Abstract
Left ventricular hypertrophy (LVH) is a major cardiac complication of hypertension. The ratio of triglycerides (TG) to high-density lipoprotein cholesterol (HDL-C) (TG/HDL-C) has been identified as a biomarker of insulin resistance and a predictor of atherosclerosis. We aimed to investigate the relationship between TG/HDL-C and LVH in hypertensive patients among the Han Chinese. Our community-based cross-sectional study recruited 4552 patients with hypertension. LVH was diagnosed by transthoracic echocardiography in these patients based on a criterion of left ventricular mass index (LVMI) over 49.2 g/m2.7 in men and 46.7 g/m2.7 in women. The independent association between the TG/HDL-C ratio quartiles and LVH prevalence was analyzed by logistic regression models. The ratio of TG/HDL-C was higher (1.27 ± 1.26, 1.15 ± 1.07, P = 0.001) in LVH patients. The prevalence of LVH with the first quartile of TG/HDL-C (Q1: < 0.61), second (Q2: 0.61 ~ 0.91), the third (Q3: 0.92 ~ 1.41), and the highest quartile (Q4: >1.41) was 36.1%, 42%, 42.6%, 44.9%, respectively. Logistic regression analysis suggested that the ratio of TG/HDL-C was independently correlated with the risk of LVH with adjustment of confounding factors. The correlation was more significant in female patients rather than males. Compared to the first quartile of TG/HDL-C (Q1), the odds ratios (ORs) and their 95% confidence intervals (CIs) for LVH in the increasing quartiles (Q2-Q4) were 1.21(1.01–1.45), 1.28(1.07–1.54), and 1.48(1.23–1.78), respectively. Similar results were also observed in the subjects younger than 60 years with coronary heart disease (CAD). The ratio of TG/HDL-C may be an independent risk factor of LVH in Han Chinese with hypertension.
Similar content being viewed by others
Introduction
LVH is a severe cardiac disease characterized with ventricular wall thickness and remodeling1,2 that further lead to heart failure3. LVH often occurs in patients with hypertension associated with hyperlipidemia and coronary atherosclerosis. Thus, adequate clinical cares in hyperlipidemia would reduce the risk of cardiac morbidity and mortality with hypertension4.
Indeed, coronary heart disease is often associated with increased plasma TG levels and decreased plasma HDL-C levels5,6. The ratio of TG/HDL-C can be used as an independent predictor of CVD risk7,8,9 and is considered as a simple marker of atherosclerotic dyslipidemia and insulin resistance10,11,12,13,14,15. Insulin resistance is an important pathway leading to LVH16,17,18. However, the relationship between the ratio of TG/HDL-C and LVH in patients with hypertension has yet to be elucidated.
We speculated that the ratio of TG/HDL-C could be used as an effective indicator for the early identification of LVH risk in patients with hypertension. We therefore conducted a cross-sectional study to define the relationship between TG/HDL-C and LVH and evaluate whether other related factors impact this relationship.
Methods
Subjects
The present protocol has been reviewed and approved by the ethical committees of the Fuwai Hospital and local hospitals. The research procedures obeyed the ethically normative criteria. All the subjects have provided written informed consent and all investigators have been trained at the Cardiovascular Institute, Chinese Academy of Medical Sciences (Beijing, China) before the experiments. Throughout the research process, the researchers adhered strictly to relevant guidelines and regulations.
A cross-sectional study was conducted in rural Han Chinese residents aged 40–75 at Xinyang County, central China, from 2004 to 2005 with the utilization of a multi-stage cluster sampling method19,20,21. Total 13,444 subjects including 5270 males and 8174 females completed the survey, with a response rate of 84.9%.
5421 of the subjects have hypertension defined as diastolic blood pressure ≥ 90 mmHg (DBP) and systolic blood pressure ≥ 140 mmHg (SBP). Blood pressure was measured by trained professionals using a standard mercury sphygmomanometer. All participants were advised not to smoke, drink coffee/tea or alcohol before the measurements of blood pressure. Participants had three readings of sitting posture with 30 s intervals in between. LVM was measured by echocardiography in 4805 patients. Due to lack of blood tests, 253 subjects were excluded. Thus, eventually, total 4552 patients with complete clinical, biochemical, and echocardiographic data were recruited for the current study.
Echocardiography measurements
Transthoracic echocardiography was performed by two echocardiographers who have been trained with the echocardiographic protocol at the Cardiovascular Institute, Chinese Academy of Medical Sciences1. M-mode, 2-dimensional (2D) and color Doppler recordings from the parasternal long-axis and short-axis windows as well as 2D and color Doppler evaluations from the apical window to yield 2-, 3- and 4-chamber images with an HP 5500 (Phillips Medical System, Boston, MA, USA) or an HDI 3000 (A TL, Bothell, W A, USA). We also measured end-systolic diameter, end-diastolic diameter, septal wall thickness, and posterior wall thickness at the end of the diastole. According to the American Society of Echocardiography recommendations, LV internal dimensions and septal and posterior wall thicknesses were measured in up to three cardiac cycles at end-diastole and end-systole. A cardiologist read the images without knowing the subjects’ clinical data.
LVM was calculated by using the following equation:
\(0.{\text{8}} \times {\text{1}}0.0{\text{4}}[{({\text{IVS}}\,+\,{\text{LVEDD}}\,+\,{\text{PW}})^{\text{3}}} - {\text{LVED}}{{\text{D}}^{\text{3}}}] \times 0.{\text{6}}\)
IVS is the interventricular septum, PW is the posterior wall, and LVEDD is left ventricular end-diastolic diameter. LVM was divided by height2.7to obtain LVMI (LVMIh2.7). LVH was diagnosed with the criteria of LVM ≥ 49.2gm− 2.7 in males and 46.7 gm− 2.7in females.
Covariate measurements and definitions
Covariates including gender, age, and lifestyle were collected through a face-to-face interview by trained nurses. In the morning, blood samples were collected from the anterior cubital vein in all the patients following ~ 12 h fasting. Fasting plasma glucose (FPG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), HDL-C, TG, electrolytes, and renal function indicators were quantified and analyzed with an autoanalyzer.
Statistical analysis
SPSS (Statistical Package for the Social Sciences) software version 26 (SPSS Inc., Chicago, IL, USA) was used for data management and statistical analysis. The total experimental population was divided into four groups according to the baseline TG/HDL-C ratio (Q1: < 0.61, Q2: 0.61 ~ 0.91, Q3: 0.92 ~ 1.41, Q4: >1.41), and the parameters were compared among these four groups. Data are reported as mean ± standard deviation for continuous variables and as percentages for categorical variables. All participants were stratified by quartiles of TG/HDL-C, baseline differences in clinical variables and echocardiographic data among the groups using analysis of variance (ANOVA) for continuous variables, and Chi-squared test for categorical variables. The logistic regression analysis was used to calculate ORs and their 95% CIs. Using binary logistic regression model and multivariate analysis to evaluate the relationship between TG/HDL-C categories and LVH. Sequential models were developed. Model 1 was no adjusted variable. In Model 2, the analysis was adjusted for age, sex. In addition to the confounders analyzed in Model 2, Model 3 included the admission of smoking, drinking, SBP, DBP, waist-to-hip ratio (WHR), stroke. Model 4 added TC, LDL-C, serum uric acid (SUA), FPG, serum creatinine (SCr), blood urea nitrogen (BUN), alanine aminotransferase (ALT), aspartate aminotransferase (AST) based on Model 3. Further stratified analysis was performed by gender and age groups to obtain OR and 95% CI for LVH and TG/HDL-C in each subgroup. The differences were considered significant if a 2-tailed P value is less than 0.05.
Results
Clinical, biochemical characteristics and echocardiography data of patients by the TG/HDL-C ratio
Clinical and biochemical characteristics, and echocardiographic parameters in all participants were summarized in Table 1 based on quartiles of the TG/HDL-C ratio. All the parameters including DBP, TG, TC, LDL-C, FPG, SUA, SCr, ALT, WHR, stroke and CAD were significantly increased in parallel with the increase of TG/HDL-C from the first to the fourth quartile (Q1-Q4) (all P for trend < 0.05). On the other hand, there was an inverse association between HDL-C and TG/HDL-C ratio. In echocardiographic parameters, IVST, LVM, and LVM/H2.7 parameters were significantly increased with TG/HDL-C from Q1-Q4 (all P for trend < 0.001). Compared with patients with the lowest quartile of TG/HDL-C, the prevalence of LVH was higher for patients with higher quartile of the TG/HDL-C ratio. The prevalence of LVH in patients among those groups was 36.1%、42%、42.6%、44.9%, respectively.
Association between TG/HDL-C and LVH
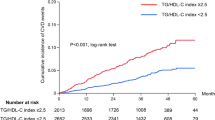
Logistic regression analysis was performed to determine the independent association between TG/HDL-C and the risk of LVH. As shown in Table 2, quartiles of TG/HDL-C were associated with increased risk of LVH in univariate analysis (model 1). After adjustment for age and sex (model 2) and further for smoking, drinking, SBP, DBP, WHR, and stroke (model 3), the ORs for LVH remained progressively increased across quartiles of TG/HDL-C. Finally, TG/HDL-C was still independently associated with the risk of LVH after further controlling for TC, LDL-C, SUA, FPG, SCr, BUN, ALT, AST (mode 4). Compared with the first quartile (Q1), the ORs (95%CI) for LVH in the increasing quartiles of TG/HDL-C (Q2-Q4) were 1.21(1.01–1.45), 1.28(1.07–1.54), and 1.48(1.23–1.78), respectively, and we found that LVH risk increased with higher quartiles of TG/HDL-C in all models (P for trend < 0.001).
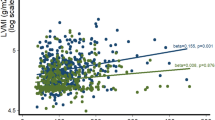
We further performed a stratified analysis of the correlation between LVH, and TG/HDL-C categories in Fig. 1. The relationship between TG/HDL-C and LVH risk was significantly different by sex, and this association was significantly higher in women compared to men. After adjustment of several covariates, women still had a strong association. In the group younger than 60 years, the highest quartile was significantly associated with LVH. This association with LVH was not significant in those patients older than 60 years. As the ratio of TG/HDL-C is related to the development of atherosclerosis, the relationship between TG/HDL-C and LVH was respectively detected in CAD and non-CAD groups. In the non-CAD group, there was no significant correlation between TG/HDL-C and LVH. However, the third and highest quartile of TG/HDL-C was significantly associated with LVH in the CAD group. In addition, we performed an interaction analysis between the TG/HDL-C categories and each stratification factor, and found that there was no interaction between them.
Discussion
We performed a cross-sectional study which analyzed the association between TG/HDL-C and LVH risk in hypertensive patients. We found that the prevalence of LVH was significantly increased accompanied with the increase of TG/HDL-C and that there was a significant independent positive association between LVH and TG/HDL-C, regardless of adjustments of potential confounders. Thus, the ratio of TG/HDL-C can be used to further identify patients who have high risk of LVH in hypertensive populations.
LVH refers to cardiac enlargement characterized with hypertrophy of cardiomyocytes and proliferation of fibrous connective tissue. LVH is one of the most common cardiac damages associated with hypertension and it increases the risk of heart failure22,23,24. However, so far, the pathogenesis of LVH is not entirely understood and it is probably related to age, sex, weight, race, genetic predisposition, metabolic status (such as insulin resistance) and hypertension etc. Leptin also increases the activity of extracellular matrix metalloproteinases and other enzymes which may induce interstitial fibrosis and participate in inflammatory response during the development of LVH25.
Lipid dysfunction accelerates atherosclerosis, resulting in decreased compliance and dilation of greater arteries. The pathological procedure leads to a stress on the left ventricular wall during the systolic period and prolongs the left ventricular ejection time, further contributing to the development of LVH. TG is involved in the synthesis of cholesterol and cholesterol esters and thus it is the major lipid component leading to atherosclerosis. Triglycerides appear to be causative of myocardial remodeling by increasing LV mass, suggesting that it influence the development of cardiovascular disease not only by atherosclerosis but also by causing adverse alterations in cardiac structure and function26. Recent studies have shown that high TG levels can lead to increased oxidative stress and inflammation, which in turn can contribute to LVH26,27. HDL-C is an anti-atherosclerotic lipoprotein that is negatively correlated with the risk of cardiovascular and cerebrovascular diseases. Previous studies indicated that the levels of serum HDL-C are usually lower in patients with hypertriglyceridemia. The low levels of serum HDL-C are probably due to the unstable structure of HDL-C particles rich in TG. Thus, compared to other lipid components, the ratio of TG/HDL-C could better reflect the comprehensive level of lipid metabolism in patients28. Recently, a study in Mexico also confirmed that the TG/HDL-C ratio is a better predictor of the prevalence of obesity and lipid triad in the Mexican population29.
The ratio of TG/HDL-C is associated with concentrations of high-risk particles that predict risk of coronary heart disease30,31. A high ratio of TG/HDL-C is also used to recognize concentric LVH and the elevated levels of TG/HDL-C are tightly associated with increased risk of concentric LVH32,33. In our present study, we observed a significant independent positive association between TG/HDL-C and LVH in Chinese Han patients with hypertension and LVH, suggesting that the high ratio of TG/HDL-C contributes to development of LVH in Chinese hypertensive patients. Following adjustments in all potential confounders, the group in the quartile with the highest TG/HDL-C levels is nearly1.5time higher chance to develop LVH than those with the lowest TG/HDLC levels. The prevalence rates of LVH were also progressively increased across the quartiles of TG/HDL-C (36.1, 42, 42.6 and 44.9%, respectively). Previous studies have shown a prevalence of 36–41% of echocardiographic LVH in all hypertensive participants, which is basically consistent with our finding34.
We next conducted a stratified analysis to explore whether the association between TG/HDL-C and LVH could be influenced by gender. In fact, the association between TG/HDL-C ratio and LVH was more profound in women rather than men. A similar finding has been also reported in the associations between TG/HDL-C and nonalcoholic fatty liver disease, chronic kidney disease, and the risk of diabetes35,36,37. The underlying mechanisms are currently unknown but one possible explanation could be the effects of sex hormones. Our current findings provide new insights into the pathophysiology of LVH in male and female patients and provide new impetus for further research. In addition, we stratified our data by age and found that the correlation between TG/HDL-C ratio and LVH in younger patients (< 60 years) seems more significant compared to older patients (> 60 years).
Although our present study recruited a large sample size of hypertensive patients, which effectively minimizes selection bias, there are still some limitations. The cross-sectional study did not carry out prospective follow-up and intervention studies. High TG/HDL-C was independently correlated with LVH, suggesting a possible mechanism of dyslipidemia contributing to the occurrence and development of LVH. However, the causal relationship between TG/HDL-C and LVH could not be concluded eventually. The proportion of male subjects in our present study was relatively low (33.3%). Thus, the impacts of gender on the correlation between TG/HDL-C and LVH need to be further clarified by expanding the proportion of male subjects.
Conclusions
Our study suggests that the ratio of TG/HDL-C is independently correlated with LVH, especially in females, suggesting that the ratio of TG/HDL-C could be used to identify patients at a higher risk of LVH in hypertensive populations.
Data availability
The research data used to support the finding of this study are available from the corresponding authors upon request.
Abbreviations
- LVH:
-
Left ventricular hypertrophy
- TG/HDL-C:
-
triglycerides to-high-density lipoprotein cholesterol
- LVM:
-
Left ventricular mass
- LVMI:
-
left ventricular mass indexed
- TG:
-
triglyceride
- HDL-C:
-
high-density lipoprotein cholesterol
- CVD:
-
cardiovascular disease
- CAD:
-
Coronary heart disease
- IVS:
-
Interventricular septum
- PW:
-
Posterior wall
- LVEDD:
-
Left ventricular end-diastolic diameter
- IVST:
-
Interventricular septal thickness
- PWT:
-
Posterior wall thickness
- RWT:
-
Relative wall thickness
- UCG:
-
Ultrasonic cardiogram
- FPG:
-
Fasting plasma glucose
- TC:
-
Total cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- SUA:
-
Sserum uric acid
- SCr:
-
Serum creatinine
- BUN:
-
Blood urea nitrogen
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- WHR:
-
Waist-to-Hip Ratio
References
Wang, S. X. et al. Prevalence and risk factors for left ventricular hypertrophy and left ventricular geometric abnormality in the patients with hypertension among Han Chinese. Chin. Med. J. (Engl). 125 (1), 21–26 (2012).
deFilippi, C. R. & Seliger, S. Malignant left ventricular hypertrophy and epidemiology 101. J. Am. Coll. Cardiol. 80 (16), 1526–1528 (2022).
Camici, P. G., Tschöpe, C., Di Carli, M. F., Rimoldi, O. & Van Linthout, S. Coronary microvascular dysfunction in hypertrophy and heart failure. Cardiovasc. Res. 116 (4), 806–816 (2020).
Gottdiener, J. S. & Kop, W. J. Body and heart: effects of Weight Gain and loss on left ventricular size and function. Circ. Cardiovasc. Imaging. 10 (3), e006084 (2017).
Jin, J. L. et al. Triglyceride glucose index for predicting cardiovascular outcomes in patients with coronary artery disease. J. Thorac. Dis. 10 (11), 6137–6146 (2018).
Abbasi, F., Kohli, P., Reaven, G. M. & Knowles, J. W. Hypertriglyceridemia: A simple approach to identify insulin resistance and enhanced cardio-metabolic risk in patients with prediabetes. Diabetes Res. Clin. Pract. 120, 156–161 (2016).
McLaughlin, T. et al. Is there a simple way to identify insulin-resistant individuals at increased risk of cardiovascular disease? Am. J. Cardiol. 96 (3), 399–404 (2005).
Sonmez, A. et al. The role of plasma triglyceride/high-density lipoprotein cholesterol ratio to predict cardiovascular outcomes in chronic kidney disease. Lipids Health Dis. 14, 29 (2015).
Kosmas, C. E. et al. The Triglyceride/High-Density Lipoprotein Cholesterol (TG/HDL-C) ratio as a risk marker for metabolic syndrome and Cardiovascular Disease. Diagnostics (Basel) 13(5). (2023).
Kim, J. S., Kang, H. T., Shim, J. Y. & Lee, H. R. The association between the triglyceride to high-density lipoprotein cholesterol ratio with insulin resistance (HOMA-IR) in the general Korean population: based on the National Health and Nutrition Examination Survey in 2007–2009. Diabetes Res. Clin. Pract. 97 (1), 132–138 (2012).
Kim-Dorner, S. J., Deuster, P. A., Zeno, S. A., Remaley, A. T. & Poth, M. Should triglycerides and the triglycerides to high-density lipoprotein cholesterol ratio be used as surrogates for insulin resistance? Metabolism 59 (2), 299–304 (2010).
Zhou, M. et al. The triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio as a predictor of insulin resistance but not of β cell function in a Chinese population with different glucose tolerance status. Lipids Health Dis. 15, 104 (2016).
Dobiásová, M. & Frohlich, J. The plasma parameter log (TG/HDL-C) as an atherogenic index: correlation with lipoprotein particle size and esterification rate in apob-lipoprotein-depleted plasma (FER(HDL)). Clin. Biochem. 34 (7), 583–588 (2001).
Lin, D. et al. Associations of lipid parameters with insulin resistance and diabetes: A population-based study. Clin. Nutr. 37 (4), 1423–1429 (2018).
Liu, H. et al. Triglyceride to High-Density Lipoprotein Cholesterol (TG/HDL-C) ratio, a simple but effective Indicator in Predicting Type 2 diabetes Mellitus in older adults. Front. Endocrinol. (Lausanne). 13, 828581 (2022).
Anan, F. et al. High-density lipoprotein cholesterol and insulin resistance are independent and additive markers of left ventricular hypertrophy in essential hypertension. Hypertens. Res. 30 (2), 125–131 (2007).
Horio, T., Miyazato, J., Kamide, K., Takiuchi, S. & Kawano, Y. Influence of low high-density lipoprotein cholesterol on left ventricular hypertrophy and diastolic function in essential hypertension. Am. J. Hypertens. 16 (11 Pt 1), 938–944 (2003).
Schillaci, G. et al. High-density lipoprotein cholesterol and left ventricular hypertrophy in essential hypertension. J. Hypertens. 19 (12), 2265–2270 (2001).
Wang, S. et al. Left ventricular hypertrophy, abnormal ventricular geometry and relative wall thickness are associated with increased risk of stroke in hypertensive patients among the Han Chinese. Hypertens. Res. 37 (9), 870–874 (2014).
Cai, S. et al. Relationship of a new anthropometric index with left ventricular hypertrophy in hypertensive patients among the Han Chinese. BMC Cardiovasc. Disord. 22 (1), 16 (2022).
Cai, S. et al. The relationship between the weight-adjusted-waist index and left ventricular hypertrophy in Chinese hypertension adults. Hypertens. Res. 46 (1), 253–260 (2023).
Maimaitiaili, R. et al. Relationship between vascular aging and left ventricular concentric geometry in Community-Dwelling Elderly: The Northern Shanghai Study. Clin. Interv Aging. 15, 853–863 (2020).
Yildiz, M. et al. Left ventricular hypertrophy and hypertension. Prog Cardiovasc. Dis. 63 (1), 10–21 (2020).
Đorđević, D. B., Koračević, G. P., Đorđević, A. D. & Lović, D. B. Hypertension and left ventricular hypertrophy. J. Hypertens. 42 (9), 1505–1515 (2024).
Alpert, M. A., Karthikeyan, K., Abdullah, O. & Ghadban, R. Obesity and cardiac remodeling in adults: Mechanisms and clinical implications. Prog Cardiovasc. Dis. 61 (2), 114–123 (2018).
Aung, N. et al. The effect of blood lipids on the left ventricle: A mendelian randomization study. J. Am. Coll. Cardiol. 76 (21), 2477–2488 (2020).
Nishida, K. & Otsu, K. Inflammation and metabolic cardiomyopathy. Cardiovasc. Res. 113 (4), 389–398 (2017).
Wakabayashi, I. & Daimon, T. Comparison of discrimination for cardio-metabolic risk by different cut-off values of the ratio of triglycerides to HDL cholesterol. Lipids Health Dis. 18 (1), 156 (2019).
Martínez-Marroquín, Y. et al. The TG/HDL-c Lipid Ratio as a Cardiovascular Risk Marker in a Mexican Urban Middle-Class Population: Do We Need a Risk Score Tailored for Mexicans? J Clin Med 12(18). (2023).
Che, B. et al. Triglyceride-glucose index and triglyceride to high-density lipoprotein cholesterol ratio as potential cardiovascular disease risk factors: An analysis of UK biobank data. Cardiovasc. Diabetol. 22 (1), 34 (2023).
Yang, T. et al. Correlation between the triglyceride-to-high-density lipoprotein cholesterol ratio and other unconventional lipid parameters with the risk of prediabetes and type 2 diabetes in patients with coronary heart disease: A RCSCD-TCM study in China. Cardiovasc. Diabetol. 21 (1), 93 (2022).
Wang, H. et al. The impact of nontraditional lipid profiles on left ventricular geometric abnormalities in general Chinese population. BMC Cardiovasc. Disord. 18 (1), 88 (2018).
Di Bonito, P. et al. Comparison of non-HDL-cholesterol versus triglycerides-to-HDL-cholesterol ratio in relation to cardiometabolic risk factors and preclinical organ damage in overweight/obese children: The CARITALY study. Nutr. Metab. Cardiovasc. Dis. 25 (5), 489–494 (2015).
Cuspidi, C., Sala, C., Negri, F., Mancia, G. & Morganti, A. Prevalence of left-ventricular hypertrophy in hypertension: An updated review of echocardiographic studies. J. Hum. Hypertens. 26 (6), 343–349 (2012).
Fukuda, Y. et al. Triglycerides to high-density lipoprotein cholesterol ratio is an independent predictor of incident fatty liver; a population-based cohort study. Liver Int. 36 (5), 713–720 (2016).
Ho, C. I. et al. Relationship between TG/HDL-C ratio and metabolic syndrome risk factors with chronic kidney disease in healthy adult population. Clin. Nutr. 34 (5), 874–880 (2015).
Wang, Y. L., Koh, W. P., Talaei, M., Yuan, J. M. & Pan, A. Association between the ratio of triglyceride to high-density lipoprotein cholesterol and incident type 2 diabetes in Singapore Chinese men and women. J. Diabetes. 9 (7), 689–698 (2017).
Acknowledgements
This study was supported by the “National Key R&D Program of China” (Funding No.2020YFC2008900), Project 166 Special Project (233-CXCY-N101-07-18-01).
Author information
Authors and Affiliations
Contributions
P.Z., S.W., R.C. and J.H. designed the research. J.H., S.C., W.Z., B.C., J.S., B.Q., Q.B. and T.H. collected the data. J.H. and S.C. wrote original draft. W.Z., B.C., J.S., B.Q., Q.B. and T.H. help optimize the research and proofread the paper. R.C. and W.Z. supplements relevant information and provide guidance. All authors critically reviewed this draft. All authors approved the final draft for submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hu, J., Cai, S., Wan, Z. et al. The relationship between the ratio of triglyceride to high-density lipoprotein cholesterol and left ventricular hypertrophy in Chinese hypertension adults. Sci Rep 15, 14252 (2025). https://doi.org/10.1038/s41598-025-90332-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-90332-5