Abstract
The atherogenic index of plasma (AIP) is a recent biomarker linked to atherosclerosis that has been validated as a novel indicator for myocardial infarction (MI). However, the relationship between AIP and the severity of coronary artery disease (CAD) in MI patients is still ambiguous, particularly among individuals with different glucose metabolic conditions. A total of 741 participants who were immediately assessed with coronary angiography upon admission and diagnosed with acute MI were recruited. The severity of CAD was assessed based on the number of narrowed vessels. AIP tertiles were used to divide the patients into three groups (T1: AIP < 0.030; T2: 0.030 ≤ AIP ≤ 0.316; T3: AIP > 0.316). The American Diabetes Association’s guidelines define three types of glucose metabolic state: diabetes mellitus (DM), prediabetes (Pre-DM), and normal glucose regulation (NGR). Logistic regression analysis was utilized to confirm an association between AIP and CAD severity in MI patients. ROC curves were employed to evaluate the diagnostic utility of AIP for CAD severity in MI patients. In MI patients, a statistically significant correlation was found between AIP and the severity of CAD, with logistic regression analysis revealing a strong association (OR: 2.055; 95% CI: 1.189–3.550; P = 0.009). Following adjustments for risk factors in the logistic regression model, AIP remained an independent predictor of multi-vessel CAD (OR: 2.902;95% CI: 1.555–5.521 ; P < 0.001). Moreover, compared with the T1 group, the odds ratios for multi-vessel CAD in the T2 and T3 groups were 2.039 (95% CI: 1.321–3.175; P = 0.001) and 2.087 (95% CI: 1.317–3.340; P = 0.001), respectively. The area under the curve for predicting CAD severity with AIP was 0.568 (95% CI: 0.520–0.616; p = 0.006). In addition, a significant association was observed between AIP and an increased risk of multi-vessel CAD in the Pre-DM group. In MI patients, AIP is closely associated with the risk of multi-vessel CAD and the prediction of the severity of CAD. In Pre-DM patients, AIP is clearly associated with the severity of CAD, whereas this association is absent in the NGR and DM groups.
Similar content being viewed by others
Introduction
Acute myocardial infarction (MI) represents a critical clinical manifestation of cardiovascular disease; despite satisfactory outcomes from targeted efforts in recent years, it still poses a serious public health burden1. Research indicates that MI patients frequently exhibit multi-vessel coronary artery disease (CAD), and in comparison to individuals with single-vessel CAD, they experience elevated mortality rates and a higher incidence of major adverse cardiovascular events2,3.Moreover, with the increasing incidence of insulin resistance (IR) and type 2 diabetes mellitus (T2DM)4,5, elevated blood glucose levels, inflammatory states, endothelial dysfunction, and dysregulated lipoprotein metabolism exacerbate not only the risk of myocardial infarction but also worsen the clinical prognosis for MI patients6,7,8.
Atherogenic index of plasma (AIP) serves as a measure of lipid metabolism, calculated as the logarithm of the ratio of triglycerides (TG) to high-density lipoprotein cholesterol (HDL-C)9. Research shows that an elevated AIP may reflect the severity of IR and is closely linked to the occurrence of IR and T2DM10. Compared with LDL-C, AIP can more comprehensively represent the overall lipid profile in diabetes mellitus (DM) patients11, and AIP is closely associated with both the reversion from pre-diabetes mellitus(pre-DM) to normal glucose regulation (NGR) and the progression from Pre-DM to DM12. Higher AIP levels and poorly managed AIP levels are linked to an increased risk of cardiovascular disease in populations with impaired glucose metabolism13. Recent studies suggest that AIP could serve as a prospective biomarker for the early diagnosis of MI14, is closely linked to the incidence of MI15, and in patients with coronary artery disease, it is significantly associated with cardiovascular outcomes16.
In addition, the AIP level is significantly correlated with the severity of CAD in patients with coronary heart disease (CHD)17,18. However, to our knowledge, no studies have specifically evaluated the association between AIP and the severity of CAD among MI patients with varying glucose metabolic statuses.
Methods
Study design and population
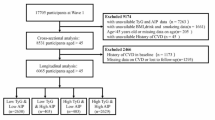
This retrospective study conforms to the Declaration of Helsinki and was approved by the Ethics Committee of the Sinopharm Dongfeng General Hospital (LW-2024-052). 1010 patients diagnosed with acute MI were hospitalized at Sinopharm Dongfeng General Hospital from January 2022 to December 2023.We excluded 152 patients without coronary angiography (CAG) data, 41 patients diagnosed with MI with nonobstructive coronary arteries following CAG, 27 patients lacking TG and HDL-C data, and 49 patients with severe infections, severe hepatic or renal dysfunction, or cancer. A total of 741 patients were included in the final statistical analysis to investigate the relationship between the AIP and the severity of CAD. Patients were divided into three groups based on the tertiles of the AIP index as follows: T1 group, AIP < 0.030; T2 group, 0.030 ≤ AIP ≤ 0.316; T3 group, AIP > 0.316(Fig. 1).
Data collection and definitions
Sociodemographic characteristics (age, gender, Body Mass Index (BMI), smoking and drinking status), medical history (hypertension, diabetes, CHD), and blood analysis results are recorded using digital medical record systems.
Fasting venous blood samples are collected in the morning after at least 8 h of fasting, analyzed by the central laboratory, and recorded for glycated hemoglobin (HbA1c), total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), hemoglobin (HB), platelet count (PLT), and estimated glomerular filtration rate (eGFR).Blood pressure is measured immediately after medical contact for all patients, and within 24 h, a bedside echocardiography is performed by experienced ultrasonologist to measure and record the left atrial diameter (LAD), left ventricular end-diastolic diameter (LVED), and left ventricular ejection fraction (LVEF).
The severity of CAD is determined by the number of coronary arteries with ≥ 50% stenosis (multi-vessel CAD is defined as two or more coronary arteries with ≥ 50% stenosis). A ≥ 50% luminal narrowing of the left main coronary artery is also considered to be multi-vessel CAD19.
The AIP was calculated using the following equation: log10 (TG [mol/L]/HDL-C [mol/L].
Based on the standards set by the American Diabetes Association, glucose metabolic states are classified into: NGR, pre-DM, and DM groups20.If the patient has no history of diabetes, the NGR group is defined as HbA1c < 5.7%, and the pre-DM group as 5.7% ≤ HbA1c < 6.5%. Individuals who have a history of diabetes or a HbA1c level of 6.5% or higher are classified in the DM group.
Statistical analysis
Continuous variables are described as medians and interquartile ranges (25–75%), while categorical variables are represented by counts or percentages. To compare continuous variables between groups, one-way ANOVA or Kruskal-Wallis tests were used, while the chi-square test was employed to compare categorical variables among groups.
To analyze the association between AIP and CAD severity in MI patients, logistic regression models including odds ratios (OR) and 95% confidence intervals (CI) were established. These models were used to examine the relationship between AIP and multi-vessel CAD across the entire population and under different glucose metabolic statuses in both crude and adjusted models. Model 1 is unadjusted, Model 2 is adjusted for age and gender, and Model 3 further adjusts for smoking, drinking, hypertension, CHD, BMI, HbA1c, LVEF, SBP, DBP, and LDL-C based on the variables in Model 2. Calculate the area under the receiver operating characteristic (ROC) curve (AUC) and 95% CI to determine the accuracy of AIP in detecting CAD severity in MI patients. Statistical analysis was performed using SPSS 26.0 (IBM, USA) and GraphPad Prism 9.0 (GraphPad Software, USA). The statistical significance threshold was set at P < 0.05.
Results
Baseline characteristics
A total of 741 patients with MI were included in this study. The median age was 66 years (IQR, 56–74 years), with a male predominance of 73.55%. Based on the CAG results, single- and multi-vessel CAD were found in 24.02% and 75.98% of patients, respectively. Based on the glucose metabolism status, the patients were divided into the NGR group (34.55%), the pre-DM group (34.82%), and the DM group (30.63%).
Table 1 shows the baseline characteristics of MI patients with single-vessel and multi-vessel CAD. Compared to the single-vessel CAD group, patients in the multi-vessel CAD group are older and have higher levels of HbA1c and TG (P < 0.05). Additionally, MI patients with multi-vessel CAD have a higher prevalence of DM and a lower prevalence of CHD (P < 0.05).
Table 2 shows the baseline characteristics according to the tertiles of AIP. Compared to the T1 group, T3 group patients are younger, with higher proportions of smoking and DM. In the T3 group, levels of BMI, HbA1c, TC, TG, LDL-C, Hb, and DBP are higher, while HDL-C and eGFR are lower(P < 0.05).Furthermore, the T3 group has a higher prevalence of multi-vessel CAD (P < 0.05).
Association between AIP and CAD severity in MI patients
Binary logistic regression analysis indicated that, compared with single-vessel CAD, age, HbA1c, and AIP were positively associated with multi-vessel CAD (P < 0.05), whereas the prevalence of CHD was negatively associated with multi-vessel CAD (P < 0.05) (Table 3).
A multivariable logistic regression model was used to verify the relationship between AIP and CAD severity in MI patients. Whether AIP was treated as a continuous or categorical variable, and whether risk factors were adjusted or not, AIP was significantly associated with CAD severity (P < 0.05).When AIP was used as a categorical variable, the incidence of multi-vessel CAD was higher in the T2 and T3 groups compared with the T1 group (P < 0.05).After adjusting for all relevant risk factors in Model 3, compared with the T1 group, the odds of multi-vessel CAD in the T2 and T3 groups were 2.039 (95% CI 1.321–3.175; P = 0.001) and 2.087 (95% CI 1.317–3.340; P = 0.001), respectively (Table 4).
As shown in Fig. 2, the ROC curve analysis of AIP for predicting multi-vessel CAD in MI patients revealed an AUC of 0.568 (95% CI 0.520–0.616), yielding an optimal cut-off value of 0.035 (Youden index: 0.137) at a sensitivity of 69.27% and a specificity of 44.38% (p = 0.006).
Relationships among AIP and CAD severity in MI patients with varying levels of glucose metabolism
Our findings indicate that, in the Pre-DM group, AIP (as a continuous variable) is an independent risk factor for multi-vessel CAD (P < 0.05).Even after adjusting for all relevant risk factors in Model 3, AIP remained significantly associated with CAD severity (OR = 5.295, 95% CI 1.545–19.483, P < 0.05).When considered as a categorical variable, the T2 group had a higher risk of multi-vessel CAD than the T1 group (P < 0.05).After adjusting all relevant risk factors in Model 3, MI patients in the T2 group had a 2.314-fold higher risk of multi-vessel CAD compared with those in the T1 group (95% CI 1.068–5.202, P < 0.05).However, in the NGR and DM groups, there was no significant association between AIP levels and CAD severity (P > 0.05) (Table 5).
Discussion
This study evaluated the relationship among AIP and the severity of CAD in MI patients across different glucose metabolic states. The main findings are: (1) In patients with MI, AIP may be a helpful marker for spotting multi-vessel CAD. (2) Compared to patients with stable CHD, AIP has a stronger correlation with multi-vessel CAD in patients with MI. (3) In the Pre-DM population, there is a strong correlation between AIP and multi-vessel CAD in MI patients, which was not observed in the NGR or DM populations.
MI patients with multi-vessel CAD had a higher complexity in both interventional and surgical treatments than those with single-vessel CAD, and they also have a comparatively worse prognosis2. Consequently, MI accompanied by multi-vessel CAD has consistently been the subject of clinical attention. Dyslipidemia is one of the main reasons why arterial diseases start and get worse, holds a prominent position among the high-risk factors for cardiovascular diseases21.AIP is considered an important marker in dyslipidemia and atherosclerosis, and compared to individual lipid values, it more comprehensively represents the overall lipid profile14.Recent studies have shown that baseline of AIP、long-term cumulative AIP exposure and its time course are closely associated with cardiovascular diseases, particularly significant in the role it plays in MI22,23.
In CHD patients, recent research indicates that AIP has a strong connection with CAD severity17,18. To our knowledge, this is the first study that investigates the link between AIP and CAD severity in MI patients (Supplemental Table S1). Unlike previous findings, our study reveals that AIP is statistically significant with CAD severity in MI patients, regardless of adjustments for confounding factors and tertiles of AIP. Compared to the lowest tertile of AIP (T1 group), the highest tertile (T3 group) shows a 1.658-fold and 2.087-fold increased risk of multi-vessel CAD before and after adjusting for confounding variables, respectively. In general, our findings indicate that CAD severity is more strongly correlated with AIP in the MI population than in the CHD population.
Previous studies have shown that AIP is not only closely related to dyslipidemia but also strongly correlated with increased IR and the onset of T2DM10. Consequently, we conducted a further assessment of the correlation between AIP and CAD severity across different glucose metabolic conditions. Ultimately, it was found that a notable association exists between AIP and CAD severity in the Pre-DM group. However, this correlation was not observed in the NGR and DM groups. This may be attributable to the fact that, during the pre-DM stage, the body typically exhibits early or mild IR, low-grade inflammation, and minor disturbances in glucose and lipid metabolism, and although these responses are weaker compared to the diabetic phase, individuals tend to overlook bodily changes and are less likely to adopt lifestyle or pharmacological interventions24.In addition, during this period, the body is in an intermediate metabolic state where IR has started to develop, but hyperglycemia has not fully manifested, and this chronic, long-term state is crucial in the formation of arteriosclerosis. In recent years, IR indicators (AIP, triglyceride glucose, etc.) have been shown to be closely linked to the risk of multi-vessel CAD as well as mortality and cardiovascular outcomes in patients with chronic coronary syndrome18,25. Meanwhile, studies reveal that in the Pre-DM population, most individuals present with abnormal lipid profiles and/or hypertension. Even though in recent years blood pressure and lipid levels have been effectively controlled, the incidence of MI remains unchanged; compared with Pre-DM patients, those diagnosed with DM have shown more improvement in cardiovascular therapy. Crucially, research has confirmed that even without progressing to DM, Pre-DM is associated with an elevated risk of both microvascular and macrovascular complications26. Previous studies have shown that relative hyperglycemia can trigger microvascular dysfunction in the coronary arteries, regardless of the presence of DM and is closely associated with poor prognosis in patients with MI27,28,29.This implies that individuals with Pre-DM often experience early atherosclerotic changes prior to full-blown dysregulation of glucose metabolism, and this stage may persist for a long time, thereby increasing the risk of multi-vessel CAD in MI patients. In contrast, in the DM population, the increased use of new antidiabetic drugs such as SGLT2 inhibitors and GLP-1 receptor agonists has been shown to alleviate, to some extent, oxidative stress, inflammatory responses, and the interactions involved in atherosclerosis development, effectively reducing cardiovascular events in DM patients30,31,32. Moreover, these patients diagnosed with DM often receive or more frequently seek consultations regarding lifestyle modifications and continuous management of cardiovascular risk factors such as blood glucose and blood lipids. In other words, compared to patients with pre-DM, those diagnosed with DM are more likely to receive guideline-recommended cardiovascular pharmacotherapy, potentially undergo more comprehensive care and treatment, and focus on achieving therapeutic goals—changes that may partially reduce their risk of multi-vessel CAD. Overall, a prolonged yet previously undetected pre-DM state may impose greater negative impacts on both microvascular and macrovascular complications, intensifying the association between AIP and the risk of multi-vessel CAD in MI patients. By contrast, individuals with NGR experience a relatively weaker impact due to their stable metabolic status, whereas in diagnosed DM patients, more consistent treatment and heightened disease awareness may weaken the link between AIP and multi-vessel CAD.
It is worth emphasizing that our study revealed an AUC of 0.568 for AIP in predicting multi-vessel CAD in MI patients, indicating potentially limited predictive capability. This may be related to the following factors: multi-vessel CAD are often the result of multiple factors (including lipid metabolism, inflammatory damage, endothelial dysfunction, and various metabolic disorders), and as a lipid-metabolism-related index, AIP alone may not sufficiently capture these complex risk factors and pathological pathways. Moreover, this study was a single-center retrospective investigation with a relatively small sample size, which may have somewhat affected AIP’s predictive efficacy. Recent research indicates that pre-DM is a high-risk state for cardiovascular events, closely associated with the risk of MI, all-cause mortality, and outcome risks33.AIP is closely linked to the risk of pre-DM34, the progression from pre-DM to DM or reversion to NGR12, and has been shown to be an independent predictor for rapid progression of coronary atherosclerosis and vulnerable plaque formation35.Therefore, even though the AUC value is relatively low, our results suggest that greater attention should still be given to AIP, especially in MI patients with pre-DM, as early decision-making and risk stratification based on AIP levels may help customize interventions, potentially slowing the progression of coronary lesions and preventing adverse cardiovascular events. Additionally, combining AIP with other biomarkers (such as stress hyperglycemia) and incorporating these into more comprehensive risk prediction models may enhance AIP’s ability to predict multi-vessel CAD in MI patients, and we will further investigate this in the future to confirm our hypothesis.
The results of this study provide additional evidence that in patients with MI, elevated AIP levels are strongly linked to multi-vessel CAD. Notably, the association between AIP levels and multi-vessel CAD is most pronounced in pre-DM patients compared to those with NGR and DM, suggesting that AIP levels in this population should be closely monitored. Based on this, AIP is expected to become an effective tool for risk stratification in pre-DM patients, helping clinicians identify high-risk MI patients earlier and develop personalized intervention strategies.
Strengths and limitations
To our knowledge, this is the first study to directly explore the effect of AIP on CAD severity among MI patients under different glucose metabolic statuses. However, this study also has certain limitations. Firstly, the AIP measurement was based on the baseline obtained at patient admission, and due to the absence of long-term follow-up data in this study, we could not assess the predictive value of changes in AIP over time for CAD severity. This deficiency partly constrains our understanding of AIP’s role in long-term risk prediction and leaves a gap in elucidating AIP’s impact at different stages of disease progression. Future extended follow-ups, incorporating more dynamic data, may prove more valuable for risk stratification of CAD severity. Secondly, we did not collect information on the duration and dosage of antidiabetic, lipid-lowering, and antihypertensive medications used by participants, nor did we have long-term monitoring data for blood pressure, blood glucose, and lipid levels, which may potentially affect our findings. Thirdly, because retrospective studies inherently have unavoidable drawbacks, we could not infer causality in this study. Moreover, although we attempted to adjust for many confounding factors, we cannot completely rule out residual confounding effects—such as diet, exercise, sleep, and stress—which may influence the results. Fourthly, this single-center study involving only the Chinese population may result in admission rate bias and has a relatively small sample size, which may limit the generalizability of the findings to broader populations. Further prospective, large-scale, multicenter randomized controlled trials may make our conclusions more reliable, and future research should account for these factors to enhance the accuracy and validity of the findings.
Conclusion
Our results indicate that AIP is associated with multi-vessel CAD in MI patients, and an elevated AIP can identify MI patients with multi-vessel CAD. In the pre-DM group, there is an association between AIP and the severity of CAD, regardless of adjustments for confounding factors, while no such correlation is observed in the non-DM and DM groups. Monitoring AIP may serve as a risk management strategy for MI patients, particularly in the pre-DM population, offering new preventive strategies for clinical management.
Data availability
The datasets generated and analyzed in this study are not publicly available due to privacy and ethical constraints; however, they can be obtained from the corresponding author.
Abbreviations
- AIP:
-
Atherogenic index of plasma
- CAD:
-
coronary artery disease
- MI:
-
myocardial infarction
- CAG:
-
coronary angiography
- NGR:
-
normal glucose regulation
- Pre-DM:
-
prediabetes mellitus
- DM:
-
diabetes mellitus
- ROC:
-
receiver operating characteristic
- AUC:
-
area under the ROC curve
- IR:
-
insulin resistance
- T2DM:
-
type 2 diabetes mellitus
- TC:
-
total cholesterol
- TG:
-
triglycerides
- HDL-C:
-
high-density lipoprotein cholesterol
- LDL-C:
-
low-density lipoprotein cholesterol
- HbA1c:
-
glycated hemoglobin
- HB:
-
hemoglobin
- PLT:
-
platelet count
- eGFR:
-
estimated glomerular filtration rate
- LAD:
-
left atrial diameter
- LVED:
-
left ventricular end-diastolic diameter
- LVEF:
-
left ventricular ejection fraction
References
Benjamin, E. J. et al. Heart Disease and Stroke Statistics-2019 update: a Report from the American Heart Association. Circulation 139, e56–e528 (2019).
Shiyovich, A. et al. Temporal trends of patients with acute coronary syndrome and multi-vessel coronary artery disease - from the ACSIS registry. Int. J. Cardiol. 304, 8–13 (2020).
Widimsky, P. Holmes. How to treat patients with ST-elevation acute myocardial infarction and multi-vessel disease? Eur. Heart J. 32, 396–403 (2011).
Ormazabal, V. et al. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 17, 122 (2018).
La Sala, L. & Prattichizzo, F. Ceriello. The link between diabetes and atherosclerosis. Eur. J. Prev. Cardiol. 26, 15–24 (2019).
Arnett, D. K. et al. 2019 ACC/AHA Guideline on the primary Prevention of Cardiovascular Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice guidelines. J. Am. Coll. Cardiol. 74, e177–e232 (2019).
Nauta, S. T., Deckers, J. W., Akkerhuis, K. M. & van Domburg, R. T. Short- and long-term mortality after myocardial infarction in patients with and without diabetes: changes from 1985 to 2008. Diabetes Care. 35, 2043–2047 (2012).
Su, L., Mittal, R., Ramgobin, D. & Jain, R. & R. Jain. Current Management Guidelines on Hyperlipidemia: The Silent Killer. Journal of Lipids. 9883352 (2021). (2021).
Dobiásová, M. & Frohlich, J. The plasma parameter log (TG/HDL-C) as an atherogenic index: correlation with lipoprotein particle size and esterification rate in apob-lipoprotein-depleted plasma (FER(HDL)). Clin. Biochem. 34, 583–588 (2001).
Yin, B. et al. Non-linear association of atherogenic index of plasma with insulin resistance and type 2 diabetes: a cross-sectional study. Cardiovasc. Diabetol. 22, 157 (2023).
Qin, Z. et al. The atherogenic index of plasma plays an important role in predicting the prognosis of type 2 diabetic subjects undergoing percutaneous coronary intervention: results from an observational cohort study in China. Cardiovasc. Diabetol. 19, 23 (2020).
Yang, H. et al. Evaluation of the role of atherogenic index of plasma in the reversion from Prediabetes to normoglycemia or progression to diabetes: a multi-center retrospective cohort study. Cardiovasc. Diabetol. 23, 17 (2024).
Min, Q. et al. Association between atherogenic index of plasma control level and incident cardiovascular disease in middle-aged and elderly Chinese individuals with abnormal glucose metabolism. Cardiovasc. Diabetol. 23, 54 (2024).
Fernández-Macías, J. C., Ochoa-Martínez, A. C. & Varela-Silva, J. A. Pérez-Maldonado. Atherogenic index of plasma: Novel Predictive Biomarker for Cardiovascular illnesses. Arch. Med. Res. 50, 285–294 (2019).
Zhang, Y. et al. Elevated atherogenic index of plasma increased the risk of myocardial infarction in a general population. Ann. Epidemiol. 90, 1–8 (2024).
Rabiee Rad, M., Ghasempour Dabaghi, G., Darouei, B. & Amani-Beni, R. The association of atherogenic index of plasma with cardiovascular outcomes in patients with coronary artery disease: a systematic review and meta-analysis. Cardiovasc. Diabetol. 23, 119 (2024).
Li, Y. et al. The atherogenic index of plasma (AIP) is a predictor for the severity of coronary artery disease. Front. Cardiovasc. Med. 10, 1140215 (2023).
Wu, X. et al. Associations of the triglyceride-glucose index and atherogenic index of plasma with the severity of new-onset coronary artery disease in different glucose metabolic states. Cardiovasc. Diabetol. 23, 76 (2024).
Zhao, S. et al. Comprehensive analysis of the association between triglyceride-glucose index and coronary artery disease severity across different glucose metabolism states: a large-scale cross-sectional study from an Asian cohort. Cardiovasc. Diabetol. 23, 251 (2024).
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 36 (Suppl 1), 67–74 (2013).
Berberich, A. J. Hegele. A Modern Approach to Dyslipidemia. Endocr. Rev. 43, 611–653 (2022).
Kim, S. H. et al. Association of the atherogenic index of plasma with cardiovascular risk beyond the traditional risk factors: a nationwide population-based cohort study. Cardiovasc. Diabetol. 21, 81 (2022).
Zhang, Y. et al. Association between cumulative atherogenic index of plasma exposure and risk of myocardial infarction in the general population. Cardiovasc. Diabetol. 22, 210 (2023).
Echouffo-Tcheugui, J. B. Selvin. Prediabetes and what it means: the Epidemiological evidence. Annu. Rev. Public Health. 42, 59–77 (2021).
Otsuka, K. et al. Impact of diabetes mellitus and triglyceride glucose index on mortality and cardiovascular outcomes in patients with chronic coronary syndrome undergoing coronary computed tomography angiography. Int. J. Cardiol. Cardiovasc. Risk Prev. 20, 200250 (2024).
Rooney, M. R. et al. Prediabetes is associated with elevated risk of clinical outcomes even without progression to diabetes. Diabetologia https://doi.org/10.1007/s00125-024-06315-0 (2024).
Mone, P. et al. Hyperglycemia drives Stent Restenosis in STEMI patients. Diabetes Care. 44, e192–e193 (2021).
Lee, T. F. et al. Relative hyperglycemia is associated with complications following an acute myocardial infarction: a post-hoc analysis of HI-5 data. Cardiovasc. Diabetol. 16, 157 (2017).
Mone, P. et al. Stress hyperglycemia drives the risk of hospitalization for chest Pain in patients with ischemia and nonobstructive coronary arteries (INOCA). Diabetes Care. 46, 450–454 (2023).
McGuire, D. K. et al. Association of SGLT2 inhibitors with Cardiovascular and kidney outcomes in patients with type 2 diabetes: a Meta-analysis. JAMA Cardiol. 6, 148–158 (2021).
Sattar, N. et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabets Endocrionol. 9, 653–662 (2021).
Paolisso, P. et al. Infarct size, inflammatory burden, and admission hyperglycemia in diabetic patients with acute myocardial infarction treated with SGLT2-inhibitors: a multicenter international registry. Cardiovasc. Diabetol. 21, 77 (2022).
Echouffo-Tcheugui, J. B., Perreault, L. & Ji, L. Dagogo-Jack. Diagnosis and management of prediabetes: a review. JAMA 329, 1206–1216 (2023).
Zheng, X., Zhang, X., Han, Y., Hu, H. & Cao, C. Nonlinear relationship between atherogenic index of plasma and the risk of prediabetes: a retrospective study based on Chinese adults. Cardiovasc. Diabetol. 22, 205 (2023).
Won, K. B. et al. Atherogenic index of plasma and the risk of rapid progression of coronary atherosclerosis beyond traditional risk factors. Atherosclerosis 324, 46–51 (2021).
Funding
This work was supported by the National Key R&D Program of China (2020YFC2008000), the National Natural Science Foundation of China (81400288, 81573244), Hubei Provincial Natural Science Foundation (2022CFB453), Foundation of Health Commission of Hubei (WJ2021M061), Natural Science Foundation of the Bureau of Science and Technology of Shiyan City (grant no. 21Y71), Faculty Development Grants from Hubei University of Medicine (2018QDJZR04), Hubei Key Laboratory of Wudang Local Chinese Medicine Research (Hubei University of Medicine) (Grant No. WDCM2024020), and Advantages Discipline Group (Medicine) Project in Higher Education of Hubei Province (2021–2025) (Grant No. 2024XKQT41).
Author information
Authors and Affiliations
Contributions
KQJ, ZJM and CLZ designed the study, analysed the data, and wrote the manuscript; KQJ, ZJM, CLZ, XTZ, HX, DFL, XWM, HDY, WWW, JXZ collected and interpreted the data; JSC and JC critically revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The Ethics Committee of the Sinopharm Dongfeng General Hospital approved this investigation, which was conducted in accordance with the Declaration of Helsinki. Due to the retrospective nature of the study, the Ethics Committee of Sinopharm Dongfeng General Hospital waived the need of obtaining informed consent.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jin, K., Ma, Z., Zhao, C. et al. The correlation between the atherogenic index of plasma and the severity of coronary artery disease in acute myocardial infarction patients under different glucose metabolic states. Sci Rep 15, 6128 (2025). https://doi.org/10.1038/s41598-025-90816-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-90816-4