Abstract
Individual differences have been observed in side effects after vaccination for COVID-19, and host genetic factors have been suggested as a contributing factor. Here, we conducted a genome-wide association study (GWAS) involving 2,554 Japanese corporate employees who received a third booster dose of BNT162b2/Pfizer or mRNA-1273/Moderna vaccine. Although no genome-wide significant association was found for the presence of adverse symptoms, the GWAS for severity revealed six associated loci. The most significant association was observed between the severity of swelling of lymph nodes and chromosome 2q12 locus, including the IL1RL1, IL18R1, and IL18RAP genes (lead variant: rs76152249; P = 1.46 × 10−9). Pathway analysis suggested associations between immune pathways related to the MHC locus, including HLA genes, and the occurrence and severity of fever, and the NF-κB binding pathway and those of itching at the injection site. In addition, a meta-analysis of previous GWAS studies for the primary first or second dose of COVID-19 vaccine revealed 818 variants from 72 loci that demonstrated genome-wide significant associations with any of 12 symptoms, and pathway analysis identified immune pathways related to the MHC locus, suggesting shared genetic risks among primary and booster vaccinations. These results may help control side effects following COVID-19 vaccination.
Similar content being viewed by others
Introduction
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by the novel coronavirus, severe acute respiratory syndrome coronavirus 2. Following a report by the World Health Organization in January 2020, COVID-19 was declared a global pandemic in March 20201.
The primary symptoms of COVID-19 include fever, fatigue, and dry cough, which can progress to severe respiratory distress requiring treatment with a ventilator. In response to the spread of COVID-19, global vaccination efforts were undertaken. In particular, mRNA-based vaccines, including the BNT162b2 vaccine developed by Pfizer and the mRNA-1273 vaccine developed by Moderna, have been widely used because of their rapid development and high efficacy in preventing infection. These vaccinations induce high levels of spike-specific antibodies and have been shown to be 90% effective in preventing subsequent disease2. However, antibody titers are reported to decline markedly in the first few months after vaccination, with efficacy dropping to 60% at 6 months after vaccination2,3. Considering the issue of antibody persistence, multiple vaccinations have been considered effective and booster injections have been administered. After booster vaccination, serum antibody levels rise again and plateau at a higher baseline than before4. On the other hand, COVID-19 vaccines can cause rare severe adverse symptoms or relatively frequent mild adverse symptoms. The frequency of adverse reactions between the primary first or second dose and booster vaccinations has been reported to be comparable5,6,7,8,9,10. These side effects include both local symptoms centered around the injection site and systemic symptoms. The occurrence of these common side effects varies widely among individuals, with a higher incidence among young individuals and females11,12,13.
However, individual differences in the occurrence of side effects that cannot be explained solely by demographic factors, such as age, sex, ethnicity, and medical history suggest the involvement of genetic factors14. According to government reports, the second dose vaccination rate in Japan is 79.5%, with a notably high rate of 92.8% among those aged ≥ 65 years. Although the COVID-19 pandemic is on the decline, regular booster vaccinations are still considered important for older adults and other groups that are particularly susceptible to infection. Therefore, identifying genetic high-risk groups for adverse vaccine reactions is particularly important for the ongoing administration of booster vaccinations.
Genome-wide association studies (GWASs) are highly efficient for comprehensive searches of the genetic factors associated with relatively frequent traits such as vaccine side effects. Several GWASs conducted on side effects after the first and second vaccination doses have reported associations with human leukocyte antigen (HLA) genes13,15,16. The association of the HLA-A*03:01 allele has been reported in European GWAS reports, but HLA-A*03:01 is rare (< 1%) in the Japanese population. A GWAS involving Japanese subjects suggested the involvement of HLA-A*11:01 and an allele of the HLA Class II gene, HLA-DQA1*03:01, indicating that HLA is an important factor affecting susceptibility to vaccination side effects13. However, all of these previous studies focused on side effects up to the second dose, and to our knowledge, no GWAS has been conducted on side effects after booster vaccinations.
Given this background, in the present study, to explore the genetic factors associated with the occurrence or severity of these side effects in a cohort of corporate employees in Japan, we conducted an online survey of side effects experienced after a third booster vaccination and a GWAS.
Materials and methods
Study subjects and ethical statement
Toshiba has established a health-care database focusing on “Research aimed at realizing precision medicine that can select optimal preventive and treatment methods”. This database includes genomic, health checkup, questionnaire, and prescription data of their employees who have individually informed consented. A survey on side effects following COVID-19 booster vaccination was conducted using an online questionnaire system for participants who gave their informed consent based on an explanatory document titled “Basic research on emerging and reemerging infectious diseases and research and development to contribute to improving the efficacy and safety of vaccination”. The protocol for this study was approved by the Ethics Committees of Toshiba (IRB No. AN-2021-08) and the National Center for Global Health and Medicine (approval No. 150), and all experiments were performed in accordance with relevant guidelines and regulations.
Collection of vaccination side effects data via a self-report questionnaire
A questionnaire on side effects experienced after booster vaccination was based on a previous study17 and modified for online use (Supplementary data). Data on sex, age, type of vaccine received, and local and systemic adverse reactions (including the severity of adverse reactions) were collected from each participant. Participants who received a booster dose of BNT162b2 (Pfizer-BioNTech) or mRNA-1273 (Moderna) according to standard protocols were included in this study. The questionnaire included items on common local symptoms at the injection site (redness/erythema, swelling, induration, pain, burning sensation, itching), lymph nodes (swelling of lymph nodes/lymphadenopathy), and systemic symptoms (fever, fatigue, headache, muscle pain/myalgia, nausea, and nasal discharge). Absence from work following booster vaccination was also surveyed. The severity of redness, swelling, and induration was assessed according to the following size (diameter) of the event: grade 1, 0–2.0 cm; grade 2, 2.1–5.0 cm; and grade 3, > 5.0 cm. The severity of injection site burning sensation and itching was classified into two categories: grade 1, side effects not requiring medication, and grade 2, side effects requiring medication. The severity of injection site pain was classified into three categories: grade 1, side effects not requiring medication; grade 2, requiring one dose of medication; and grade 3, requiring two or more doses of medication. Fever was graded into five levels based on self-reported body temperature: grade 1, 37.5–38.0 °C; grade 2, 38.1–38.5 °C; grade 3, 38.6–39.0 °C; grade 4, 39.1–39.5 °C; and grade 5, ≥ 39.6 °C. No systemic symptoms other than fever were graded. The duration of side effects was also evaluated, but some survey responses were excluded because of discrepancies between answers regarding the occurrence and duration of the event. Symptoms with at least 100 cases were included in our GWAS. The correlation coefficients of each symptom were analyzed using R Statistical software18 to examine correlations between age, sex, booster vaccine type, and each adverse symptom after booster vaccination.
Genotyping and quality control (QC) of samples and variants
A total of 2,751 participants were genotyped using the Affymetrix Axiom Japonica array NEO (Santa Clara, CA, USA)19 and 666,890 variants from human genome reference GRCh37 (hg19), including chromosomes X, Y, and MT. For QC, five samples that showed a mismatch with the inferred gender from the genomic data and self-reported gender, one that showed close relatedness with PI_HAT > 0.1875, 175 who did not respond to the online questionnaire, and 10 who answered an unknown or minor vaccine type for booster vaccination were excluded from the subsequent analysis. Six samples that deviated from the HapMap Asian cluster in principal component analysis (PCA) were also excluded (Supplementary Fig. S1a online). The remaining samples were clustered with the Hapmap Japanese samples and no outliers were observed in the remaining samples in the PCA (Supplementary Fig. S1bc online). Therefore, in total, 2,554 samples and 620,417 autosomal variants passed QC filtering with a minor allele frequency (MAF) > 0.01, missing rate < 0.03, and a Hardy–Weinberg equilibrium (HWE) test P-value > 1 × 10−6. All QC procedures and association analyses were performed using PLINK (version v1.90b4.8)20. We applied whole genome imputation to our filtered dataset using BEAGLE (version 5.2)21 and a phased reference panel of whole genome sequencing data composed of 419 in-house healthy controls and 2,504 public 1000 Genome project samples22,23. The 7,382,264 imputed autosomal variants satisfied the following QC conditions: quality score > 0.9, MAF > 0.01, missing rate < 0.03, and HWE test P-value > 1 × 10−6.
Statistical analysis
The associations between genotypes and the occurrence of each adverse event were examined using logistic regression analysis adjusted for age, sex, and the type of booster vaccine as covariates. The associations between genotypes and event severity were further examined using linear regression analysis for the categorical variable of symptom severity adjusted for age, sex, and the type of booster vaccine as covariates. Among the moderately associated variants with a P-value < 1 × 10−3 for the occurrence of each adverse event, overlap with previously reported variants was analyzed. In short, variants at the same position and alleles on GRCh37 (hg19) were extracted and a meta-analysis was performed using the meta-analysis option in PLINK with a random effect model. Variants with a P-value < 1 × 10−5 were considered to be suggestively associated in this study. For the multiple testing correction in GWAS, a threshold of P = 5 × 10−8 was set to declare significance at the single variant level, considering that the symptoms evaluated were partially correlated; thus, many association tests were not independent. For the multiple testing correction in the gene-set enrichment tests, the Benjamini–Hochberg false discovery rate was considered.
Validation of candidate variants utilizing previous GWAS datasets on vaccine side effects
We compared candidate variants in our GWAS with those of previous GWASs exploring genetic factors associated with mRNA vaccine side effects related to COVID-1913,15, even though those studies did not focus on booster vaccination. As shown in Supplementary Table S5 online, an overview of these previous datasets reflected variation in vaccine type, number of doses administered, participant characteristics, and the prevalence of each adverse event. GWAS summary statistics from the Italian GWAS study on the NHGRI-EBI Catalog of human genome-wide association studies (https://www.ebi.ac.uk/gwa/deposion) with reference numbers from GCST90256681 to GCST90256698, and those from the Japanese GWAS study on Zenodo (https://doi.org/10.5281/zenodo.8381695), were downloaded and utilized for the validation of moderately associated variants in our GWAS study for booster vaccination. We matched the chromosomal position and allele of each variant on GRCh37 (hg19) and searched for variants with a P-value < 0.05 in the previous study and < 1E-3 in the present study, with matching odds in the same direction. Variants that met the above criteria in at least one sample set analysis were selected for the meta-analysis using a random effects model, and the results with the lowest P-value in the previous study were adopted in the meta-analysis for variants detected in multiple analyses. For the summary statistics derived from the regression analysis, we calculated odds ratios (ORs) and standard errors (SEs) for the meta-analysis as follows: OR = exp(beta) and SEOR = exp(beta) * SEbeta. To count the genome-wide significant variants that originated from different loci, variants that were < 250 kb maximum from each other were merged into a single locus.
Identification of lead variants and symptom-related pathways
The FUMAGWAS web platform (version 1.5.2)24 was utilized to identify lead variants and define the pathways associated with adverse symptoms, modifying the maximum P-value threshold of lead single nucleotide polymorphisms (SNPs) at 1 × 10−5 and the reference panel population to consider the linkage disequilibrium (LD) as 1000G Phase3 East Asians. Suggestively associated variants with a P-value < 1 × 10−5 in the logistic regression analyses for the prevalence of an adverse event, linear regression analyses for symptom severity, or meta-analyses for the prevalence of an adverse event together with the previous GWAS summary statistics, were applied to the SNP2GENE process in FUMAGWAS, and genes that were in expression quantitative trait loci (eQTL) with the candidate variants were extracted. Relationships between the mapped genes were analyzed using STRING database (version 12.0) with minimum required interaction score at median (default) and visualized with hiding disconnected nodes in the network25. The eQTL gene list was further applied to the GENE2FUNC process in FUMAGWAS to identify symptom-associated pathways. Geno Ontology (GO) annotations supported by multiple independent loci are reported in this study. Because variants in the MHC locus are likely to be linked with many genes because of strong LD, we defined the region from chr6:25,000,000–34,000,000 (hg19) as the MHC locus and show the genes contained therein collectively in the Tables.
Results
Clinical characteristics of the corporate genome cohort dataset and correlation between each side effect and age, sex, or booster vaccine type
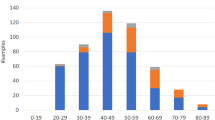
The characteristics of the participants and each side effect are summarized in Table 1. A unique age and sex distribution derived from our corporate cohort was observed, with a median age of 53 years and a lower percentage of females (15.3%). The proportion of booster vaccinations using Pfizer/BNT162b1 was 41.7%. Six local symptoms (pain, redness/erythema, swelling, induration/lump, itching, and burning sensation at the injection site) and six systemic symptoms, including swelling of the lymph nodes, fever ≥ 37.5 °C, fatigue, headache, and muscle pain, were observed in over 100 participants. The incidence rate of each adverse event was comparable to those reported in previous studies6,7,8 and observed lower proportion of side effects in male may partially explain the lower incidence in this study (Supplementary Table S1 online). The absence rate from work after booster vaccination was also surveyed and found to be relatively high. The duration of side effects was most frequently within 1 to 2 days (Supplementary Table S1 online). Depending on the booster vaccine type, the greatest difference in the appearance of side effects was observed for fever ≥ 37.5 °C (Pearson correlation coefficient (r) = 0.142; 42.6% for Pfizer/BNT162b1 and 57.4% for Moderna/mRNA-1273). Some pairs showed a correlation with r > 0.2 and the strongest correlation was observed between redness and swelling at the injection site (r = 0.471) (Supplementary Table S2 online). In addition to vaccine type, age and sex also showed weak correlations (r > 0.1) with some symptoms; therefore, we considered the influence of these factors in our subsequent association analyses.
GWAS for side effects after COVID-19 booster vaccination
In total, 2,554 Japanese participants with 7,382,264 autosomal variants with an MAF > 0.01 were included in our GWAS. First, we performed GWAS for the presence or absence of local or systemic side effects, adjusting for age, sex, and booster vaccine type. No genome-wide significant associations (P < 5 × 10−8) were observed for the presence of each local or systemic side effect or absence from work (Supplementary Fig. S2 online). Next, to target variants that showed associations but did not reach genome-wide significance, we focused on independent lead variants with a P-value < 1 × 10−5 and searched for genes in eQTLs to identify pathways and networks associated with the symptoms and supported by independent genomic loci. The analysis revealed the association of “fever” and immune response-related pathways, including MHC locus genes (minimum P = 5.49 × 10−15), “itching at the injection site”, and the NF-κB binding pathway (P = 1.16 × 10−3) (Table 2, Supplementary Fig. S3 online).
Analysis of the severity of side effects after COVID-19 booster vaccination
We further conducted a linear regression analysis to assess the association between genotype and side effect severity, utilizing the size of swelling at the injection site or lymph nodes, redness or induration at the injection site, or necessity of medication for injection site pain, itching, or burning sensation, or fever at 0.5 °C intervals as categorical variables. Our analysis adjusted for age, sex, and booster vaccine type identified genome-wide significant associations (P < 5 × 10−8) of six loci with swelling of the lymph nodes, redness, induration, or itching at the injection site (Table 3; Fig. 1, Supplementary Fig. S4 online). The most significant association was observed between the severity of the swelling of the lymph nodes and the lead variant rs76152249 on chromosome 2q12 located at the intronic region of the IL18R1 gene (P = 1.46 × 10−9, β = 0.253, Supplementary Fig. S5a online). The lead variant was in eQTL with several genes, including IL1RL1, IL18R1, and IL18RAP. Among the other genome-wide significant variants, the lead variant rs67334974 associated with the severity of swelling of the lymph nodes was in eQTL with the LPIN1, ATP6V1C2, GREB1, and RP11-245G13.2 genes (Table 3, Supplementary Fig. S5b online), and the lead variant rs12956144 associated with the severity of induration at the injection site was in eQTL with the PTPRM gene (Table 3, Supplementary Fig. S5e online). We did not detect any eQTL associations with the remaining three variants. We observed an increase in the minor allele-positive genotype in the lead variants that increased the severity of each associated symptom (Supplementary Fig. S6 online). Pathway and network analysis based on the mapped genes in eQTL with the associated variants (P < 1 × 10−5) in our linear regression analysis against the severity of candidate symptoms also detected associations between the MHC locus-related pathways for severity of fever (minimum adjusted P = 2.75 × 10−13 for LEUKOCYTE_MEDIATED_IMMUNITY) and the NF-κB binding pathway for itching at the injection site (adjusted P = 8.69 × 10−3) (Table 4, Supplementary Fig. S7 online).
Manhattan plot for the association with the severity of local side effects or fever after booster vaccination with genome-wide significant associations. (A) Severity of swelling of lymph nodes (N = 2,459). (B) Severity of itching at injection site (N = 2,503). (C) Severity of induration at injection site (N = 2,213). (D) Severity of redness at injection site (N = 2,201). The red line indicates the genome-wide significance threshold (P = 5.0 × 10−8) and the blue line indicates the suggestive association threshold (P = 1.0 × 10−5).
Validation of overlap with previous GWASs on vaccine side effects in candidate regions
Our study focuses on side effects from booster vaccination. To our knowledge, no studies have specifically examined the genetic factors associated with side effects after booster vaccinations. Although some genetic factors specifically may be associated with side effects during the booster shot, given the mechanism of side effect development, genetic factors associated with side effects during the first and second doses are likely to be associated with side effects during the booster shot. Two GWASs reported on local or systemic side effects associated with the COVID-19 mRNA vaccine, one on genetic testing service participants in the Japanese population, and the other on health-care providers in the Italian population13,15 (Supplementary Table S5 online). Here, we conducted a meta-analysis focusing on variants that met the condition of P < 0.05 in a previous study and P < 1 × 10−3 in our study, with matching odds in the same direction. As a result, by combining all side effects and analyzing the sample sets, a total of 818 variants from 72 loci demonstrated genome-wide significant associations (Supplementary Table S6 online). All of these variants were detected in a meta-analysis with the Japanese study, and none were detected in a meta-analysis with the Italian study, possibly because of its smaller sample size (N = 854). None of the genome-wide significant variants reached the genome-wide significance threshold in the Japanese study, and most were associated with the presence or absence of fever ≥ 37.5 °C after two doses of the Pfizer/BNT162b1 vaccine; however, most were variants in the MHC locus (Supplementary Table S7 online). Although a considerable number of genome-wide significant variants were detected, many of them, especially those in the MHC locus, exhibited strong LD with neighboring genome-wide significant variants and could be grouped together into a single locus (Supplementary Table S8 online). We also applied pathway and network analysis to the candidate variants in the meta-analysis. The number of independent lead variants with a P-value < 1 × 10−5 after the meta-analysis was the lowest for the presence of any of systemic symptoms, at 24, and the highest for fever ≥ 37.5 °C, at 62 (Table 5, Supplementary Fig. S8 online). Greater variation was seen in the number of genes linked by eQTL to those variants, primarily because of the variants in the MHC locus being linked to a large number of genes belonging to the gene family. The pathway analysis revealed genetic associations between immune-related pathways, including the MHC locus genes, and the presence of pain, redness, itching, fever, and fatigue (Table 5, Supplementary Table S9 online). Particularly strong associations were observed with pathways related to fever, fatigue, and pain. For instance, the “adaptive immune response” pathway showed the strongest association with fever ≥ 37.5 °C (P = 1.50 × 10-21), and the regulation of T-cell activation and MHC class II protein complex binding pathways showed the strongest associations with fatigue and pain, respectively (P = 9.86 × 10−8 and P = 1.55 × 10−7, respectively) (Table 5).
Discussion
In this study, we first performed GWAS of common side effects from COVID-19 booster vaccination utilizing a corporate genome cohort in the Japanese population. Although no genome-wide significant associations were observed for the occurrence of side effects, our analysis focusing on the severity of the local adverse reactions revealed six genetic loci showing genome-wide significant associations (P < 5 × 10−8) for increased symptom severity. We also performed validation utilizing the results from previous GWASs for side effects after the first or second dose of COVID-19 vaccination. The meta-analysis revealed shared genetic risk factors showing genome-wide significant associations for the occurrence of local or systemic side effects after COVID-19 vaccination. The prevalence of side effects has been suggested to be almost comparable among booster and second dose vaccinations5,6,7,8,9,10; therefore, shared genetic risk factors might exist. On the other hand, booster vaccination-specific genetic risk factors might have been included in our GWAS but not validated in the independent dataset. Whether the variants that were not validated in our meta-analysis with previous GWASs are specific for booster vaccination or false positives requires further validation based on independent datasets assessing adverse symptoms after booster vaccination. Especially, swelling of the lymph nodes is suggested to be more frequent after booster vaccination9,10, and more participants in our study reported that this symptom was worse at the first than at the second dose, in contrast to other local symptoms, which remained mostly the same or became milder. Our analysis identified three genetic risk loci increasing the severity of swelling of the lymph nodes; however, whether these factors contribute to the development of swelling of the lymph nodes requires further validation.
Regarding the identified genes and pathways through our GWAS, the pathway detected from the mapped genes in eQTL with the suggestively associated variants with the occurrence of adverse symptoms indicated an association of MHC locus genes, including HLA genes with fever ≥ 37.5 °C. This result was consistent with previous GWAS reports in the Italian and Japanese populations13,15. Our analysis of the severity of fever further suggested an association of MHC locus genes with fever. An association between HLA polymorphisms and the level of immunoglobulin G (IgG) production has been suggested in previous studies17,26,27, and a higher level of IgG production has been shown to be associated with the occurrence of local or systemic adverse reactions, including fever ≥ 37.5 °C17,28. Thus, one possible mechanism underlying the association of MHC locus genes with the occurrence and severity of fever is the degree of IgG response through the HLA genes.
In addition to the association of MHC locus genes, our pathway analysis revealed an association of NF-κB binding function mediated by the ANXA4, PSMA6, APEX1, and NFKBIA genes with occurrence and severity of itching at the injection site. NF-κB is a transcription factor that enhances the production of inflammatory cytokines and chemokines, and activated NF-κB exacerbates allergic inflammation of the skin and tumors29. The degree of inflammation of the skin could be a plausible mechanism to induce itching at the injection site.
Three genetic loci with genome-wide significant associations were identified for the severity of swelling of the lymph nodes after booster vaccination. The most significant variant was located on chromosome 2q12 containing the IL1RL1, IL18R1, and IL18RAP genes encoding the interleukin (IL)-1 receptor family proteins30. These genes contribute to the NAD+ nucleosidase activity of the Toll/interleukin-1 receptor (TIR) domains and are primary mediators of inflammation. It has been reported that ST2 (encoded by IL1RL1) forms a complex with IL-33 and recruits the shared receptor subunit IL-1RAP to initiate IL-33-mediated signal transduction. IL18R1 is a receptor that specifically binds IL-18, and IL-18RAP enhances their binding to initiate IL-18-mediated signal transduction. IL-18 and IL-33 reinforce the TH1 and TH2 cell subsets, respectively. Interestingly, IL-18 and IL-1β upregulate the expression of co-stimulatory molecules by Langerhans cells and enable the migration of Langerhans cells out of the skin to the draining lymph node, where a genome-wide significant association with severity of swelling was observed in this study, resulting in the presentation of antigens to T cells30. On the other hand, IL-33 mediates local pain and inflammation in gouty arthritis by boosting the macrophage production of cytokines31. Besides the IL-1 family genes, AC007278.2 and AC007278.3, which are long noncoding RNAs specific in T helper 1 lineage and whose expression levels have been reported to show correlations with IL18R1 and IL18RAP in patients with multiple sclerosis32, were also in eQTL with the lead variant on chromosome 2q12. The other eQTL genes included SLC9A4 and SLC9A2, which are sodium-hydrogen exchanger (NHE) family proteins, and noncoding RNAs or uncharacterized proteins, although their association with swelling or inflammation has not been well established.
The second significant association was located within the LPIN1 gene, which encodes Lipin-1, which has phosphatidate phosphatase activity and contributes to sterol regulatory element-binding protein-dependent gene transcription promoted by mTORC1 and participates in the development of inflammatory and metabolic diseases33,34. The lead variant was also in eQTL with the GREB1 gene, which plays a role in estrogen-stimulated cell proliferation35, and the ATP6V1C2 gene, which produces a proton gradient through its vacuolar proton pump activity, which uses the energy from ATP hydrolysis and plays a key role in bone resorption in osteoclasts36; however, their role in swelling and inflammation has not been established. Another genome-wide significant association was detected for the severity of induration at the injection site. The lead variant was in eQTL with the PTPRM gene. PTPRM is a receptor protein-tyrosine phosphatase that mediates homotypic cell–cell interactions in epithelial and cancer cells, among others. Interestingly, downregulation of PTPRM has been reported to promote keratinocyte proliferation through excessive ERK1/2 signaling37, and the severity increasing allele for induration downregulates the expression of the PTPRM gene. No genes in eQTL were detected in the registered eQTL dataset for the remaining three lead variants showing genome-wide significance with local symptom severity.
Our validation analysis utilizing a previous GWAS dataset identified 818 variants from 72 loci for various adverse symptoms, suggesting shared genetic risk factors between primary and booster vaccinations. MHC locus genes that were suggested from the pathway analysis based on the suggested variants in our GWAS for fever after booster vaccination were replicated for the occurrence of fever and showed genome-wide significant associations with various local symptoms (e.g., pain, redness, itching) and systemic symptoms (e.g., fatigue) besides fever.
Conclusions
Our GWAS involving 2,554 Japanese participants for adverse symptoms after COVID-19 booster vaccination identified genetic factors associated with the severity of adverse symptoms that surpassed the genome-wide significance threshold. The results of our GWAS also suggested the absence of strong genetic risk factors for the common adverse symptoms detected in our sample. To our knowledge, no GWAS on COVID-19 booster vaccination has been conducted; thus, genetic risk factors specific for booster vaccination were not validated in this study. Besides the difference in the number of vaccines, our cohort was male-dominated and the symptoms might involve a self-reporting bias, which could have caused under- or over-reporting of specific adverse events. We also identified multiple genetic factors shared among first or second primary and booster vaccinations. Along with HLA-mediated immunity, other immune system associations, such as NF-κB signaling, were suggested. The present results are expected to provide a useful basis for the improved control of side effects after COVID-19 vaccination.
Data availability
The summary statistics supporting the conclusions of this article have been deposited into the GWAS catalog website (https://www.ebi.ac.uk/gwas/) with reference numbers from GCST90454398 to GCST90454418 and embargo until date of publication.
References
COVID-19 epidemiological update—16 February 2024. World Health Organization (WHO). https://www.who.int/docs/default-source/coronaviruse/situation-reports/20240216_covid-19_epi_update_164.pdf
Thomas, S. J. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N. Engl. J. Med. 385, 1761–1773. https://doi.org/10.1056/NEJMoa2110345 (2021).
Krammer, F. & Ellebedy, A. H. Variant-adapted COVID-19 booster vaccines. Science 382, 157–159. https://doi.org/10.1126/science.adh2712 (2023).
Meng, H., Mao, J. & Ye, Q. Strategies and safety considerations of booster vaccination in COVID-19. Bosn. J. Basic Med. Sci. 22, 366–373. https://doi.org/10.17305/bjbms.2021.7082 (2022).
Nixon, D. F., Schwartz, R. E. & Ndhlovu, L. C. Booster vaccines for COVID-19 vaccine breakthrough cases? Lancet 399, 1224. https://doi.org/10.1016/S0140-6736(22)00044-7 (2022).
Naito, T. et al. Reactogenicity and immunogenicity of BNT162b2 or mRNA-1273 COVID-19 booster vaccinations after two doses of BNT162b2 among healthcare workers in Japan: a prospective observational study. Expert Rev. Vaccines 21, 1319–1329. https://doi.org/10.1080/14760584.2022.2093722 (2022).
Interim report after the third dose. of mRNA vaccination for primary mRNA vaccine recipients. Ministry of Health, Labour and Welfare Web site. https://warp.da.ndl.go.jp/info:ndljp/pid/12499217/www.mhlw.go.jp/content/10601000/001011553.pdf
Hause, A. M. et al. Safety monitoring of an additional dose of COVID-19 Vaccine - United States, August 12-September 19, 2021. MMWR Morb. Mortal. Wkly. Rep. 70, 1379–1384. https://doi.org/10.15585/mmwr.mm7039e4 (2021).
El-Shitany, N. A. et al. The adverse reactions of Pfizer BioNTech COVID-19 vaccine booster dose are mild and similar to the second dose responses: A retrospective Cross-Sectional study. Int. J. Gen. Med. 15, 6821–6836. https://doi.org/10.2147/ijgm.S376316 (2022).
Amer, S. A. et al. Exploring the reported adverse effects of COVID-19 vaccines among vaccinated Arab populations: a multi-national survey study. Sci. Rep. 14, 4785. https://doi.org/10.1038/s41598-024-54886-0 (2024).
Menni, C. et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID symptom study app in the UK: a prospective observational study. Lancet Infect. Dis. 21, 939–949. https://doi.org/10.1016/S1473-3099(21)00224-3 (2021).
Baden, L. R. et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 384, 403–416. https://doi.org/10.1056/NEJMoa2035389 (2021).
Nogawa, S. et al. A web-based genome-wide association study reveals the susceptibility loci of common adverse events following COVID-19 vaccination in the Japanese population. Sci. Rep. 13, 20820. https://doi.org/10.1038/s41598-023-47632-5 (2023).
Atmar, R. L. et al. Homologous and heterologous Covid-19 booster vaccinations. N. Engl. J. Med. 386, 1046–1057. https://doi.org/10.1056/NEJMoa2116414 (2022).
Magri, C. et al. Genome-wide association studies of response and side effects to the BNT162b2 vaccine in Italian healthcare workers: increased antibody levels and side effects in carriers of the HLA-A*03:01 allele. HLA 102, 707–719. https://doi.org/10.1111/tan.15157 (2023).
Bolze, A. et al. HLA-A∗03:01 is associated with increased risk of fever, chills, and stronger side effects from Pfizer-BioNTech COVID-19 vaccination. HGG Adv. 3, 100084. https://doi.org/10.1016/j.xhgg.2021.100084 (2022).
Khor, S. S. et al. An association study of HLA with the kinetics of SARS-CoV-2 Spike specific IgG antibody responses to BNT162b2 mRNA vaccine. Vaccines (Basel) 10 https://doi.org/10.3390/vaccines10040563 (2022).
R Core Team. R: A Language and Environment for Statistical Computing (2023).
Sakurai-Yageta, M. et al. Japonica array NEO with increased genome-wide coverage and abundant disease risk SNPs. J. Biochem. 170, 399–410. https://doi.org/10.1093/jb/mvab060 (2021).
Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575. https://doi.org/10.1086/519795 (2007).
Browning, B. L., Zhou, Y. & Browning, S. R. A One-Penny imputed genome from Next-Generation reference panels. Am. J. Hum. Genet. 103, 338–348. https://doi.org/10.1016/j.ajhg.2018.07.015 (2018).
Kawai, Y. et al. Mapping of susceptible variants for cold medicine-related Stevens-Johnson syndrome by whole-genome resequencing. NPJ Genomic Med. 6. https://doi.org/10.1038/s41525-021-00171-2 (2021).
Byrska-Bishop, M. et al. High-coverage whole-genome sequencing of the expanded 1000 genomes project cohort including 602 trios. Cell 185, 3426–3440.e3419. https://doi.org/10.1016/j.cell.2022.08.004 (2022).
Watanabe, K., Taskesen, E., van Bochoven, A. & Posthuma, D. Functional mapping and annotation of genetic associations with FUMA. Nat. Commun. 8, 1826. https://doi.org/10.1038/s41467-017-01261-5 (2017).
Szklarczyk, D. et al. The STRING database in 2023: protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 51, D638–d646. https://doi.org/10.1093/nar/gkac1000 (2023).
Mentzer, A. J. et al. Human leukocyte antigen alleles associate with COVID-19 vaccine immunogenicity and risk of breakthrough infection. Nat. Med. 29, 147–157. https://doi.org/10.1038/s41591-022-02078-6 (2023).
Bian, S. et al. Genetic determinants of IgG antibody response to COVID-19 vaccination. Am. J. Hum. Genet. 111, 181–199. https://doi.org/10.1016/j.ajhg.2023.12.005 (2024).
Otani, J., Ohta, R. & Sano, C. Association between Immunoglobulin G levels and adverse effects following vaccination with the BNT162b2 vaccine among Japanese healthcare workers. Vaccines (Basel). https://doi.org/10.3390/vaccines9101149 (2021).
Barnes, P. J. & Karin, M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 336, 1066–1071. https://doi.org/10.1056/nejm199704103361506 (1997).
Sims, J. E. & Smith, D. E. The IL-1 family: regulators of immunity. Nat. Rev. Immunol. 10, 89–102. https://doi.org/10.1038/nri2691 (2010).
Fattori, V.et al. IL-33 enhances macrophage release of IL-1β and promotes pain and inflammation in gouty arthritis. Inflamma. Res. 69, 1271–1282. https://doi.org/10.1007/s00011-020-01399-x (2020).
Hosseini, A. et al. LncRNAs associated with multiple sclerosis expressed in the Th1 cell lineage. J. Cell. Physiol. 234, 22153–22162. https://doi.org/10.1002/jcp.28779 (2019).
Peterson, T. R. et al. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell 146, 408–420. https://doi.org/10.1016/j.cell.2011.06.034 (2011).
Balboa, M. A., de Pablo, N., Meana, C. & Balsinde, J. The role of lipins in innate immunity and inflammation. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids. 1864, 1328–1337. https://doi.org/10.1016/j.bbalip.2019.06.003 (2019).
Rae, J. M. et al. GREB 1 is a critical regulator of hormone dependent breast cancer growth. Breast Cancer Res. Treat. 92, 141–149. https://doi.org/10.1007/s10549-005-1483-4 (2005).
Henriksen, K. et al. Ion transporters involved in acidification of the resorption lacuna in osteoclasts. Calcif. Tissue Int. 83, 230–242. https://doi.org/10.1007/s00223-008-9168-8 (2008).
Rioux, G. et al. Gene profiling of a 3D psoriatic skin model enriched in T cells: downregulation of PTPRM promotes keratinocyte proliferation through excessive ERK1/2 signaling. Cells. https://doi.org/10.3390/cells11182904 (2022).
Acknowledgements
The authors thank all the people who participated in this study.
Author information
Authors and Affiliations
Contributions
Designed the study: Y.O., SS.K., M.S, T.Y. and K.T. Performed the data analyses: Y.O.,SS.K., M.S. and Y.K. Drafted the manuscript: Y.O., SS.K. M.S., T.Y. and K.T. Collected samples: T.Y., M.Y. and M.E. Supervised the survey. JS.T., T.M. and W.S. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Omae, Y., Khor, SS., Shimada, M. et al. Genome-wide association study of common side effects following COVID-19 booster vaccination in a cohort of corporate employees in Japan. Sci Rep 15, 12728 (2025). https://doi.org/10.1038/s41598-025-90787-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-90787-6