Abstract
Neuropathic pain (NP) is caused by primary damage and dysfunction of nervous system, in which spinal cord injury (SCI) is a common cause of NP. Evidence shows that neuroinflammation and oxidative stress are related to the pathophysiology of NP, in which the activation of microglia and astrocytes in spinal is significant. Therefore, understanding the molecular mechanism of NP after SCI is of great significance. The rat model of SCI was established and BV2 cell was treated with LPS. The exosomes derived from astrocytes were extracted by centrifugation. The morphology of the exosomes was observed by electron microscope and the surface markers were detected by Western blot. LncRNA in astrocytes and astrocyte-derived exosomes were detected by qRT-PCR. The expression of microglia activation markers CD68 and Iba-1 was detected by immunohistochemistry. The von Frey test was applied to assess mechanical hypersensitivity. The heat plate analgesia instrument was used to evaluate Paw withdrawal latency (PWL). QRT-PCR used to detect expression of LncRNA49rik and miR-10a-5p. Western blot was used to detect MAPK/PI3K/AKT / mTOR signal pathway and COX2, iNOS. The content of MDA and the activity of SOD were detected by oxidative stress kit. The concentrations of IL-6, IL-1β, IL-18 and IFN-αwere detected by ELISA. The targeting relationship between LncRNA49rik and miR-10a-5p was analyzed by bioinformatics and double luciferase activity, Rip and FISH experiments. LncRNA49rik was highly expressed in astrocytes and its derived exosomes. SCI stimulated astrocytes to release exosome containing LncRNA49rik and promote microglia activation to increase inflammatory response. At the same time, overexpression of LncRNA49rik increased the incidence of NP and aggravated the level of inflammation and oxidative stress in rats with SCI. MiR-10a-5p is the target of LncRNA4933431K23Rik. Overexpression of LncRNA49rik significantly inhibited the up-regulation of miR-10a-5p. Overexpression of miR-10a-5p inhibited hyperalgesia and inflammation in SCI rats. In addition, transfection of miR-10a-5p mimics significantly inhibited the expression of MAPK/PI3K/AKT and up-regulated the expression of mTOR. Mechanism studies have shown that overexpression of miR-10a-5p weakens the phenotypic induction of microglia induced by LncRNA4933431K23Rik. LncRNA4933431K23Rik regulates microglial phenotype through inhibiting miR-10a-5p, which is responsible for NP induced by SCI.
Similar content being viewed by others
Introduction
Neuropathic pain (NP) is caused primary damage and dysfunction of the nervous system1. NP can be divided into two categories: peripheral pain (including post-herpetic neuralgia, trigeminal neuralgia, glossopharyngeal neuralgia, etc.) and central pain (including Parkinson’s pain, post-stroke pain, spinal cord injury pain, etc.)2,3. Clinically, NP is characterized by spontaneous persistent pain or shooting pain, as well as amplified pain responses induced by stimuli4. NP is usually caused by a variety of mechanisms, including peripheral sensitization, central sensitization, disability of descending inhibitory system, activation of microglia, sympathetic sprouting, changes of ion channels, and so on5. Spinal cord injury (SCI) is the common cause of NP. More and more evidence shows that neuroinflammation and oxidative stress are related to the pathophysiology of NP, in which the activation of microglia and astrocytes in the spinal dorsal horn is significant6. Therefore, understanding the molecular mechanism of NP after SCI is of great significance to the treatment and prognosis.
Exosomes (Exo) are vesicles with lipid bilayer membrane structure, which are synthesized and secreted by cells with a diameter of about 30-200 nm7. Exosomes contain a variety of protein and nucleic acid, among which exosome LncRNA has significant value in controlling cell development, tumorigenesis, cell differentiation, immune inflammation and so on8. For example, studies have shown that exosome LncRNAHOTAIR promotes the progression of endometriosis and angiogenesis through miR-761/HDAC1 axis and STAT3-mediated inflammatory activation9. In addition, exosome LncRNAMir9-3hg inhibits cardiomyocyte iron death by regulating the Pum2/PRDX6 axis, thereby reducing ischemia reperfusion induced cardiac injury and improving cardiac function10. LncRNA4933431K23Rik is a member of the LncRNA family. Studies have shown that astrocyte-derived exosomes LncRNA4933431K23Rik can reduce neuroinflammation and protect neuronal function in mice with traumatic brain injury by regulating Smad7 to regulate microglia phenotype11. However, the role and mechanism of LncRNA4933431K23Rik in the progression of NP caused by SCI are not clear.
MicroRNA (miR) is another group of 18–22 nucleotides non-coding RNA. Studies have reported that miR can act as a direct target for LncRNA, thus mediating the function of LncRNA to participate in the progression of a variety of diseases12. For example, WuJ found that LncRNAMALAT1 promotes the progression of rat NP through influencing miR-154–5/AQP9 axis in rats with chronic compressive injury13. In addition, LncRNAMEG3 aggravates NP and astrocyte activation by regulating the miR-130a-5p/CXCL12/CXCR4 axis14. MiR-10a-5p is a member of the miR family. Studies have shown that LncRNASNHG5 inhibits chondrocyte apoptosis and reduces inflammatory response in osteoarthritis by regulating the miR-10a-5p/H3F3B axis15. Inhibition of LncRNAMIAT improves myocardial dysfunction induced by myocardial infarction through miR-10a-5p/EGR2 axis16. However, the role of LncRNA in regulating miR-10a-5p in the progression of NP has not been reported (Table 1).
Glial cells include astrocytes, oligodendrocytes and microglia. Glial cells, as the main supporting cells of the central nervous system (CNS), play an important role in maintaining brain homeostasis17. Microglia are the main immune cells in the CNS, which can show different phenotypes such as anti-inflammatory or pro-inflammatory according to different stimuli. Studies have found that microglia is one of the most important factors of CNS inflammation after injury. Studies also show that astrocytes interact with microglia and regulate the phenotype of microglia through exosomes containing LncRNA19. In this study, the rat model of SCI and the BV2 microglia model in vitro were established to explore the role and mechanism of exosomes LncRNA4933431K23Rik in NP caused by SCI, so as to provide more references for NP treatment.
Materials and methods
SCI model
Forty adult SD rats (female, 4 weeks old, 180-200 g) were purchased from Nanjing Animal Experimental Center and randomly divided into sham operation group (n = 10), SCI group (n = 10), SCI + LV-NC group (n = 10) and SCI + LV-LncRNA49rik group (n = 10). The rats were raised in a standard plastic cage with a temperature of 24 ± 1 °C, a humidity of 50–70%, and a light and dark cycle of 12 h. They are free to drink and eat. Follow the guidelines for the care and use of experimental animals at the National Institutes of Health to reduce discomfort or stress. The rats were deeply anesthetized with pentobarbital sodium (40 mg/kg), and then laminectomy was performed at the T9-T10 level of the rat spine to completely expose the dorsal side of the spinal cord. Next, a rat spinal cord impactor (RWD,Cat#ModelIII,USA, 2 mm in diameter, 12.5 g and 20 mm in height) was used to cause moderate contusions on the surface of the T9-T10 spinal cord. Sham-operated rats received the same treatment in addition to spinal cord contusion. After operation, there were hemorrhage on the surface of spinal cord, straightening of legs and prolapse of tail, which indicated that the rat model of moderate spinal cord injury was established successfully. The muscles, fascia and skin of rats were sutured and disinfected with iodine. For the next few days, the bladder was manually emptied every day until the rats regained spontaneous micturition function. The experiment was conducted in accordance with the project license granted by the ethics committee of Hospital and in accordance with the guidelines for animal care and use in Hospital.
Spinal lipid extract (SLE)
SLE is generated as described by GilbertO et al.20. To put it simply, the rat spinal cord samples were put into a glass grinder to grind the tissue, and then 350xg centrifuged 5 min at 4 ℃ to remove cells. The sample passes through a 0.22 μ m filter unit (Cat#SLGP033RB;Millipore) to remove bacteria. Next, the supernatant of 1 ml was introduced into the 1.5 ml LEP tube and stored at-80 ℃ for further experiments.The protein concentration of SLE was determined using the BCA reaction system (Cat# G2026- 497 200 T; Wuhan Servicebio Technology) via a microplate reader (Infinite F50; Tecan 498 Group, Männedorf, Switzerland) at a wavelength of 570 nm. The SLE was used to treated control or transfected astrocytes for 6 h, and the supernatant was collected for further experiments.
Intrathecal injection
Intrathecal injection refer to the study of Hu Y et al.21. The PE-10 polyethylene catheter was inserted into the sheath and injected with lidocaine to paralyze both hind limbs for intrathecal implantation. Then fix the catheter and close the incision. In terms of gene delivery, LV- NC, LV-LV-LncRNA49rik lentiviraLVectors (1 × 107/0.1 mL) (Shanghai Genome Pharmaceutical Co., Ltd.) were injected into the spinal cord sheath through a microsyringe connected to the intrathecal catheter 3 days before the establishment of the model.
The Basso, Beattie, and Bresnahan (BBB) score
The BBB score is a 21-point scale defined by surgery that follows the recovery process from complete paralysis to normal movement22. The BBB score contains 3 stage, including the joint activity of the hind limbs (0–7 points), the gait and hindlimb coordination (8–13 points), and the free movement of the claws(14–21 points). On the 0th, 3rd, 7th, 14th and 21st day after SCI, the animals of each group were placed in the open field and evaluated by two observers for 5 min. The rats with BBB score less than 7 did not participate in the follow-up experiment. Since spontaneous bladder contraction usually affects the movement of the hind limbs, the bladder has been emptied before the test.
Mechanical allodynia (Von Frey Test)
The Von Frey test was applied to assess mechanical hypersensitivity, paw withdrawal threshold (PWT), referencing to previous report23. Before the experiment, the rats were placed in a transparent plastic box (20–17-13 cm) away from the table and adapted to the environment for 15 min. The calibrated Von Frey fiber (Electronic von Frey 2393; IITC, Woodland Hills,CA,USA) was taken to apply pressure the plantar surface of the rat’s hind paw. On day 0, day 3, day 7, day 14 and day 21 after SCI, the researchers recorded the size of the flaments as the claws.
Thermal hyperalgesia (Hot Plate Test)
The heat plate analgesia instrument (Bio-chp, Bioseb,France) was used to evaluate Paw withdrawal latency (PWL), reference to24. The rats were placed on a hot plate (53 ± 1 ℃), one at a time. We recorded the latency of jump response or hindpaw response. Each rat was measured 3 times with an interval of 10 min. In the case of tissue damage, the time is shortened by 30 s.
Exosome isolation
Exosome derived from astrocytes were extracted by ExoQuick Solution(EXOQ20A-1, SystemBiosciences, USA ) with differential centrifugation. All centrifugation steps are carried at 4 °C. Firstly, cells were centrifuged 300 g for 10 min to absorb the supernatant, then ExoQuick Solution was added and 2000 g centrifugation for 10 min. After that, 10000 g high-speed centrifugation for 30 min to absorb the supernatant, and 140000 g ultracentrifugation 90 min; to remove the supernatant, the precipitate obtained was exosomes. After washing and re-suspension with PBS buffer, 1400 g was centrifuged again for 90 min, and 100 μ L PBS buffer was re-suspended and stored at-80 ℃.
Cell Culture and transfection
BV-2 microglia were purchased from the Shanghai Cell Bank (Chinese Academy of Sciences,Shanghai,China) of the Chinese Academy of Sciences. Normal astrocytes (NHAs, isolated from the spinal cord) are provided by the American typical Culture Preservation Center (ATCC, Rockville,MD,USA). The cells are cultured in DMEM medium (Thermo Fisher HyClone,Utah,USA) containing 5% FBS (Thermo Fisher Scientific,MA,USA), in the incubator 5%CO2 and 37 ℃. The medium is changed every 2 days and passaged once every 5 days. The experiment is carried out when the cells grow to 90%. The overexpression plasmid of LncRNA 4933431K23Rik, the mimic of miR-10a-5p (miR-10a-5p), and the corresponding control group (NC) were obtained by GenePharma (Shanghai,China). Briefly, the cells were seeded into a 6-well plate, and then Lipofectamine 3000(Invitrogen, USA), according to the manufacturer’s instructions, was used to transfect the plasmid into the cells at a concentration of 2.5 μg per well. After 6 h, the culture medium was removed. After transfection, cells were cultured 24 h, and total RNA was extracted and real qRT-PCR was used to detect the expression of target gene.
qRT-PCR
Total RNA was extracted by Trizol reagent (Invitrogen, LA, USA) according to the instruction and its concentration was determined. Primer amplification was carried out according to the manufacturer (Takara,Tokyo, Japan). QRT-PCR adopts Bio-Rad CFX96 quantitative PCR system(USA) and SYBR, according to the manufacturer’s regulations. The conditions of qRT-PCR are as follows: pre-denatured 5 min at 95℃, then denatured at 95℃ for 15 s, and annealed at 60℃ for 30 s. GAPDH is the internal reference of LncRNA49rik, MAPK, IL-6, IL-1β, IL-18, IFN-α, U6 is the internal reference of miR-10a-5p, and 2 (- ΔCt) method is used for statistics. Repeat all qRT-PCR responses three times. All the primers were designed and synthesized by Guangzhou Ruibo Company (China).
Western blot
BV2 cells were collected and washed with cold PBS for 3 times. 100 ~ 200 μL RIPA lysate (Beyotime Biotcchnology,Shanghai,China) was added. The cells were lysed by ultrasonic in ice water. The protein of T10 spine was obtained by RIPA lysate. The protein concentration was determined by Bradford method. The same amount of protein was taken from each group for 10% SDS-P AGE electrophoresis, and the proteins on the gel were transferred to PVDF membrane (Millipore,Bedford, MA, USA). The membranes were sealed at 4 ℃ for 1 h, followed by the addition of primary antibody (1:1000), Anti-p-p38 MAPK antibody (ab4822), Anti-p38 MAPK antibody (ab31828), Anti-PI3K antibody (ab191606), Anti-PI3k (phosphoY607) antibody (ab182651), Anti-pan-AKT antibody (ab18785), Anti-AKT (phospho T308) antibody (ab38449), Anti-mTOR antibody (ab32028), Anti-p-mTOR antibody (ab109268), Anti-COX2 antibody (ab179800), Anti-iNOS antibody (ab15323), Anti-GAPDH antibody (ab181602), and overnight at 4 ℃. After washing the membrane with TBST for 2 times, the membrane was incubated with second antibody sheep anti-rabbit (ab205718,1:2500) at RT for 1 h. All the above antibodies came from (abcam,Abcam,Cambridge,UK). Membranes were exposed with ECL chromogenic agent (Millipore,Bedford,MA,USA) after washing for 3 times, and the images were obtained by film scanner.
Immunohistochemistry
Slices were incubated with 15min in methanol solution containing 0.3%H2O2 at 37 ℃ to eliminate the activity of endogenous peroxidase. After the slices were washed by PBS 3 times, slices were incubated with 10% normal goat serum for 15min at 37 ℃. Then, slices were incubated with primary antibody like mouse anti-CD68 antibody (ab283654,Abcam,Cambridge,UK), anti-Iba-1 antibody (ab178876,Abcam,Cambridge,UK), overnight in wet box at 4 ℃. Then, the slices were washed by PBS 3 times, the biotin labeled sheep anti-rat IgG (1:200, Beijing Zhongshan Biotechnology Co., Ltd.) was added. After washed by PBS, ABC solution (Beijing Zhongshan Biotechnology Co., Ltd.) was added and incubated 30min at 37 ℃. After washed by PBS, gradient alcohol dehydration, dimethylbenzene transparent, neutral gum sealed tablets, observed under light microscope.
Evaluation of oxidative stress (OS)
According to the manufacturer’s agreement, a specific detection kit was used to determine the content of malondialdehyde (malondialdehyde,MDA) and the activity of superoxide dismutase (superoxide dismutase,SOD). The oxidative stress index detection kits were purchased from Nanjing Jiancheng Biological Company (NanJing JianCheng Bioengineering Institute).
Enzyme-linked immunosorbent assay (ELISA)
According to the manufacturer’s recommendation, use commercial ELISA kits (rotated System, Minneapolis, MN, USA) to explore the concentration of IL-6, IL-1 β, IL-18, IFN- α in the supernatant of BV2 cell culture. Use Power Wave Microplate Reader (Bio-TEK, USA) to text the OD level.
Dual-luciferase reporter assay
The LncRNA49rik or MAPK 3′UTR fragments containing miR-10a-5p target sites (wild-type or mutant-type) were inserted to pmirGLO dual-luciferase reporter vectors (Promega, Madison, WI), which were then named as LncRNA49rik-WT/MUT. The vectors were co-transfected with miR-10a-5p mimics or NC mimics into BV2 cells. Their luciferase intensity was individually estimated using dual-luciferase reporter assay system (Promega).
RNA immunoprecipition (RIP)
RIP assay uses Magna RIP RNA Immunoprecipitation Kit (Millipore, Billerica, MA, USA) to determine the endogenous interaction between miR-10a-5p and LncRNA49rik or MAPK. In short, microglia were transfected with miR-NC or miR-10a-5p mimics and then cleaved in a cold lytic buffer. The supernatant was incubated with anti-Argonaute 2 (anti-Ago2, Cell Signaling T echnology) or IgG-coupled protein G beads. The co-precipitated RNA was digested with Proteinase K. the total RNA was extracted and LncRNA49rik or MAPK mRNA enrichment was detected by qRT-PCR assay.
Fluorescence in situ hybridization (FISH) analysis
LncRNA49rik and miR-10a-5p were captured by Cy3 labeled probe (RiboBio,Guganghzou,China) and Alexa 488 labeled probe (RiboBio), respectively.FISH experiment was conducted with Fluorescent Hybridization Kit (No. C10910, RiboBio, Guangzhou, China), according to the guidelines. DAPI was utilized to stain the nuclei. Subsequently,LncRNA49rik and miR-10a-5p were observed through a confocal microscope (LSM 880 with Airyscan, Carl Zeiss, Germany).
Data analysis
The data were analyzed by SPSS20.0 statistical software (SPSSInc.,Chicago,IL,USA). The measurement data are expressed by the average ± standard deviation (x ± s). Multiple factors were compared by single factor analysis of variance, and independent sample t-test was used between the two groups. The difference was statistically significant (P < 0.05).
Results
LncRNA4933431K23Rik was highly expressed in exosomes
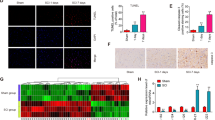
The morphology of exosomes was observed by transmission electron microscope. It was found that most of the vesicles ranged in size from 50 to 150 nm (Fig. 1A). The expression of exosome markers was further confirmed by Westernblot analysis. These results showed that the exosomes have been isolated successfully (Fig. 1B). QRT-PCR was used to detect the expression of 15 LncRNA in astrocytes and exosomes, which was treated with SLE (5 μg / mL) for 6 h. The results of thermographic analysis showed that LncRNA49rik were highly expressed in astrocytes and its exosomes, and LncRNA49rik expression was the increased significantly than that of other LncRNA (Fig. 1C,D, P < 0.05). In order to further determine the effect of exosomes containing LncRNA49rik on the microglia. Firstly, the astrocytes were treated LncRNA49rik or control. Then, the normal astrocytes or transfected astrocytes were treated with SLE for 6 h, and the supernatant were collected respectively and exsome was obtained. The exsome from conditioned astrocytes supernatant was used to treat microglia for 6 h. We observed that the exosomes overexpressing LncRNA49rik significantly increased the expression of SLE-induced pro-inflammatory factors (such as TNF- α, IL-6 and IL-1 β) in microglia (P < 0.05,Fig. 1E). The results of immunohistochemistry also showed that exosomes LncRNA49rik significantly increased the activation of pro-inflammatory microglia labeled by CD68 and Iba-1 (P < 0.05, Fig. 1F). The results of these suggest that exosomes from astrocytes contains LncRNA49rik. LncRNA49rik could promote microglia activation to increase their related inflammatory response.
LncRNA 4933431K23Rik is highly expressed in astrocyte-derived exosomes. The morphology of the exosome from astrocytes was observed by transmission electron microscope (× 100000). (B). Western blotting analysis of exosomes surface markers (CD63,CD9, CD81, Alix, and GM130). (C,D). The expression of LncRNA in astrocytes and astrocyte-derived exosomes was detected by qRT-PCR. The exsome form supernatant of astrocytes, which from the normal astrocytes or transfected astrocytes were treated with SLE for 6 h, was used to treat microglia for 6h. (E). The expression of proinflammatory factors (such as TNF- α, IL-6 and IL-1 β) in microglia was detected. F. The activation of microglia labeled by CD68 and Iba-1 after overexpression of exosomes LncRNA49rik was detected by immunohistochemistry. NS P > 0.05,**P < 0.01, ***P < 0.001.N = 3.
Expression of LncRNA 4933431K23Rik increased in T10 with SCI
In order to explore the role of LncRNA49rik in SCI, we first established a rat model of SCI. The expression of LncRNA49rik in T10 spinal cord was detected by qRT-PCR at 0,3,7,14 and 21 days after SCI. The results showed that compared with sham group, the expression of LncRNA49rik increased significantly after SCI (P < 0.05,Fig. 2A). The activation of microglia in T10 spinal cord was detected by immunohistochemical method. The results showed that compared with the sham group, the number of Iba-1 labeled cells in the spinal cord of SCI injured rats increased gradually, meanwhile, the more process and bigger cell body in SCI rats (P < 0.05, Fig. 2B). Furthermore, the WB results showed that the expression of Iba-1 in T10 spinal cord of SCI rats increased with the time (P < 0.05, Fig. 2C). BV2 microglia were treated with LPS (100 ng/mL) for 6 h-14 days. At the same time, the expression of LncRNA49rik and Iba-1 in BV2 cells was detected. The results showed that compared with con group, the expression of LncRNA49rik and Iba-1 in LPS treated group increased gradually after treatment (P < 0.05,Fig. 2D,E). These findings suggest that LncRNA49rik is involved in the activation of microglia after SCI.
The increased expression of LncRNA 4933431K23Rik in T10 spinal cord. Firstly established the rat model of SCI. (A). The expression of LncRNA49rik in T10 spinal cord was detected by qRT-PCR at 0,3,7,14 and 21 days after spinal cord injury. (B). The activation of microglia in T10 spinal cord of rats with SCI was detected by immunohistochemistry. (C).The expression of Iba-1 in T10 spinal cord of rats with SCI was detected by WB. BV2 were treated with LPS (100ng/mL) for 6h-14 days. (D). The expression of LncRNA49rik in BV2 cells was detected by qRT-PCR. E. The expression of Iba-1 protein in BV2 cells was detected by WB. NS P > 0.05,**P < 0.01, ***P < 0.001.N = 3.
Overexpression of LncRNA 4933431K23Rik aggravates neuropathic pain in rats with SCI
In order to further study the function of LncRNA49rik in SCI, recombinant lentivirus LV-LncRNA49rik or LV-NC was injected into the spinal cord of SCI rats. Using qRT-PCR to detect the LncRNA49rik level in T10 spinal cord, we found that LncRNA49rik in SCI + LV-LncRNA49rik group was significantly higher than that in SCI + LV-NC group (P < 0.05,Fig. 3A). The activation of microglia was detected by immunohistochemistry and WB. The results showed that injection of LV-LncRNA49rik significantly increased the expression of Iba-1 labeled cells and Iba-1 in T10 spinal cord (P < 0.05,Fig. 3B,C). Then, the BBB motor score scale was used to monitor the neurological function. It was found that the BBB motor score of rats in SCI + LV-LncRNA49rik group was significantly lower than that in SCI + LV-NC group (P < 0.05,Fig. 3D). PWT and PWL were used to evaluate neuropathic pain. The results showed that after injection of LV-LncRNA49rik, the PWT and PWL of SCI + LV-LncRNA49rik rats were higher than those of SCI + LV-NC rats, indicating that the hyperalgesia was further enhanced (P < 0.05, Fig. 3E-F). These showed that the overexpression of LncRNA49rik in SCI increases the incidence of neuropathic pain.
Overexpression of LncRNA 4933431K23Rik aggravated neuropathic pain SCI rats. Recombinant LV-LncRNA49rik or LV-NC was injected into the spinal cord sheath of SCI rats. (A). The level of LncRNA49rik in T10 spinal cord was detected by qRT-PCR in 21d after SCI. (B,C). The activation of microglia was detected by immunohistochemistry and WB in 21d after SCI. (D). The neurological function of rats was monitored by BBB motor score scale in 21d after SCI. E.In the evaluation of pain-related behaviors, mechanical hypersensitivity reactions in each group were determined by the withdrawal threshold in 21d after SCI. F.Temperature hypersensitivity was analyzed by withdrawal threshold in 21d after SCI. NS P > 0.05,**P < 0.01, ***P < 0.001.N = 3.
Overexpression of LncRNA 4933431K23Rik promoted the inflammatory factors and oxidative stress in SCI
Firstly, the expression of COX2 and iNOS was evaluated by WB in vivo. The results showed that compared with sham group, the expression of COX2 and iNOS in SCI + LV-NC group was up-regulated. While, the expression of COX2 and iNOS in T10 spinal cord of SCI rats was further up-regulated in SCI + LV-LncRNA49rik group, as compared with that of SCI + LV-NC group (P < 0.05,Fig. 4A). ELISA results showed that compared with SCI + LV- NC group, the levels of IL-6, IL-1 β, IL-18, IFN-α and MDA in SCI + LV-LncRNA49rik group were significantly up-regulated, while the expression level of SOD was significantly down-regulated (P < 0.05,Fig. 4B-C). Oe-NC and oe-LncRNA49rik were transfected into LPS-treated microglia. Similarly in vivo, up-regulation of LncRNA49rik enhanced the expression of COX2, iNOS, IL-6, IL-1β, IL-18, IFN-α, MDA and decreased the expression level of SOD (P < 0.05,Fig. 4D-F). The results indicated that overexpression of LncRNA49rik aggravated the levels of inflammation and oxidative stress in SCI.
Overexpression of LncRNA 4933431K23Rik promoted inflammatory response and oxidative stress in SCI. Recombinant lentivirus LV-LncRNA49rik or LV-NC was injected into the spinal cord sheath of SCI rats, and the samples were collected at 21d after SCI. (A). The expression of COX2 and iNOS was evaluated by WB. (B,C).ELISA was used to detect the expression of inflammatory cytokines IL-6, IL-1β, IL-18, IFN-αand oxidative stress factors MDA and SOD in T10 spinal cord. (D,E). BV2 microglia were treated with LPS (100ng/mL) for 6h. Oe-NC and oe-LncRNA49rik were transfected into microglia treated with LPS. The expression of COX2, iNOS, IL-6, IL-1 β, IL-18, IFN- α, MDA and SOD was detected by WB and ELISA.NS P > 0.05,**P < 0.01, ***P < 0.001.N = 3.
MiR-10a-5P is the target of LncRNA 4933431K23Rik
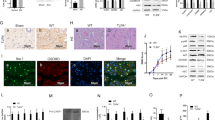
In the ENCORI (http://starbase.sysu.edu.cn/) database, we found that LncRNA49rik can target the 3 'UTR of miR-10a-5p (P < 0.05,Fig. 5A). The results of double luciferase activity assay showed that miR-10a-5p significantly inhibited the luciferase activity of microglia transfected with LncRNA49rik-WT, but had no significant effect on LncRNA49rik-MUT (P < 0.05,Fig. 5B). The results of Rip assay showed that after microglia were transfected with miR-10a-5p, the amount of LncRNA 4933431K23Rik precipitated by Ago2 antibody group was significantly higher than that in IgG group, suggesting that LncRNA49rik binds to Ago2 protein through miR-10a-5p (P < 0.05,Fig. 5C). Furthermore, the RNA FISH assay indicated a high degree of colocalization between LncRNA49rik and miR-10a-5p in BV2 cells (P < 0.05, Fig. 5D). Furthermore, qRT-PCR found that compared with the control group, overexpression of LncRNA49rik significantly inhibited the up-regulation of miR-10a-5p expression (P < 0.05, Fig. 5F). These results suggest that LncRNA49rik targets and inhibits the expression of miR-10a-5p.
MiR-10a-5P is the target of LncRNA 4933431K23Rik. (A): In the ENCORI (http://starbase.sysu.edu.cn/) database, the bounding relationship between LncRNA49rik and miR-10a-5p. (B,C): The targeting relationship between miR-10a-5p and LncRNA49rik was verified by double luciferase activity test and Rip experiment in microglia. (D):FISH revealed colocalization between miR-10a-5p and LncRNA49rik in microglia. LncRNA49rik probes were labeled with Alexa Fluor 555. MiR-10a-5p probes were labeled with Alexa Fluor 488. Nuclei were stained with DAPI. (E). The expression of miR-10a-5p after LncRNA49rik over expression was detected by qRT-PCR. NS P > 0.05,**P < 0.01, ***P < 0.001.N = 3.
Overexpression of miR-10a-5p inhibits inflammatory response and reduces NP in SCI rats
Previous studies have shown that there is a binding relationship between LncRNA49rik and miR-10a-5p, but the function of miR-10a-5p in SCI rats is not clear. Therefore, we injected LV-miR-10a-5p into the spinal cord sheath of SCI rats. Firstly, qRT-PCR was used to detect the mRNA expression of miR-10a-5p in T10 spinal cord of rats with SCI. The results showed that miR-10a-5p in SCI + LV-miR-10a-5p group was significantly higher than that in SCI + LV- NC group (P < 0.05, Fig. 6A). The activation of microglia in T10 spinal cord of was detected by immunohistochemistry and WB. The results showed that the number and protein level of Iba-1 cells in SCI + LV-miR-10a-5p group were lower than those in SCI + LV-NC group (P < 0.05, Fig. 6B,C). In addition, with the up-regulation of miR-10a-5p, the motor score of rats increased significantly (P < 0.05, Fig. 6D). Additionally, the PWT and PWL increased significantly (P < 0.05and Fig. 6E,F). The results of WB and ELISA showed that the levels of COX2, iNOS, IL-6, IL-1 β, IL-18, IFN-α and MDA in SCI + LV-miR-10a-5p group were significantly lower than those in SCI + LV-NC group, while the level of SOD in SCI + LV-NC group was significantly higher than that in SCI + LV-NC group (P < 0.05, Fig. 6G-I). These results suggest that overexpression of miR-10a-5p inhibits hyperalgesia and inflammation in SCI rats.
MiR-10a-5p overexpression suppressed inflammatory response in SCI rats and reduced neuropathic pain. LV-miR-10a-5p was injected into the spinal cord of SCI model rats. (A). QRT-PCR was used to detect the mRNA expression of miR-10a-5p in T10 spinal cord of rats with SCI on the 21st day after operation. (B,C). The activation of microglia in T10 with SCI was detected by immunohistochemistry and WB on the 21st day after operation. (D). The BBB movement scale was used to monitor the neurological function of rats. (E).Pain-related behaviors, mechanical hypersensitivity reactions were determined by the withdrawal threshold. F. Temperature hypersensitivity was analyzed by withdrawal threshold. (G–I). The expressions of COX2, iNOS, IL-6, IL-1 β, IL-18, IFN-α, MDA and SOD were detected by WB and ELISA on the 21st day after operation. NS P > 0.05,**P < 0.01, ***P < 0.001.N = 5.
Overexpression of miR-10a-5p attenuated the activation of microglia
In order to further study the specific mechanism of SCI and polarization of microglia, we added LncRNA49rik overexpression plasmid and/or miR-10a-5p mimic to BV2 induced by LPS. Firstly, the expression of LncRNA49rik and miR-10a-5p was detected by qRT-PCR assay, and it was found that compared with the LPS + NC group, the expression of LncRNA49rik was up-regulated while miR-10a-5p was down-regulated after overexpression of LncRNA49rik. Moreover, compared with LPS + miR-10a-5p group, the expression of LncRNA49rik was up-regulated and the expression of miR-10a-5p was down-regulated in LPS + LncRNA49rik + miR-10a-5p group (P < 0.05,Fig. 7A-B). Furthermore, the WB assay results showed that compared with LPS + NC group, the protein expression of COX2 and iNOS was significantly up-regulated after overexpression of LncRNA49rik, while COX2 and iNOS was significantly down-regulated after overexpression of miR-10a-5p. At the same time, compared with LPS + miR-10a-5p group, the protein expression of COX2 and iNOS in LPS + LncRNA49rik + miR-10a-5p group was significantly up-regulated (P < 0.05,Fig. 7C). Otherwise, the results also showed that compared with LPS + NC group, the levels of IL-6, IL-1 β, IL-18, IFN-α and MDA were significantly up-regulated and the levels of SOD were significantly down-regulated after overexpression of LncRNA49rik. After overexpression of miR-10a-5p, the levels of IL-6, IL-1β, IL-18, IFN-α and MDA were significantly down-regulated, and the level of SOD was significantly up-regulated. Compared with LPS + miR-10a-5p group, the levels of above-mentioned inflammatory factors and MDA in LPS + LncRNA49rik + miR-10a-5p group were significantly up-regulated, while the level of SOD was significantly down-regulated (P < 0.05,Fig. 7D-E). The results of WB showed that compared with LPS + NC group, the protein expression of MAPK/PI3K/AKT was up-regulated,while the mTOR was down-regulated after overexpression of LncRNA49rik. However, after overexpression of miR-10a-5p, the protein expression of MAPK/PI3K/AKT was down-regulated, but the mTOR was up-regulated. More importantly, compared with LPS + miR-10a-5p group, the protein expression of MAPK/PI3K/AKT was up-regulated and the protein expression of mTOR was down-regulated in LPS + LncRNA49rik + miR-10a-5p group (P < 0.05, Fig. 7F). These suggested that astrocyte-derived exosomes LncRNA49rik could regulate microglia phenotype in SCI through sponge miR-10a-5p.
Overexpression of miR-10a-5p attenuated the activation of microglia. LncRNA49rik overexpression plasmid and/or miR-10a-5p mimic transfected BV2 cell, were treated with LPS (100ng/mL) for 6h. (A,B). The expression of LncRNA49rik and miR-10a-5p was detected by qRT-PCR assay. C.The protein expression of COX2 and iNOS was detected by WB. (D,E). The expressions of IL-6, IL-1β, IL-18, IFN-α, MDA and SOD were detected by ELISA. (F).The protein expression of MAPK/PI3K/AKT was detected by WB. NS P > 0.05,**P < 0.01, ***P < 0.001.N = 3.
Discussion
NP is a common symptom in patients after SCI. Although great progress has been made in its treatment, there are still large amount of patients with chronic pain. The molecular mechanism of NP remains to be clarified. Some studies have confirmed that overactivation of spinal dorsal horn neurons after SCI can accelerate the progress of NP26. GilronI et al. found that inactivated astrocytes can reduce NP27. In addition, activation of microglia and astrocytes can also lead to excessive production of inflammatory mediators such as TNF-α, IL-1β and IL-6. Inhibition of microglial activation or astrocyte activation can effectively alleviate the progression of NP28,29. It is reported that LPS has been widely used to activate glial cells in vitro to mimic the SCI28,29. In this study, we used SCI to establish a rat model of NP and LPS in BV2 to build an model in vitro. The results found that the expression of LncRNA49rik was up-regulated in spinal cord tissue of SCI rats and microglia induced by LPS. In addition, LncRNA49rik activates MAPK/PI3K/AKT/mTOR pathway by sponging miR-10a-5p, which aggravates NP in SCI rats.
It is reported that astrocytes communicates with microglia via exosomes containing non-coding RNA30,31. In order to systematically understand the exosomes relationship between astrocytes and LncRNA, we detected the expression of LncRNA in astrocytes and exosomes derived from astrocytes. The results showed that 15 LncRNA were highly expressed in astrocytes and their derived exosomes, and the increase of LncRNA49rik expression was more significant than other LncRNA. Therefore, in order to further explore the biological function of LncRNA49rik, we further discussed this problem by generating exosomes containing LncRNA49rik.
Previous studies have shown that a variety of LncRNA are involved in the development of NP. For example, studies have found that the expression of LncRNASNHG1 is up-regulated in rats undergoing spinal nerve ligation, and inhibition of SNHG1 reduces NP32. At the same time, the LncRNAGAS5 was down-regulated in rats with chronic compressive injury, and overexpression of GAS5 inhibited the expression of inflammatory factors in spinal cord tissue and reduced NP33. In addition, LncRNASNHG1634 and LncRNADILC35 are all involved in the progress of NP. At present, our studies have shown that LncRNA49rik significantly increased the expression of SLE-induced pro-inflammatory cytokines in microglia and increased CD68 and Iba-1-labeled proinflammatory microglia, suggesting that LncRNA49rik may play a role in NP. Further studies found that exosomes LncRNA49rik increased significantly after SCI, and in vitro experiments also showed that exosomes LncRNA49rik was involved in the activation of microglia. Then, we found that overexpression of LncRNA49rik weakened the neurological function of rats, aggravated NP, promoted the inflammation and enhanced the oxidative stress.
We also found that miR-10a-5p is predicted to be the target of LncRNA49rik. It is reported that miR-10a-5p is associated with a variety of inflammatory diseases. Studies have found that inhibition of miR-10a-5p weakens the damage of cell and slows down the disease progression of acute pancreatitis36. In addition, the expression of miR-10a-5p is up-regulated in osteoarthritis tissues, which promotes the apoptosis of osteoarthritis chondrocytes by targeting HOXA137. More importantly, XuC et.al found that mesenchymal stem cells can up-regulate the expression of brain-derived neurotrophic factor (BDNF) by inhibiting miR-10a-5p, thus improving the therapeutic potential of mesenchymal stem cells in the treatment of SCI38. However, our research has produced different results, which is related to the different downstream targets of miR-10a-5p. In this study, we found that overexpression of miR-10a-5p attenuated the activation of microglia and inhibited hyperalgesia and inflammation in NP. In addition, miR-10a-5p was negatively regulated by LncRNA49rik, and the direct correlation between them has been verified in our investigation. More importantly, overexpression miR-10a-5p could reverse the induction of injury and inflammation induced by overexpression of LncRNA49rik.
Some mRNA transcripts are involved in the development of NP, such as MAPK and mTOR. Studies have shown that Formononetin inhibits microglial inflammation by targeting EGFR/MAPK pathway and participates in the repair of SCI39. In addition, in the rat model of chronic contractile injury, NP was alleviated by inhibiting the MAPK/ERK pathway. YeoJH et.al also found that inhibiting mTOR by regulating MKK4/MAPK pathway could reduce the activation of microglia and relieve trigeminal pain41. This study found that up-regulation of miR-10a-5p inhibited the expression of MAPK/PI3K/AKT and promoted the up-regulation of mTOR. In microglia, the protein expression of MAPK/PI3K/AKT was up-regulated while mTOR was down-regulated after overexpression of LncRNA49rik. Overexpression of miR-10a-5p reversed the effect of LncRNA49rik, which indicated that miR-10a-5p regulated MAPK/PI3K/AKT/mTOR was involved in the progression of NP.
In conclusion, this study firstly demonstrated the imbalance of LncRNA49rik and miR-10a-5p/MAPK/PI3K/AKT/mTOR derived from astrocytes and their clinical significance in SCI. It was found that LncRNA49rik promoted the phenotype of microglia in NP by regulating the miR-10a-5p/MAPK/PI3K/AKT/mTOR axis, and provided a new strategy for the treatment of SCI-induced NP. However, this study still has some limitations, such as the sample size is very limited, whether there are other related mechanisms, and so on, which we will further study in the follow-up experiments.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Ying, Ye. et al. Neuropathic Pain Induced by Spinal Cord Injury from the Glia Perspective and Its Treatment. Cell. Mol. Neurobiol. https://doi.org/10.1007/s10571-024-01517-x (2024).
Bouhassira, D. Neuropathic pain: Definition, assessment and epidemiology. Rev. Neurol. https://doi.org/10.1016/j.neurol.2018.09.016 (2019).
Rosenberger, D. C., Blechschmidt, V., Timmerman, H., Wolff, A. & Treede, R. D. Challenges of neuropathic pain: focus on diabetic neuropathy. J. Neural. Transm. https://doi.org/10.1007/s00702-020-02145-7 (2020).
Szok, D., Tajti, J., Nyári, A. & Vécsei, L. Therapeutic Approaches for Peripheral and Central Neuropathic Pain. Behav. Neurol. 21(2019), 8685954 (2019).
Yinuo, Liu,Xintong, Cai,Bowen, Shi et al. Mechanisms and Therapeutic Prospects of Microglia-Astrocyte Interactions in Neuropathic Pain Following Spinal Cord Injury. Mol. Neurobiol., (2024).
Anjum, A. et al. Spinal Cord Injury: Pathophysiology, Multimolecular Interactions, and Underlying Recovery Mechanisms. Int. J. Mol. Sci. 21(20), 7533 (2020).
Li, W. et al. Role of exosomal proteins in cancer diagnosis. Mol. Cancer. https://doi.org/10.1186/s12943-017-0706-8 (2017).
Kulkarni, B. et al. Exosomal miRNA in chemoresistance, immune evasion, metastasis and progression of cancer. Drug. Discov. Today. 24(10), 2058–2067. https://doi.org/10.1016/j.drudis.2019.06.010 (2019).
Zhang, L. et al. Exosomal lncRNA HOTAIR Promotes the Progression and Angiogenesis of Endometriosis via the miR-761/HDAC1 Axis and Activation of STAT3-Mediated Inflammation. Int. J. Nanomed. 16(17), 1155–1170. https://doi.org/10.2147/IJN (2022).
Zhang, J. K. et al. The BMSC-derived exosomal lncRNA Mir9-3hg suppresses cardiomyocyte ferroptosis in ischemia-reperfusion mice via the Pum2/PRDX6 axis. Nutr. Metab. Cardiovasc. Dis. 32(2), 515–527. https://doi.org/10.1016/j.numecd.2021.10.017 (2022).
He, X. et al. Astrocyte-derived exosomal lncRNA 4933431K23Rik modulates microglial phenotype and improves post-traumatic recovery via SMAD7 regulation. Mol. Ther. https://doi.org/10.1016/j.ymthe.2023.01.031 (2023).
Zhao, L. et al. The lncRNA SNHG5/miR-32 axis regulates gastric cancer cell proliferation and migration by targeting KLF4. FASEB J. 31(3), 893–903. https://doi.org/10.1096/fj.201600994R (2017).
Wu, J., Wang, C. & Ding, H. LncRNA MALAT1 promotes neuropathic pain progression through the miR-154–5p/AQP9 axis in CCI rat models. Mol. Med. Rep. 21(1), 291–303. https://doi.org/10.3892/mmr.2019.10829 (2020).
Dong, J., Xia, R., Zhang, Z. & Xu, C. lncRNA MEG3 aggravated neuropathic pain and astrocyte overaction through mediating miR-130a-5p/CXCL12/CXCR4 axis. Aging https://doi.org/10.18632/aging.203592 (2021).
Jiang, H. et al. LncRNA SNHG5 promotes chondrocyte proliferation and inhibits apoptosis in osteoarthritis by regulating miR-10a-5p/H3F3B axis. Connect Tissue Res. 62(6), 605–614. https://doi.org/10.1080/03008207.2020.1825701 (2021).
Cao, X. et al. Silencing long non-coding RNA MIAT ameliorates myocardial dysfunction induced by myocardial infarction via MIAT/miR-10a-5p/EGR2 axis. Aging https://doi.org/10.18632/aging.202785 (2021).
Spurgat, M. S. & Tang, S. J. Single-Cell RNA-Sequencing: Astrocyte and Microglial Heterogeneity in Health and Disease. Cells 11(13), 2021. https://doi.org/10.3390/cells11132021 (2022).
Xie, L. et al. Inflammatory factors and amyloid β-induced microglial polarization promote inflammatory crosstalk with astrocytes. Aging. https://doi.org/10.18632/aging.103663 (2020).
Li, S. et al. Microglial NLRP3 inflammasome activates neurotoxic astrocytes in depression-like mice. Cell. Rep. https://doi.org/10.1016/j.celrep.2022.111532 (2022).
Gilbert, O. et al. Serum lipid concentrations among persons with spinal cord injury - a systematic review and meta-analysis of the literature. Atherosclerosis 232(2), 305–312. https://doi.org/10.1016/j.atherosclerosis.2013.11.028 (2014).
Hu, Y. et al. Intrathecal Injection of Ropivacaine Reduces Cervical Resistance in Late-Pregnant Rats. Drug. Des. Devel. Ther. 26(16), 1183–1189. https://doi.org/10.2147/DDDT.S352411 (2022).
Junrui Jonathan, Hai,Weishi, Liang,Duan, Sun et al. Rutin Attenuates Distraction Spinal Cord Injury by Inhibiting Microglial Inflammation Through Downregulation of P38 MAPK/NF-κB/STAT3 Pathway,.Mol. Neurobiol., (2024).
Lolignier, S., Eijkelkamp, N. & Wood, J. N. Mechanical allodynia. Pflugers Arch. https://doi.org/10.1007/s00424-014-1532-0 (2015).
Nagasaka, K., Takashima, I., Matsuda, K. & Higo, N. Pharmacological inactivation of the primate posterior insular/secondary somatosensory cortices attenuates thermal hyperalgesia. Eur. J. Pain. 26(8), 1723–1731. https://doi.org/10.1002/ejp.1996 (2022).
Rekker, K. et al. Comparison of serum exosome isolation methods for microRNA profiling. Clin. Biochem. 47(1–2), 135–138. https://doi.org/10.1016/j.clinbiochem.2013.10.020 (2014).
Cavalli, E., Mammana, S., Nicoletti, F., Bramanti, P. & Mazzon, E. The neuropathic pain: An overview of the current treatment and future therapeutic approaches. Int. J. Immunopathol. Pharmacol. https://doi.org/10.1177/2058738419838383 (2019).
Gilron, I., Baron, R. & Jensen, T. Neuropathic pain: principles of diagnosis and treatment. Mayo Clin. Proc. 90(4), 532–545. https://doi.org/10.1016/j.mayocp.2015.01.018 (2015).
Jun-Xiang, Liao,Qi-Ming, Huang,Zhi-Cheng, Pan et al. The anti-inflammatory and immunomodulatory effects of olfactory ensheathing cells transplantation in spinal cord injury and concomitant pathological pain .Eur. J. Pharmacol., (2024).
Shixue, Huang,Yinuo, Zhang,Haoming, Shu et al. Advances of the MAPK pathway in the treatment of spinal cord injury .CNS Neurosci. Ther., (2024).
Ying, Ye,Hao, Cheng,Yan, Wang et al. Macrophage: A key player in neuropathic pain..Int. Rev. Immunol., (2024).
Cui, S. Y. et al. Knockdown of long non-coding RNA LEF1-AS1 attenuates apoptosis and inflammatory injury of microglia cells following spinal cord injury. J. Orthop. Surg. Res. 16(1), 6. https://doi.org/10.1186/s13018-020-02041-6 (2021).
Zhang, J. Y. et al. LncRNA SNHG1 attenuates neuropathic pain following spinal cord injury by regulating CDK4 level. Eur. Rev. Med. Pharmacol. Sci. 24(23), 12034–12040. https://doi.org/10.26355/eurrev_202012_23992 (2020).
Tian, Y., Sun, L. & Qi, T. Long noncoding RNA GAS5 ameliorates chronic constriction injury induced neuropathic pain in rats by modulation of the miR-452-5p/CELF2 axis. Can. J. Physiol. Pharmacol. 98(12), 870–877. https://doi.org/10.1139/cjpp-2020-0036 (2020).
Li, H., Fan, L., Zhang, Y., Cao, Y. & Liu, X. SNHG16 aggravates chronic constriction injury-induced neuropathic pain in rats via binding with miR-124-3p and miR-141-3p to upregulate JAG1. Brain Res. Bull. 165, 228–237. https://doi.org/10.1016/j.brainresbull.2020.09.025 (2020).
Liu, Y., Feng, L., Ren, S., Zhang, Y. & Xue, J. Inhibition of lncRNA DILC attenuates neuropathic pain via the SOCS3/JAK2/STAT3 pathway. Biosci. Rep. https://doi.org/10.1042/BSR20194486 (2020).
Huang, H. et al. Circ_0000284 Promoted Acute Pancreatitis Progression through the Regulation of miR-10a-5p/Wnt/β-Catenin Pathway. Chem. Biodivers. https://doi.org/10.1002/cbdv.202101006 (2022).
Ma, Y. et al. miR-10a-5p Promotes Chondrocyte Apoptosis in Osteoarthritis by Targeting HOXA1. Mol. Ther. Nucleic Acids. https://doi.org/10.1016/j.omtn.2018.12.012 (2019).
Xu, C. et al. MicroRNA-10a, -210, and -563 as circulating biomarkers for ossification of the posterior longitudinal ligament. Spine J. 19(4), 735–743. https://doi.org/10.1016/j.spinee.2018.10.008 (2019).
Fu, H. et al. Formononetin Inhibits Microglial Inflammatory Response and Contributes to Spinal Cord Injury Repair by Targeting the EGFR/MAPK Pathway. Immunol. Invest. https://doi.org/10.1080/08820139.2023.2183135 (2023).
Liu, M. et al. PACAP inhibition alleviates neuropathic pain by modulating Nav1.7 through the MAPK/ERK signaling pathway in a rat model of chronic constriction injury. Neuropeptides https://doi.org/10.1016/j.npep.2023.102327 (2023).
Yeo, J. H. & Roh, D. H. The mTOR inhibitor rapamycin suppresses trigeminal neuropathic pain and p-MKK4/p-p38 mitogen-activated protein kinase-mediated microglial activation in the trigeminal nucleus caudalis of mice with infraorbital nerve injury. Front Mol Neurosci. 14(16), 1172366 (2023).
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not- for- profit sectors.
Author information
Authors and Affiliations
Contributions
Changhui You,Mengying Hu,Bo Xu conceived and designed the experiments.Changhui You, Waiping Zhou,Ping Ye,Li Zhang,Wenchao Sun,Lili Tian,Bocheng Peng,Mengying Hu performed the experiments. Waiping Zhou,Ping Ye,Li Zhang,Wenchao Sun,Lili Tian,Bocheng Peng did the statistical analysis. Changhui You,Bo Xu wrote the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
Our study was approved by the Ethics Review Board of General Hospital of Southern Theatre Command of PLA.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
You, C., Zhou, W., Ye, P. et al. LncRNA 4933431K23Rik modulate microglial phenotype via inhibiting miR-10a-5p in spinal cord injury induced neuropathic pain. Sci Rep 15, 11620 (2025). https://doi.org/10.1038/s41598-025-91021-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-91021-z