include dimethyl succinate and dimethyl glutarate, generated in the temperature range of 200–600 ℃. Under aerobic conditions, the main pyrolysis temperature ranges are 30–600 ℃, 400–600 ℃, 200–800 ℃, and 200–800 ℃. All four sample products include ethylene oxide and 2-methylbutane as the main pollutants, which are generated above 200 ℃.
The volatile organic compounds (VOCs) in casting sand pose a serious threat to human health and the environment. The study of pollutants generated by the pyrolysis of resins and their binders is of great significance for environmental protection. By targeted degradation of VOCs in molding sand, not only can the design of molding sand be optimized and production efficiency improved, but also the environment can be effectively protected, promoting the sustainable development of the casting industry.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Lee, J., Lee, C., Kwag, J., Buglass, A. & Lee, G. Determination of optimum conditions for the analysis of volatile components in pine needles by double-shot pyrolysis–gas chromatography–mass spectrometry. J. Chromatogr. A 1089 (1), 227–234 (2005).
Wang, J., Jiang, H. & Jiang, N. Study on the pyrolysis of phenol-formaldehyde (PF) resin and modified PF resin. Thermochim. Acta 496 (1), 136–142 (2009).
Zhang, H. et al. Diminishing hazardous air pollutant emissions from pyrolysis of furan no-bake binders using methanesulfonic acid as the binder catalyst. J. Therm. Anal. Calorim. 116 (1), 373–381 (2014).
Dungan, R. S. & Reeves Iii, J. B. Pyrolysis of foundry sand resins: a determination of organic products by mass spectrometry. J. Environ. Sci. Health Part. A 40 (8), 1557–1567 (2005).
Effendi, A., Gerhauser, H. & Bridgwater, A. V. Production of renewable phenolic resins by thermochemical conversion of biomass: a review. Renew. Sustain. Energy Rev. 12 (8), 2092–2116 (2008).
Kim, S. W. & Lee, G. H. Analysis of t-butylphenol acetylene condensed resin with methyl-methine linkages in vulcanized rubber by pyrolysis-gas chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 13 (18), 1855–1860 (1999).
Obzina, T. et al. Researchers from VSB-technical university of ostrava report recent findings in metals research (technological and quality aspects of the use of innovative inorganic binders in the production of castings metals). (2021).
Wang, Y., Cannon, F. S. & Li, X. Comparative Analysis of hazardous air pollutant emissions of casting materials measured in analytical pyrolysis and conventional metal pouring emission tests. Environ. Sci. Technol. 45 (19), 8529–8535 (2011).
Jiang, H. et al. The pyrolysis mechanism of phenol formaldehyde resin. Polym. Degrad. Stab. 97 (8), 1527–1533 (2012).
Xing, X. et al. Pyrolysis mechanism of phenylboronic acid modified phenolic resin. Polym. Degrad. Stab. 191, 109672 (2021).
Pienihäkkinen, E. et al. Production of pyrolytic lignin for the phenolic resin synthesis via fast pyrolysis. J. Anal. Appl. Pyrol. 176, 106239 (2023).
Ren, X., Cai, H., Du, H. & Chang, J. The preparation and characterization of pyrolysis bio-oil-resorcinol-aldehyde resin cold-set adhesives for wood construction polymers. (2017).
Wu, D., Ding, G., Chi, W. & Jiang, L. Research on the pyrolysis kinetics of resin powder on waste printed circuit board with different particle sizes at different heating rates: inspiration for the pyrolysis mechanism. J. Therm. Anal. Calorim. 147 (14), 8047–8059 (2022).
Ma, C., Sánchez-Rodríguez, D. & Kamo, T. A comprehensive study on the oxidative pyrolysis of epoxy resin from fiber/epoxy composites: product characteristics and kinetics. J. Hazard. Mater. 412, 125329 (2021).
Kmita, A., Benko, A., Roczniak, A. & Holtzer, M. Evaluation of pyrolysis and combustion products from foundry binders: potential hazards in metal casting. J. Therm. Anal. Calorim. 140 (5), 2347–2356 (2020).
Holtzer, M., Dańko, R., Żymankowska-Kumon, S., Bobrowski, A. & Kubecki, M. Assessment of the harmfulness of moulding sands with alkyd resin subjected to the high temperature influence. Arch. Metall. Mater. 61 (4), 2171–2176 (2016).
Liang, B. et al. TG-MS-FTIR study on pyrolysis behavior of phthalonitrile resin. Polym. Degrad. Stab. 169, 108954 (2019).
Gao, R., Liu, Y. & Xu, Z. Synthesis of oil-based resin using pyrolysis oil produced by debromination pyrolysis of waste printed circuit boards. J. Clean. Prod. 203, 645–654 (2018).
Juang, R. S. & Lee, T. S. Oxidative pyrolysis of organic ion exchange resins in the presence of metal oxide catalysts. J. Hazard. Mater. 92 (3), 301–314 (2002).
Zheng, F. et al. Elucidating multiple-scale reaction behaviors of phenolic resin pyrolysis via TG-FTIR and ReaxFF molecular dynamics simulations. J. Anal. Appl. Pyrol. 157, 105222 (2021).
Chen, S., Zhang, J., Xu, K. & Xu, Q. Thermal decomposition behaviour of foundry sand for cast steel in nitrogen and air atmospheres. Math. Prob. Eng., 2020, 8121276. (2020).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 12375253), the Key R&D project in Hangzhou city (Grant No. 2024SZD1B26), the Science Foundation of Zhejiang Sci-Tech University (Grant Nos. 19022108-Y, 21022310-Y and 21022092-Y), and The State Environmental Protection Key Laboratory of Odor Pollution Control (Grant No. 20230801).
Author information
Authors and Affiliations
Contributions
X. Zhu and X. Zhang guided the research work. Y. Liu prepared tables and figures. L.Zhang and Y. Lu did laboratory experiments. D. Wang and K. Li wrote the main manuscript text. J. Han revised the manuscript and prepared submission materials. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, D., Liu, Y., Zhang, L. et al. Thermal decomposition behavior of casting raw materials under different atmospheres and temperatures. Sci Rep 15, 5992 (2025). https://doi.org/10.1038/s41598-025-90297-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-90297-5