Abstract
Diabetic kidney disease (DKD) is a main complication of diabetes mellitus. experimental in vitro validation and Network pharmacology were used in this study to explore the potential mechanism of Huangkui capsules (HKC) in treating DKD. First, we used CCK8 to analyze the optimal drug concentration of HKC. Next, we used flow cytometry, ELISA, Scratch test, and immunofluorescence to examine the apoptosis, oxidative stress, inflammatory factors, and fibrotic factors (FN and α-SMA) expression in HK-2 cells. Thereafter, in order to determine the potential molecular mechanisms underlying the therapeutic effect of HKC in DKD. Compounds contained in HKC were explored by UPLC-Q-TOF-MS/MS. SwissTargetPrediction was utilized for predicting potential gene targets of these compounds. OMIM, DisGeNet and GeneCards databases were employed to identify DKD-related genes. Meanwhile, the association of compounds with DKD genes was examined by protein-protein interaction, GO and KEGG analysis. Finally, molecular docking and molecular dynamics simulation were adopted for further validation. The results showed that HKC had 40 active ingredients, 1051 possible gene targets, and 133 DKD-HKC intersection genes. IL6, TNF, GAPDH, AKT1, PPARG, and TP53 were candidate hub genes by which HKC exerted its anti-DKD function based on molecular docking, molecular dynamics simulation and experimental results. To conclude, this study sheds more lights on the possible pharmacological activities of HKC in DKD and a foundation for further clinical application.
Similar content being viewed by others
Introduction
Diabetic kidney disease (DKD) is a frequently seen complication of diabetes mellitus and 25-40% of diabetics will experience DKD. Chronic hyperglycemia and insulin resistance in DKD patients induce renal oxidative stress, inflammatory damage, protein kinase pathway activation, and advanced glycation end products activation(AGEs), causing widespread glomerular fibrosis and sclerosis, and renal dysfunction1. A series of symptoms including proteinuria, edema, hypertension and even renal failure gradually appear. If poorly controlled, it will do harm to human health and gradually develop into chronic kidney disease2,3.
DKD is a main factor inducing kidney failure in the USA, occupying 47% of kidney failure cases in 20194.With the increasing aging population and the decreasing mortality related to diabetes complications, this will be a significant factor driving the increasing DKD incidence and mortality in the USA and globally5. Clinical treatment of DKD focuses more on the blood glucose control, urinary protein reduction, and renal microcirculatory improvement, etc. However, strict blood glucose/lipid control cannot change DKD outcome; besides, these treatments usually have chronic side effects6,7. Considering the limitations of current treatments, complementary and alternative medicine is the potential effective choice for intervening with DKD.
With the gradual deepening of research on traditional Chinese medicine (TCM) in recent years, the advantages of multi-target, and multi-link whereas fewer side effects of natural compounds on preventing and treating DKD have been discovered. Abelmoschus Manihot (L.) Medic (AM) is an annual or perennial herbaceous flowering plant, In addition to its edible value in daily life, AM is also crucial in the modern food industry. Abelmoschus manihot gum (AMG) can be isolated from the AM stems and roots, and is used as the stabilizer and thickener in the food industry8. Huangkui capsule (HKC), as an AM extract, possesses anti-inflammatory, antioxidant, free radical scavenging, renal tubule epithelial cell protection, and anti-fibrosis effects9,10,11. Recently, HKC has been widely adopted for the treatment of kidney disease. Numerous clinical trials have demonstrated that HKC significantly reduces levels of proteinuria and serum creatinine (Scr), while also improving insulin resistance (IR) and urinary microalbumin levels in patients with early DKD12,13,14. A meta-analysis showed that the combination of HKC and ACEI/ARB is considered a potential first choice intervention to reduce BUN levels in patients with DKD15. Furthermore, the therapeutic efficacy of HKC is likely due to the synergistic action of multiple bioactive compounds rather than isolated components. In the field of network pharmacology, the emphasis is on investigating the interconnected effects of various components on multiple targets within the biological network, rather than isolating individual compounds. Therefore, the introduction will concentrate on the comprehensive therapeutic properties of HKC, positioning it as a holistic treatment approach for DKD.
Due to the complexity of Chinese medicine and the lack of reference compounds, the research of Chinese medicine is hindered by a lot of problems. Ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry(UPLC-Q-TOF-MS/MS) technology can efficiently separate and characterize the components of TCM, which is considered as an accurate method for HKC analysis. In full scan mode, accurate mass number of group denominator ions and fragment ions can be obtained, while enhancing the reliability of analysis results. Because of its high selectivity and high sensitivity, it has been used in the analysis of other TCM and other natural medicines16.Network pharmacology is a new discipline to understand the interaction between drugs and diseases, which has outstanding advantages in the efficacy research, mechanism elucidation, target prediction and so on17.
Therefore, we used human proximal renal tubular epithelial cells (HK-2) as experimental model to investigate whether HKC can regulate the levels of inflammatory factors, oxidative stress status and fibrosis levels to attenuate HG-mediated damage, therefore protecting renal tubular epithelial cells18. UPLC-Q-TOF-MS/MS was adopted for analyzing HKC components, combined with network pharmacology technology for preliminary analysis of the HKC-DKD associated mechanism. Figure 1 displays the complete research procedure.
Methods and materials
In vitro experiment
Cell culture
HK-2 (iCell-h096) cells in iCell Bioscience Inc. (China) were obtained and cultivated in DMEM including 10% fetal bovine serum (FBS, FG701-01, TRANSGEN, China) in a 37 °C, 5% CO2 cell incubator (BB150, Thermo Scientific, USA).
The optimum HKC concentration in HK-2 cells selected by CCK-8 assay
To study the function of HKC in HK-2 cells, HK-2 cells were cultured in 5.5mmol/L normal glucose with/without 0.3, 0.6, 0.9, 1.2–1.5 g/L HKC for 24 and 48 h, respectively. Afterwards, the 96-well plates were introduced with HK-2 cell suspensions (1 × 105 cells/mL) after trypsinization, and then further cultured into the cell incubator. After 24 h and 48 h, cell treatment was performed according to each group as described above. Subsequently, cells were treated with CCK-8 reagent (10 µL, C0039, Beyotime, China) and put into the cell incubator for 2 h. Finally, a microplate reader (CMaxPlus, MD, USA) was used for determining absorbance (450 nm).
Cell grouping and apoptosis analysis
In order to analyze the function of HKC in HG-induced HK-2 cell injury, cells were categorized into four groups: (i) control group (Control): 5.5 mmol/L D-glucose; (ii) high-glucose group (HG): 30 mmol/L D-glucose; (iii) HKC group (HKC): 30 mmol/L D-glucose + 0.6 mg/ml HKC; and (iv)mannitol group (MG): 5.5 mmol/L D-glucose + 30 mmol/L mannitol. After a 48-h culture, the cells and supernatants were collected from each group. Apoptosis was identified by Annexin V-FITC/propidium iodide (PI) kit (556,547, MD, USA). The cells (1.2 × 106 cells/mL) were treated in line with corresponding groups. After cell harvesting, washing and centrifugation, cell concentration was adjusted with 1× binding buffer. Every sample tube was introduced with AnnexinV-FITC (5 µL) and PI (10 µL), followed by the 15-min treatment at 37 °C. After the addition of 1 × binding buffer to each tube, flow cytometry (C6-BD, USA) was performed to determine apoptosis.
Cell viability assay
The four groups, including control, HG, HKC, and mannitol groups were set as described above. Cells (5 × 103/well) were inoculated in the 96-well plates, followed by overnight incubation at 37 °C. On the following day, corresponding intervention was added for 48 h cell treatment. Then, every well was introduced with CCK-8 solution (10µL) for 2-h treatment. The spectrophotometer was used to determine absorbance at 450 nm.
Wound healing assay
HK-2 cells (5 × 105/well) were placed onto 6-well plates. Then, 3 scratches were made using the 200-µL sterile pipette tip on the cell monolayer. Later, cells were rinsed three times by PBS to remove cellular debris. Subsequently, the scratched area was photographed and monitored after 0 h and 24 h of treatment respectively. Finally, a microscope was utilized to monitor and photograph treated cells, while ImageJ software was applied to determine migration distance.
Determination of oxidative stress index and pro-inflammatory factors
Human tumor necrosis factor-alpha (TNF-α) (EK182), interleukin-1β (IL-1β) (EK101B), and IL-6 (EK106) kits were provided by Multi (Hangzhou, China). Malondialdehyde (MDA) Content Assay Kit was provided by Solarbio (Beijing, China). Human reduced glutathione (GSH) ELISA scientific research kit and Human superoxide dismutase (SOD) ELISA scientific research kit were acquired from Meimian (Jiangsu, China). Then, cell culture supernatants from four groups were harvested for 20-min centrifugation (2000–3000 rpm / min). IL-6, IL-1β, TNF-α, MDA, SOD and GSH contents were identified by taking and using the supernatant and Elisa.
Immunofluorescence assay
HK-2 cells (3 × 104/ml) were inoculated in the 6-well cover plate until reaching a 60% cell fusion rate. After different treatments, cells were fixed with 4% paraformaldehyde (P1110, Solarbio, China), followed by complete washing using phosphate buffer (PBS, FG701-01,TransGen, China) thrice.Thereafter, 0.5%TritonX-100 (P0096,Beyotime, China) was added for cell membrane permeation in every well, cells were blocked for 30 min using the blocking solution containing 3% BSA (B265993, Aladdin, China), followed by overnight reaction with α-SMA(1:200, AF1032, Affinity Biosciences LTD, CHINA) and fibronectin(FN) antibodies(1:200, AF5335, Affinity Biosciences LTD, CHINA) under 4 °C, as well as additional incubation with goat anti-rabbit IgG H&L (1:500, ab150080, abcam, UK) and reaction with DAPI. Finally, the cover glass was mounted and the results were observed using inverted fluorescence microscope (Ts2-FC, Nikon, Japan).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
To quantify gene expression levels, RT-qPCR was conducted. Total RNA was isolated from podocytes using a total RNA isolation kit following the manufacturer’s protocol. The extracted RNA was then used to synthesize cDNA with the Verso cDNA synthesis kit (Thermo Scientific) using 1 µg of RNA per sample. The RT-qPCR mixture contained cDNA templates, primers, and SYBR Green qPCR Master Mix (Thermo Scientific), and amplification was carried out with conditions of 95 °C for 30 s and 40 cycles. The results were analyzed using the ΔΔCt method to determine fold change compared to the control group, with β-actin as internal controls for mRNA normalization, respectively. Refer to Table 1 for primer details.
Pharmaceutical composition analysis
HKC were purchased from Jiangsu Suzhong Pharmaceutical Group. The composition of HKC was examined using UPLC-Q-TOF-MS/MS. First, the drug capsule shell is peeled off. Then, two solvents, methanol and methylene chloride, were used to extract the drug components. After drying at a low temperature, the extract was dissolved again with methanol. Finally, UPLC-Q-TOF-MS/MS was performed to study the resultant solution19.
UPLC-Q-TOF-MS/MS analysis procedure: The Waters e2695 Ultra-HPLC (Waters, USA) was used for LC separation using the C18 column (Fortis type, 7.5 × 250 mm, 1.8 μm; temperature 40 °C). Mobile phase was comprised by acetonitrile (A) and water (B) (containing 0.1% formic acid, v/v). Elution conditions were shown below: (1) 0–5 min, 1–5% B; (2) 5–55 min, 5–28% B; (3) 55–58 min, 28–40% B; (4) 58–63 min,40–95% B; (5) Run at 95% B until terminated. The sample injection volume and flow rate were 6 µl and 0.35 ml/min, respectively. The Thermo Velos mass spectrometer (Thermo, CA, United States) was utilized for mass spectrometry in the full-scan and both positive-negative modes.
Target prediction of potential active compounds
The 2D structure, 3D structure, InChI, and canonical SMILES profiles of drug ingredients were obtained in pubchem database(https://pubchem.ncbi.nlm.nih.gov/). Then, they were imported into SwissTarget Prediction platform (http://www.swisstargetprediction.ch/) for the prediction of compound targets. All chemical structure files were in SDF format. Besides, species were restricted to “Homo sapiens” to predict its targets.
DKD-related target screening
To find the relevant disease targets more comprehensively and accurately, information on DKD-associated target genes was collected from the following 3 databases: The human disease gene databases Genecards (http://www.genecards.org/), OMIM (https://omim.org/) and Disgenet (https://www.disgenet.org/). With “diabetic nephropathy” and “diabetic kidney disease” as the search terms, DKD-related targets were searched. In order to obtain all DKD-related targets, we summarized all acquired disease targets and removed duplicate values. Then, Wayne 2.1.0 platform was employed to acquire HKC-DKD cross targets.
Mapping the Protein-Protein interaction (PPI) network
The cross-target PPI networks were constructed and analyzed based on STRING 12.0 database (https://cn.string-db.org/). The species restriction was set to “Homo sapiens”, and the minimum interaction score was > 0.4 (greatest confidence level). Cytoscape 3.9.1 was used for PPI network plotting and visualization. To form an integrative PPI protein interaction map, the core network was filtered and refined in line with Degree, Closeness, and Betweenness.
GO and KEGG analyses
DAVID 6.8 platform was used for GO and KEGG analyses. Using HKC for DKD, the top 6 significantly enriched biological properties and the 10 significantly enriched pathways were visualized. GO and KEGG images were drawn with bioinformatics online website (http://www.bioinformatics.com.cn/)20.
Molecular Docking validation
Molecular docking was employed to investigate the association of HKC active compounds with HKC-DKD intersection genes derived from this study because it could predict the binding mode and affinity between receptors and ligands. Additionally, protein 3D structure was acquired based on Protein Database (PDB) (https://www.rcsb.org/). In addition, ingredient was downloaded in PubChem database to find the chemical and conformational content. Compounds and intersection genes were used as ligands and receptors, respectively. PyMOL (v 2.3.0) and AutoDockTools (v1.5.7) were used to demonstrate the key targets of herbal medicine and molecular docking of active components. Binding energy is the molecular docking result and is applied to evaluate the potential of ligand-receptor binding (typically, ≤−5 kcal/mol)21.
Molecular dynamics simulation analysis
Molecular dynamics simulation (MD) is an effective approach for determining the affinity of small compounds inside a binding site and investigating complex stability. It gives a temporal scale for conformational changes, allowing us to determine if the target ligand compound is stable or unstable. In this investigation, 50ns molecular dynamics simulations of GAPDH-Quercetin complexes acquired from molecular docking were done using Gromacs v2022.03 software, and the force field adopted from CHARMM36. The procedures and situations for molecular dynamics simulation were as follows: (1) The complex’s “pdb” format was converted to“gro”format, and the complex was treated as the initial structure for MD simulation, (2) The Generalized Amber Force Field force field is applied to Quercetin using AmberTools22 software. The Gaussian 16 W is used to execute hydrogenation and compute RESP potential operations on Quercetin, and the potential data is uploaded to the molecular dynamics system topology file. (3) The complexes were dissolved in a three-point transferable intermolecular potential solvent, with protein atoms at least 1.2 nm (12Å) from the water box edge. Meanwhile, the system charge was simulated by adding Na + and Cl- (concentration: 0.154 M), (4) To achieve a stable system, energy minimization is done out utilizing the steepest descent algorithm, (5) Following that, the solute was contained in an isothermal isovolumetric system by gradually heating the system from 0 K to 300 K, and then equilibrated in an isothermal isobaric system at a temperature of 300 K and a pressure of 1 Bar, (6) Finally, 50ns temporal molecular dynamics simulations were performed on the complexes, with the simulation traces preserved for further study. Based on the MD simulation findings, we estimated RMSD (root mean square deviation), RMSF (root mean square fluctuation), Rg (radius of gyration), SASA (solution accessible surface area), and hydrogen bonds (H-bonds) for the complexes. And ten, the RMSD and Rg value were used to compute Gibbs free energy using Gromacs v2022.03’s built-in “g_sham” and “xpm2txt.py” scripts. In addition, we used the “MMPBSA.py v.16.0” script to compute the molecular mechanics/Poisson-Boltzmann surface area to estimate the thermodynamic stability of complexes22,23.
Statistical analysis
SPSS 22.0 (SPSS, Inc., Chicago, IL, USA) was used to perform statistical analysis. For two-group analysis, we used Student’s t test, Among-group differences were compared by one-way ANOVA. Results were represented by mean ± standard deviation. P < 0.05 stood for indicated statistical significance.
Results
The results of cell experiments
The impact of HKC on HK-2 cell viability
HG-stimulated HK-2 cells have been widely used as cell models of DKD to evaluate the anti-inflammatory activity of drugs. Firstly, the impacts of HKC at varying concentrations on HK-2 cells treated for 24 h and 48 h were examined, respectively. It was found that the cell viability of HKC (0.6 g/L) increased significantly compared with the carrier group, and decreased when the concentration was higher than 1.2 g/L. Similar results were obtained after 48-h HKC treatments of HK-2 cells at varying concentrations. Therefore, 0.6 g/L HKC was chosen for follow-up experiment (Fig. 2).
HK-2 cells in 5 mmol/L normal glucose group were treated with or without varying concentrations of HKC (0.3, 0.6, 0.9, 1.2, 1.5 g/L) for 24 (A) and 48 h (B). CCK-8 assay was carried out to identify cell viability. Data are indicated by mean ± standard deviation (SD) from three independent experiments. Related to control group, ** p < 0.01. Related to HG group, ## p < 0.01.
HKC suppressed HG-induced HK-2 cell apoptosis
HK-2 cells were treated using HKC and/or high glucose. From Fig. 3, no significant change was found in HK-2 cell apoptosis in mannitol group, whereas HG treatment markedly promoted HK-2 cell apoptosis. Typically, we found that HKC could greatly reverse HG-induced HK-2 cell apoptosis.
HKC reduced MDA and pro-inflammatory cytokine levels and enhanced SOD and CAT activities in HG-induced HK-2 cells
The function of HKC in pro-inflammatory cytokines was examined; therefore, TNF-α, IL-1β, and IL-6 contents of HG group significantly increased when compared with control group (Fig. 4A–C). HKC treatment obviously decreased their levels induced by HG. Then, the function of HKC in oxidative stress was determined; thus, mannitol treatment made no difference to MDA content, SOD or CAT activity in relative to control group. Furthermore, the MDA content increased significantly and the SOD and CAT activities decreased significantly in HG-treated HK-2 cells, while these changes were weakened in HKC-treated HK-2 cells (Fig. 4D–F). The results demonstrate that HKC may suppress inflammation and oxidant stress.
The impact of HKC on HG-induced inflammatory cytokine generation(A–C) and oxidative stress (D–F) of HK-2 cells. A–F. The functions of HKC in IL-6, IL-1β, TNF-α, MDA, SOD and GSH levels were analyzed using enzyme linked immunosorbent assay (ELISA). Data are indicated by mean ± standard deviation (SD) from three independent experiments. Relative to control group, ** p < 0.01. Compared with HG group; ## p < 0.01.
HKC treatment reversed the HG-induced weakening of HK-2 cell migration ability
As shown in Fig. 5, relative to control group, HK-2 cell migration of HG group extremely significantly decreased (P < 0.01), that is, cell lateral migration ability was weakened; however, relative to HG group, HK-2 cell migration rate exhibits a significant increase after treatment with HKC(P > 0.05).
The enhancing effect of HG on up-regulating FN and α-SMA was mitigated by HKC intervention
FN and α-SMA are key genes which can reflect renal fibrosis. In Fig. 6, we examined FN and α-SMA levels through immunofluorescence assay, as a result, FN and α-SMA levels were up-regulated after HG treatment. Additionally, HKC treatment suppressed the HG-induced FN and α-SMA expression.
Qualitative study on the HKC chemical components based on UPLC-Q-TOF-MS/MS technology
UPLC-Q-TOF-MS/MS technology was performed to explore the active components of HKC. We eliminated some components that may have interference and have no relevant structures after searching the TCM database, TCM integrated database, TCM information database, and related literature. Finally, totally 40 chemical components of HKC were enrolled for further analysis. Details were presented in Supplementary Table S2.
Prediction of HKC targets and screening of DKD-associated targets
At first, the pubchem database was applied to find the Canonical SMILES of drug ingredients, potential targets of the HKC pharmacodynamic chemical components were obtained based on SwissTargetPrediction databases. The predicted targets of 40 drug ingredients were summarized. Then, the duplicates were removed. Finally, totally 611 final targets were obtained. Next, search findings were combined, and 1051 DKD-associated genes were obtained from Genecards, OMIM, and DisGeNet (Supplementary Table S3). Compound-target genes were intersected with disease-related genes to produce HKC target and DKD-associated gene set (Fig. 7).
Functional enrichment analysis of common targets
To explore the mechanisms related to the impact of HKC against DKD, GO annotation was performed on the 133 predicted targets, containing biological processes (BP), cellular components (CC) and molecular functions (MF)(Supplementary Table S4) .The GO results showed 808 GO terms, of which 614 were associated with BP, such as negative regulation of apoptotic process, positive regulation of gene expression, and positive regulation of protein kinase B signaling, 66 GO terms were related to CC, including macromolecular complex, receptor complex, and plasma membrane, and 128 GO terms were correlated with MF, including RNA polymerase II transcription factor activity, protein binding, and ligand-activated sequence-specific DNA binding (Fig. 8A). KEGG analysis was carried out at the significance level of p<0.01 on 133 potential anti-DKD targets of HKC(Fig. 8B). Those 10 most significant KEGG pathways included Lipid and atherosclerosis(hsa05417), Pathways in cancer(hsa05200), AGE-RAGE pathway in diabetic complications(hsa04933), Kaposi sarcoma-associated herpesvirus infection(hsa05167), EGFR tyrosine kinase inhibitor resistance(hsa01521), Hepatitis B(hsa05161), Prostate cancer(hsa05215), HIF-1 pathway(hsa04066), TNF pathway(hsa04668), and Proteoglycans in cancer(hsa05205).
PPI network analysis on 133 possible targets
To construct the PPI network, 133 potential anti-DKD targets of HKC were imported to the STRING database (Fig. 9). In addition, MCODE module in Cytoscape was carried out for clustering analysis to generate closely connected sub-networks. The network was consisted of 133 nodes and 2101 edges. Our analysis showed that the average degree value was 31.6, and 54 nodes had degree levels above the average. It was found that the top 6 targets (IL6、TNF、GAPDH、AKT1、PPARG and TP53) were potential hub genes related to the effect of HKC on DKD.
Molecular docking analysis
Molecular docking was applied to deeply explore the possibility of interaction between core targets (IL6, TNF, GAPDH, AKT1, PPARG and TP53) and key drug components of HKC. Our results suggest that the core targets have a strong affinity with ViscidulinI, Robinetin and quercetin (Fig. 10). Details were presented in Supplementary Rar S1. The smaller compound-target binding energy indicated the higher binding activity. Figure 11 shows the free binding energies of the components of HKC with the target.
MD simulation
MD simulation is a means of simulating the movement of small molecules in the body environment by computer. To confirm the ligand-receptor binding stability, we performed MD simulations using the GAPDH-Quercetin.
MD simulation of GAPDH-quercetin complex
To validate the ligand-receptor binding stability, we conducted 100ns MD simulations on the GAPDH-Quercetin complex generated from molecular docking. The results of the MD simulations, in particular the RMSD, RMSF, Rg value, SASA, and H-bonds, participate an important role in determining the stability of chemical compounds and proteins.
The RMSD was utilized to examine the mobility of the receptor-ligand complex, showing protein conformational changes. As shown in Fig. 12A, the RMSD curve of the GAPDH-Quercetin complex was in equilibrium at the 16-48ns stage system, with an average RMSD value of 0.35 nm. The RMSF can be utilized to illustrate variations in the complex at the residue level. As presented in Fig. 12B, RMSF analysis revealed increased flexibility of the GAPDH-Quercetin complex systems LYS194, ARG197, and SER288 during the 50ns simulation. The Rg value shows the degree of system constraint and binding tightness, as well as reflecting protein folding. A higher Rg value indicates a greater possibility of creating flexible ligands. Thus, the stability decreases as the Rg value increases. In contrast, a lower Rg value suggests a densely packed system. Figure 12C presented the Rg curve of the GAPDH-Quercetin complex progressively equilibrated the system after 20 ns, with the average Rg value being 2.07 nm. In addition, SASA analysis was performed to detect the degree of exposure of the receptor to surrounding solvent molecules during the simulation24. SASA research demonstrated that the GAPDH-Quercetin complex gradually equilibrated the system after 20 ns, with the average SASA value of 158 nm2 (Fig. 12D). Finally, Hydrogen Bonds (HBs), as a strong non-covalent interaction, play a role in facilitating the interaction between protein surface atoms and solvent molecules25. To further explore the frequency of hydrogen bonding in the GAPDH-Quercetin complex, we analyzed the hydrogen bonds formed by the GAPDH-Quercetin complex during the 50ns molecular simulations. The results indicated that the hydrogen bond numbers for the GAPDH-Quercetin complex was 1–5, respectively (Fig. 12E).
The 50ns MD simulation of the GAPDH-Quercetin complex. (A) The RMSD plot of the GAPDH-Quercetin complex. (B) The RMSF plot of the GAPDH-Quercetin complex. (C) The Rg plot of the GAPDH-Quercetin complex. (D) The SASA plot of the GAPDH-Quercetin complex. (E) The number of hydrogen bonds in the GAPDH-Quercetin complex.
Gibbs free energy analysis of GAPDH-quercetin complex
In this study, we utilized the built-in scripts “g_sham” and “xpm2txt.py” in Gromacs v2022.03 to calculate the Gibbs free energy of the GAPDH-Quercetin complex based on the RMSD and Rg values. Gibbs free energy and plotted with RMSD value, Rg value and Gibbs free energy to obtain Gibbs free energy 3D and 2D morphology maps. The Gibbs free energy maps depict the stability of the receptor-ligand combination. Regions with blue and purple hues indicate that the steady state conformation of the complex can be depicted at lower energies within the minimum free energy region. If the protein-ligand interaction is weak or unstable, multiple and surface-rough minimum energy clusters appear in the free energy landscape map; in contrast, strong and stable interactions can form nearly single and smooth energy clusters in the potential energy distribution. As shown in Fig. 13A,B, there is one single and sharp minimum energy region in the Gibbs free energy 3D and 2D landscape maps of the GAPDH-Quercetin complex. Therefore, visualization of the conformation of the GAPDH-Quercetin complex at the energy minimum moment (48ns) in this study revealed that the GAPDH-Quercetin complex could form 5 hydrogen bonds (Fig. 13C,D). Binding free energy calculations of GAPDH-Quercetin complex were presented in Supplementary Table S5. Amino acid binding free energy decomposition of the GAPDH-Quercetin complex were presented in Supplementary Figure S1.
Gibbs free energy analysis of GAPDH-Quercetin complex. (A, B) Gibbs free energy 3D and 2D landscape of the GAPDH-Quercetin complex, (C) Conformational global map of the energy minimum moment of the GAPDH-Quercetin complex, (D) 2D interaction map of the energy minimum moment of the GAPDH-Quercetin complex.
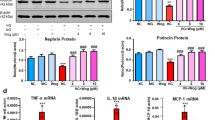
Effect of HKC on hub genes
We evaluated the effect of HKC on candidate hub genes in the high-glucose HK2 model using RT-qPCR. Our results indicated that HKC significantly reduced the expression levels of IL-6, TNF, AKT1, and TP53, while it increased the expression of PPARG and GAPDH (Fig. 14).
The effects of candidate hub genes of HKC on high glucose (HG)-induced HK-2 cells were evaluated using quantitative qPCR to measure the mRNA levels of IL6, TNF, GAPDH, AKT1, PPARG, and TP53 (A–F).Statistical significance was determined relative to the control group, with **p < 0.01 and ***p < 0.001.
Discussion
TCM is widely used to treat many disorders and its efficacy has been recognized in China. However, the underlying therapeutic mechanisms are not easy to clarify from a modern medical perspective. The reason may be that the ingredients of TCM are relatively complex and different active ingredients interact; in addition, the pharmacological functions are not just the superposition of single ingredients. Fortunately, network pharmacology provides the novel method for investigating the compatibility mechanism in TCM.
DKD can be frequently seen after diabetes, which is a major factor inducing chronic kidney disease across the world. Regardless of aggressive treatment, a large number of DKD patients inevitably progress to end-stage renal disease. As the complex disease, DKD involves multiple pathophysiological processes, which include the glomerular hypertension, glomerular hypertrophy, tubular basement membrane thickening, podocyte loss fibrosis, mesangial expansion, inflammation, and oxidative stress26,27. As a result, it is of great significance to search for anti-DKD drugs regulated by multi-target network. While numerous drugs are currently utilized in the management of DKD, including SGLT2 inhibitors and ACEI, each has its own limitations. Previous studies have demonstrated that HKC can improve DKD through various mechanisms, including the modulation of mitochondrial autophagy, inhibition of inflammatory body activation, and regulation of intestinal flora11,28,29. Our study, for the first time, utilizing network pharmacology and kinetic simulations, found that HKC can ameliorate the progression of DKD through multiple mechanisms. This study establishes a theoretical foundation for the application of HKC in the treatment of DKD.
Chronic hyperglycemia has been determined as a major triggering factor for the DKD pathophysiology, which leads to AGEs deposition within kidney tissue, therefore activating multiple signaling pathways, including protein kinase C, nrf2/keap1, and polyol pathways30,31,32,33. The signaling pathways drive renal inflammation, oxidative stress, and fibrosis, resulting in kidney injury or even kidney failure. In addition, oxidative stress induced by hyperglycemia contributes to producing reactive oxygen species(ROS), which can thus activate some pro-inflammatory pathways, like nuclear factor-κB and mitogen-activated protein kinase pathways34. The pathways accelerate the generation of chemokines, cytokines, and adhesion molecules for recruiting inflammatory cells into kidney tissue and amplifying inflammatory response and other processes to aggravate kidney damage35,36,37. ROS can trigger the STAT kinases activities (JAK2 and TYK2), while JAK/STAT pathway activation further promotes the excess glomerular mesangial cell proliferation, resulting in DKD38,39.
Hyperglycemia is a major factor which induces DKD occurrence. The results of this study confirmed that the HK-2 cell viability was weakened and that apoptosis elevated following HG stimulation, conforming to the findings proposed by Deng et al.40,41. The increases levels of inflammatory factors and ROS caused by hyperglycemia will accelerate DKD genesis and development42,43, and make a vital impact on inducing apoptosis and producing cytotoxicity44.
It was found in this study that co-incubation of HKC with HG could reverse the HG-mediated expression of fibrotic factors (FN and α-SMA) and change the expression of MDA, SOD, GSH, IL-6, IL-1β, and TNF-α in HK-2 cells. content. This indicates that the use of HKC can reduce the oxidative stress state, inflammatory factor content, and fibrosis levels of HG-treated HK-2 cells45,46,47. This shows that the use of HKC can reduce the oxidative stress state, inflammatory factor levels, and fibrosis levels in HG-induced HK-2 cells. During network pharmacology analysis, GO and KEGG analyses were performed on 23 intersection targets of HKC-DKD. The KEGG enrichment analysis revealed pathways related to lipid metabolism, atherosclerosis, and AGE-RAGE signaling, among others. The accumulation of AGEs associated with hyperglycemia is crucial in the pathogenesis of DKD. The interaction between AGE receptors and their ligands induces oxidative stress in renal tissue, resulting in chronic inflammation and fibrosis, which ultimately leads to renal dysfunction. Quercetin has the potential to reduce atherosclerotic lesions by modulating gut microbiota and decreasing atherogenic lipid metabolites. Additionally, Quercetin forms stable interactions with AKT1 and TNF, making it a potential therapeutic target for DKD. Quercetin may regulate these core pathways by targeting AKT1 and TNF, influencing AGEs and lipid metabolism. Therefore, it could be beneficial for the treatment of DKD48,49. Meanwhile, results consistent with the cell experiments were obtained. Therefore, pathways modulated via HKC effective ingredients are closely associated with inflammatory response, oxidative stress, and fibrosis pathways, consistent with other studies on DKD-related factors and the associated mechanisms9,10,11,50,51.
The main targets of HKC in treating DKD include AKT1, IL-6, TNF, GAPDH, TP53 and PPARG. Research has indicated that compared to α-lipoic acid, HKC reduces renal fibrosis by down-regulating phosphorylated Akt, phosphorylated p38MAPK, TNF-α, and TGF-β1 protein levels in kidney tissues in DKD model rats52. HKC inhibits the expression of NOX4 which is a major ROS source in the kidney, and excessive ROS will promote nuclear translocation of NF-κB, inducing the production of several inflammatory factors (including IL-6, IL-1β, and TNF-α) and adhesion molecules (including ICAM-1, VEGF, and VCAM-1), eventually aggravating inflammatory responses53. Inhibiting ROS/NF-κB/NLRP3 pathway may delay the development of progressive kidney injury by reducing the inflammation54. The study performed by Yang et al. discovered that NF-κB was further activated and TNF-α production increased during DKD; besides, podocyte damage was related to NF-κB/TNF-α pathway activation in macrophages43. As the NF-κB pathway downstream molecule, TNF-α makes a vital impact on DKD development as the primer inducer of renal micro-inflammation. The up-regulation of NF-κB and TNF-α expression induces podocyte damage and proteinuria of DKD55.
Molecular docking is a predictive tool that can reflect the strength of the interaction between drugs and target molecules. The results of molecular docking are evaluated mainly by minimum binding energy. The lower the binding energy, the less energy is required for the drug molecule to bind to the target, indicating a greater likelihood and stability of binding. For GAPDH with Quercetin, we found Quercetin formed conventional hydrogen bond with GLY10, ASN34, ASN9 and GLU79, formed pi-alkyl bond with PRO36, and formed pi-sigma bond with THR99. The results showed that GAPDH with Quercetin had relatively good binding activity. Utilizing RMSD, RMSF, Rg, SASA, and H-bonds, we demonstrated that the binding of Quercetin and GAPDH is remarkably stable through molecular dynamics simulations. The RMSD curves of the Quercetin and GAPDH complexes reached equilibrium during the 16–48 ns period. The Rg reflects the tightness of the binding, with higher Rg values positively correlating with increased flexibility of ligands. Consequently, higher Rg values correspond to lower stability. As illustrated in Fig. 12, the average Rg value of the Quercetin-GAPDH complex is 2.07 nm, indicating a high level of stability in their interaction. The number of hydrogen bonds is a critical index for assessing the binding stability between the protein and ligand. During the molecular dynamics simulation from 0 to 50 ns, the number of hydrogen bonds stabilized between 1 and 5. Fluctuation. RMSF primarily reflects the flexibility of amino acid residues in proteins, indicating increased flexibility in LYS194, ARG197, and SER288 within the Quercetin-GAPDH system.
DKD is characterized by excess ROS generation and destructed antioxidant defense mechanisms56. The antioxidant enzyme system is saturated after the production of excess ROS. As a result, the redundant ROS attacks membranes, enzymes, DNA, proteins, and lipids, resulting in cell injury and impairment. Inflammation and oxidative stress are closely associated with the DKD pathogenesis, and these two factors depend on each other57,58. The production of ROS contributes to recruiting numerous inflammatory cells and producing inflammatory factors, transcription factors, and growth factors associated with the progression of DKD into renal inflammation and fibrosis among diabetics59,60. GSH acts as an antioxidant scavenges oxygen free radicals under the action of glutathione peroxidase or glutathione transferase and is oxidized to GSSG at the same time; then, GSSG reduced to GSH under the action of glutathione reductase, forming an oxidation-reduction cycle61. Quercetin (one of the main components of HKC) increases the antioxidant capacity of the human body by regulating endogenous antioxidant contents which include glutathione, SOD and CAT62,63. In addition, the molecular docking and experimental results of this study also demonstrate this conclusion.
Our findings highlight that HKC exhibits significant antioxidant and anti-inflammatory properties, which may complement traditional therapies by offering additional renal protection through mechanisms such as reducing oxidative stress and inflammation. The natural origin and perceived safety profile of HKC may enhance patient compliance and acceptance, particularly among individuals who prefer herbal treatments to synthetic medications. The multifaceted approach of HKC, which addresses both hyperglycemia and nephropathy, may provide synergistic benefits that enhance overall diabetes management. The efficacy of HKC may vary significantly among individuals due to genetic, dietary, and lifestyle differences, potentially resulting in inconsistent outcomes across diverse patient populations. Although HKC appears to be well-tolerated, comprehensive long-term safety data and potential interactions with conventional drugs are necessary to fully validate its use in clinical settings. Current studies have primarily involved preclinical research or small sample sizes, necessitating larger, well-designed clinical trials to establish robust efficacy and safety profiles64.
This study investigated the therapeutic effects of HKC on DKD using network pharmacology that encompasses multi-component, multi-pathway, and multi-target approaches. These findings provide a scientific foundation for the clinical application of HKC in DKD treatment and establish a framework for potential combination therapies with other anti-DKD drugs in the future.
However, this study still has the following limitations. At first, some ingredients may have been neglected during the process of component identification because of their complex structures. Second, the network pharmacology database is under constant update. Therefore, the ingredient targets might not be completely included. Furthermore, this study analyzed the possible mechanism of HKC in the treatment of DKD via molecular docking and conventional experimental verification. In addition, further animal experiments and clinical research verification should be carried out to study the possible molecular mechanism of HKC in treating DKD.
Conclusions
Totally 40 HKC chemical components were analyzed with UPLC-Q-TOF-MS/MS technique. It was concluded that therapeutic targets for HKC against DKD are screened comprehensively via network pharmacology, molecular docking simulation, and in vitro cell evaluations. HKC can reduce inflammation, oxidative stress and renal fibrosis by regulating the levels of AKT1 and other genes. These findings comprehensively indicate the potential therapeutic targets for HKC against DKD and provide a solid foundation for future application. Additionally, relevant studies can offer a novel direction for the development of natural medicines.
Data availability
Data is provided within the manuscript .
References
Torban, E. et al. From podocyte biology to novel cures for glomerular disease. Kidney Int. 96(4), 850–861 (2019).
Jung, C. Y. & Yoo, T. H. Pathophysiologic mechanisms and potential biomarkers in diabetic kidney disease. Diabetes Metab J. 46(2), 181–197 (2022).
Anders, H. J., Huber, T. B., Isermann, B. & Schiffer, M. CKD in diabetes: Diabetic kidney disease versus nondiabetic kidney disease. Nat Rev Nephrol 14(6), 361–377 (2018).
Ríos Burrows, N., Koyama, A. & Pavkov, M. E. Reported cases of end-stage kidney disease-United States, 2000–2019. Am. J. Transplant. 22(5), 1483–1486 (2022).
McCullough, K. P., Morgenstern, H., Saran, R., Herman, W. H. & Robinson, B. M. Projecting ESRD incidence and prevalence in the United States through 2030. J. Am. Soc. Nephrol. 30(1), 127–135 (2019).
Chen, X. et al. Klotho-derived peptide 6 ameliorates diabetic kidney disease by targeting Wnt/β-catenin signaling. Kidney Int. 102(3), 506–520 (2022).
Flyvbjerg, A. The role of the complement system in diabetic nephropathy. Nat. Rev. Nephrol. 13(5), 311–318 (2017).
Yuan, D. et al. In-depth insight into the mechanism of incorporation of Abelmoschus manihot gum on the enhancement of gel properties and in vitro digestibility of frankfurters. Foods 12(7), 1507 (2023).
Wu, W. et al. Inhibition of Akt/mTOR/p70S6K signaling activity with Huangkui capsule alleviates the early glomerular pathological changes in diabetic nephropathy. Front. Pharmacol. 9, 443 (2018).
Ge, J., Miao, J. J., Sun, X. Y. & Yu, J. Y. Huangkui capsule, an extract from Abelmoschus manihot (L.) medic, improves diabetic nephropathy via activating peroxisome proliferator-activated receptor (PPAR)-α/γ and attenuating endoplasmic reticulum stress in rats. J. Ethnopharmacol. 189, 238–249 (2016).
Han, W. et al. Huangkui capsule alleviates renal tubular epithelial-mesenchymal transition in diabetic nephropathy via inhibiting NLRP3 inflammasome activation and TLR4/NF-κB signaling. Phytomedicine: Int. J. Phytotherapy Phytopharmacol. 57, 203–214 (2019).
Zhang, L. et al. Efficacy and safety of Abelmoschus manihot for primary glomerular disease: A prospective, multicenter randomized controlled clinical trial. Am. J. Kidney Dis 64(1), 57–65 (2014).
Chen, Y. Z. et al. Efficacy and safety of Flos Abelmoschus manihot (Malvaceae) on type 2 diabetic nephropathy: A systematic review. Chin. J. Integr. Med. 21(6), 464–472 (2015).
Wu, W. et al. Multi-targeted therapeutic effects of Huangkui Capsules on insulin resistance and urine microalbumin in early diabetic kidney disease patients. Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China journal of Chinese materia medica 45(23), 5797–5803 (2020).
Zhao, J., Ai, J., Mo, C., Shi, W. & Meng, L. Comparative efficacy of seven Chinese patent medicines for early diabetic kidney disease: A Bayesian network meta-analysis. Complement. Ther. Med. 67, 102831 (2022).
Xu, L. et al. Rapid identification of chemical profile in Gandou decoction by UPLC-Q-TOF-MS(E) coupled with novel informatics UNIFI platform. J. Pharm. Anal. 10(1), 35–48 (2020).
Li, X. et al. Network pharmacology approaches for research of traditional Chinese medicines. Chin. J. Nat. Med. 21(5), 323–332 (2023).
Valdés, A. et al. Comprehensive metabolomic study of the response of HK-2 cells to hyperglycemic hypoxic diabetic-like milieu. Sci. Rep. 11(1), 5058 (2021).
Shen, Z. J. et al. Integrating network pharmacology, UPLC-Q-TOF-MS and molecular docking to investigate the effect and mechanism of Chuanxiong Renshen decoction against Alzheimer’s disease. Chin. Med. 17(1), 143 (2022).
Kanehisa, M., Furumichi, M., Sato, Y., Kawashima, M. & Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 51(D1), D587-d592 (2023).
Liu, J. et al. Network pharmacology prediction and molecular docking-based strategy to discover the potential pharmacological mechanism of Huai Hua san against ulcerative colitis. Drug Des. Devel. Ther. 15, 3255–3276 (2021).
Genheden, S. & Ryde, U. The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert. Opin. Drug. Discov. 10(5), 449–461 (2015).
Donnelly, S. M., Lopez, N. A. & Dodin, I. Y. Steepest-descent algorithm for simulating plasma-wave caustics via metaplectic geometrical optics. Phys. Rev. E 104(2–2), 025304 (2021).
Sharma, P., Joshi, T., Joshi, T., Chandra, S. & Tamta, S. Molecular dynamics simulation for screening phytochemicals as α-amylase inhibitors from medicinal plants. J. Biomol. Struct. Dyn. 39(17), 6524–6538 (2021).
Zhou, H., Bie, S., Li, J., Yuan, L. & Zhou, L. Comparison on inhibitory effect and mechanism of inhibitors on sPPO and mPPO purified from “Lijiang snow” peach by combining multispectroscopic analysis, molecular docking and molecular dynamics simulation. Food Chem. 400, 134048 (2023).
Han, Q., Zhu, H., Chen, X. & Liu, Z. Non-genetic mechanisms of diabetic nephropathy. Front. Med. 11(3), 319–332 (2017).
Kwon, G. et al. A novel pan-Nox inhibitor, APX-115, protects kidney injury in streptozotocin-induced diabetic mice: possible role of peroxisomal and mitochondrial biogenesis. Oncotarget 8(43), 74217–74232 (2017).
Shi, R. et al. Abelmoschus Manihot ameliorates the levels of circulating metabolites in diabetic nephropathy by modulating gut microbiota in non-obese diabetes mice. Microbial Biotechnol. 16(4), 813–826 (2023).
Zhu, Z. et al. Huangkui capsule attenuates diabetic kidney disease through the induction of mitophagy mediated by STING1/PINK1 signaling in tubular cells. Phytomedicine: Int. J. Phytotherapy Phytopharmacol. 119, 154975 (2023).
Fioretto, P. & Mauer, M. Histopathology of diabetic nephropathy. Semin. Nephrol. 27(2), 195–207 (2007).
Liu, Y. et al. Network pharmacology-based study on the mechanism of ShenKang injection in diabetic kidney disease through Keap1/Nrf2/Ho-1 signaling pathway. Phytomedicine 118, 154915 (2023).
Tanase, D. M. et al. Oxidative stress and NRF2/KEAP1/ARE pathway in diabetic kidney disease (DKD): New perspectives. Biomolecules 12(9), 1227 (2022).
Lu, Q. et al. Empagliflozin attenuates the renal tubular ferroptosis in diabetic kidney disease through AMPK/NRF2 pathway. Free Radic. Biol. Med. 195, 89–102 (2023).
Giacco, F. & Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 107(9), 1058–1070 (2010).
Wada, J. & Makino, H. Inflammation and the pathogenesis of diabetic nephropathy. Clin. Sci. (Lond.) 124(3), 139–152 (2013).
Baynes, J. W. & Thorpe, S. R. Role of oxidative stress in diabetic complications: A new perspective on an old paradigm. Diabetes 48(1), 1–9 (1999).
Ceriello, A. et al. Defective intracellular antioxidant enzyme production in type 1 diabetic patients with nephropathy. Diabetes 49(12), 2170–2177 (2000).
Simon, A. R., Rai, U., Fanburg, B. L. & Cochran, B. H. Activation of the JAK-STAT pathway by reactive oxygen species. Am. J. Physiol. 275(6), C1640-1652 (1998).
Marrero, M. B., Banes-Berceli, A. K., Stern, D. M. & Eaton, D. C. Role of the JAK/STAT signaling pathway in diabetic nephropathy. Am. J. Physiol. Renal. Physiol. 290(4), F762-768 (2006).
Deng, J. et al. Diosmin mitigates high glucose-induced endoplasmic reticulum stress through PI3K/AKT pathway in HK-2 cells. BMC Complement. Med. Ther. 22(1), 116 (2022).
Bernardo-Bermejo, S. et al. A non-targeted capillary electrophoresis-mass spectrometry strategy to study metabolic differences in an in vitro model of high-glucose induced changes in human proximal tubular HK-2 cells. Molecules 25(3), 512 (2020).
Xiang, E. et al. Human umbilical cord-derived mesenchymal stem cells prevent the progression of early diabetic nephropathy through inhibiting inflammation and fibrosis. Stem. Cell. Res. Ther. 11(1), 336 (2020).
Yang, H. et al. Tim-3 aggravates podocyte injury in diabetic nephropathy by promoting macrophage activation via the NF-κB/TNF-α pathway. Mol. Metab. 23, 24–36 (2019).
Tuttle, K. R. et al. Molecular mechanisms and therapeutic targets for diabetic kidney disease. Kidney Int 102(2), 248–260 (2022).
Noonin, C., Itsaranawet, T. & Thongboonkerd, V. Calcium oxalate crystal-induced secretome derived from proximal tubular cells, not that from distal tubular cells, induces renal fibroblast activation. Eur. J. Med. Res. 28(1), 150 (2023).
Islam, M. N. et al. Human mesenchymal stromal cells broadly modulate high glucose-induced inflammatory responses of renal proximal tubular cell monolayers. Stem. Cell. Res. Ther. 10(1), 329 (2019).
El-Lateef, A. E. A., El-Shemi, A. G. A., Alhammady, M. S., Yuan, R. & Zhang, Y. LncRNA NEAT2 modulates pyroptosis of renal tubular cells induced by high glucose in diabetic nephropathy (DN) by via miR-206 regulation. Biochem. Genet. 60(5), 1733–1747 (2022).
Nie, J., Zhang, L., Zhao, G. & Du, X. Quercetin reduces atherosclerotic lesions by altering the gut microbiota and reducing atherogenic lipid metabolites. J. Appl. Microbiol. 127(6), 1824–1834 (2019).
Zhang, M. Y. & Zheng, S. Q. Network pharmacology and molecular dynamics study of the effect of the Astragalus-Coptis drug pair on diabetic kidney disease. World J. Diabetes 15(7), 1562–1588 (2024).
Gu, L. F. et al. Huangkui capsule ameliorates renal fibrosis in a unilateral ureteral obstruction mouse model through TRPC6 dependent signaling pathways. Front. Pharmacol. 11, 996 (2020).
Liu, B. H. et al. Total flavones of abelmoschus manihot ameliorates podocyte pyroptosis and injury in high glucose conditions by targeting METTL3-dependent m(6)A modification-mediated NLRP3-inflammasome activation and PTEN/PI3K/Akt signaling. Front. Pharmacol. 12, 667644 (2021).
Mao, Z. M. et al. Huangkui capsule attenuates renal fibrosis in diabetic nephropathy rats through regulating oxidative stress and p38MAPK/Akt pathways, compared to α-lipoic acid. J. Ethnopharmacol. 173, 256–265 (2015).
Cepas, V., Collino, M., Mayo, J. C. & Sainz, R. M. Redox signaling and advanced glycation endproducts (AGEs) in diet-related diseases. Antioxidants (Basel) 9(2), 142 (2020).
Xu, X. et al. Adiponectin protects obesity-related glomerulopathy by inhibiting ROS/NF-κB/NLRP3 inflammation pathway. BMC Nephrol. 22(1), 218 (2021).
Zhu, W. et al. Regulation of renal lipid deposition in diabetic nephropathy on morroniside via inhibition of NF-KB/TNF-a/SREBP1c signaling pathway. Chem. Biol. Interact. 385, 110711 (2023).
Jha, J. C., Banal, C., Chow, B. S., Cooper, M. E. & Jandeleit-Dahm, K. Diabetes and kidney disease: Role of oxidative stress. Antioxid. Redox Signal 25(12), 657–684 (2016).
Rayego-Mateos, S. et al. Pathogenic pathways and therapeutic approaches targeting inflammation in diabetic nephropathy. Int. J. Mol. Sci. 21(11), 3798 (2020).
Biswas, S. K. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox?. Oxid. Med. Cell Longev. 2016, 5698931 (2016).
Jha, J. C. et al. Independent of renox, NOX5 promotes renal inflammation and fibrosis in diabetes by activating ROS-sensitive pathways. Diabetes 71(6), 1282–1298 (2022).
Jha, J. C., Ho, F., Dan, C. & Jandeleit-Dahm, K. A causal link between oxidative stress and inflammation in cardiovascular and renal complications of diabetes. Clin. Sci. (Lond.) 132(16), 1811–1836 (2018).
Ferreira, M. J. et al. The mammalian peroxisomal membrane is permeable to both GSH and GSSG - Implications for intraperoxisomal redox homeostasis. Redox Biol. 63, 102764 (2023).
Kobori, M. et al. Chronic high intake of quercetin reduces oxidative stress and induces expression of the antioxidant enzymes in the liver and visceral adipose tissues in mice. J. Funct. Foods 15, 551–560 (2015).
Yu, J. H. et al. Quercetin alleviates lipopolysaccharide-induced cardiac inflammation via inhibiting autophagy and programmed cell death. Biomed. Environ. Sci. 37(1), 54–70 (2024).
An, W. et al. Efficacy and safety of Huangkui capsule for diabetic nephropathy: A protocol for systematic review and meta-analysis. Medicine 100(42), e27569 (2021).
Funding
Suported by Scientific Research Fund of Anhui Provincial Education Department (No.2024AH050711).
Author information
Authors and Affiliations
Contributions
J.L.: Conceptualization, writing—original draft, resources, Supervision, Data curation, project administration; Z.L.: Writing—review and editing; formal analysis; Z.Z.: visualization; Z.S.: Conceptualization, methodology, investigation. All authors have read and agreed to the published version of the manuscript.
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, J., Li, Z., Zhang, Z. et al. Uncovering the mechanism of Huangkui capsule in the treatment of diabetic kidney disease based on network pharmacology and experimental validation. Sci Rep 15, 6503 (2025). https://doi.org/10.1038/s41598-025-91264-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-91264-w