Abstract
In pediatric liver recipients perioperative factors may affect respiratory and cardiac function, and prolong mechanical ventilation during post-operative period. The use of NAVA can improve the interaction between the patient and the ventilator from both a respiratory and cardiac perspective. The objective of this study is to evaluate the synchronization between the patient and the ventilator, as well as cardiac function, during the application of neurally adjusted ventilatory assist (NAVA) and pressure support ventilation (PSV) in pediatric liver transplant recipients. This is a single-center, prospective, randomized, physiological cross-over controlled trial conducted between 2021 and 2022. Children (1 month-10 years old) who underwent liver transplantation were admitted to the pediatric intensive care unit. Patients were randomised to one of two crossover sequences of ventilation trials of 40 min each (PSV/NAVA/PSV or NAVA/PSV/NAVA). Cardiac function was studied by echocardiogram. Twenty-four patients were enrolled and 21 completed the study. Primary outcomes were variation of asynchrony index (AI) and tricuspid annular plane systolic excursion (TAPSE) during the two ventilation modes. Secondary outcomes were patient-ventilator interaction parameters, gas exchange, left and right ventricular function, and hemodynamic parameters. NAVA compared to PSV: (1) improves patient-ventilator interaction reducing AI (coeff − 6.66 95% CI −11.5 to −1.78, p = 0.008); (2) does not improve TAPSE (coeff 0.62 95% CI −1.49 to 2.74, p < 0.557) No differences in terms of pulmonary gas exchange and hemodynamic parameters were detected. NAVA (when compared to PSV) improves patient-ventilator interaction in terms of asynchronies without affecting cardiac biventricular function.
Trial registration: NCT 04792788, Registration date: 2021-03-11.
Similar content being viewed by others
Introduction
Liver transplantation (LT) is the treatment of choice for acute/chronic end-stage liver disease1. In the postoperative period several risk factors such as intraoperative fluid administration, total transfusion volume, large graft size, ascites, and pleural effusion negatively affect cardiac and pulmonary function2,3 prolonging the time of dependence from mechanical ventilation.
(MV). In addition, intra-operative factors such as bilateral transection of the abdominal muscles, pain stimulation and the presence of a mesh affect the recovery of spontaneous respiratory function4. In pediatric liver recipients diaphragmatic dysfunction is associated with prolonged ventilation and pediatric intensive care unit (PICU) stay5,6. During weaning from MV, an ideal support mode should improve patient-ventilator interaction while minimizing asynchronies and detrimental hemodynamic effects induced by rising intrathoracic pressure7. Pressure support ventilation (PSV) is one of the most adopted modes of assisted spontaneous breathing to efficiently unload respiratory muscle8,9. However, PSV delivers a fixed level of assistance that is associated to higher occurrence of patient-ventilator asynchronies10,11. Neurally Adjusted Ventilatory Assist (NAVA) is a mode of assisted spontaneous ventilation that delivers positive pressure in proportion to electrical activity of the diaphragm (EAdi) thus optimizing ventilator cycling12 and reducing incidence of patient-ventilator asynchronies as compared to PSV13,14. Moreover, synchronization between positive pressure during inspiration and electrical diaphragm activity (Eadi) during NAVA leads to a reduction of pleural pressure and reduces the negative effects on cardiovascular function15. Advanced hemodynamic monitoring is essential to evaluate hemodynamic status, especially during the perioperative period of high-risk surgery16,17. Echocardiography is the most common noninvasive method to assess cardiac function18. The combination of cardiovascular and respiratory effects during NAVA is not well established, especially after major abdominal surgery as liver transplantation where several factors negatively affect respiratory system mechanics and diaphragm function. To the best of our knowledge the physiologic effects of proportional assisted mode of MV compared to PSV have not been investigated in pediatric liver recipients. The aim of this study is to evaluate patient ventilator interaction and cardiac function at the first transition to spontaneous assisted breathing of pediatric patients who underwent liver transplantation. We hypothesized that, compared to PSV, NAVA minimizes patient-ventilator asynchronies and improves cardiac performance.
Methods
Study ethics
The Use of Neurally Adjusted Ventilator Assist versus Pressure Support Ventilation During Weaning from Mechanical Ventilation in Pediatric Patients After Liver Transplantation (NAVIGATE) protocol was approved by institutional research board at Bambino Gesù Children’s Hospital (document 1695_OPBG_2018, March 19, 2019).
All research was performed in accordance with relevant guidelines/regulations, and written informed consent was obtained from parents/legal guardian. The study has been performed in accordance with the Declaration of Helsinki. No organs/tissues were procured from prisoners.
This is a single center, randomized, no profit, physiologic cross-over controlled trial comparing NAVA with PSV in children underwent liver transplantation. This study follows CONSORT recommendations.
The study was registered on ClinicalTrials.gov (Clinical Trial Number: NCT04792788, Registration date: 2021-03-11).
Protocol
All the enrolled patients were admitted to PICU in Bambino Gesù Children’s Hospital, Rome (Italy). Inclusion criteria were: (1) age between one month to 10 years of age; (2) liver recipients; (3) invasive mechanical ventilation. Exclusion criteria were: (1) neurological impairment; (2) hypotonia (by neuromuscular, mitochondrial, metabolic, or chromosomal diseases); (3) lesions of medulla; (4) hemodynamic instability requiring inotropes/vasopressors (dopamine > 6 mcg/kg/min, norepinephrine > 0.1 mcg/kg/min, epinephrine > 0.1 mcg/kg/min, dobutamine > 6 mcg/kg/min, milrinone > 0.35 mcg/kg/min) or almost one volume bolus (crystalloids/colloids > 20 ml/kg) during the past 6 h; (5) congenital cardiovascular disease; (6) patient extubated; (7) respiratory instability (paO2/FiO2 < 200; SpO2 < 90% with FiO2 0.4); (8) need of controlled mechanical ventilation; (9) intravenous infusion of benzodiazepines or propofol; (10) pneumonia, pneumothorax, massive pleural effusion; 11) patient placed on extracorporeal circuit (continuous renal replacement therapy, extracorporeal membrane oxygenation, apheresis); 12) contraindications to insert nasogastric tube; 13) not expected to survive beyond 24 h; 14) parental/legal guardian refusal.
After enrollment, the standard nasogastric tube of each patient was replaced with a specific nasogastric tube (Edi catheter) with an array of eight bipolar electrodes mounted at its distal end (Getinge Critical Care, Solna, Sweden). The description of verification of nasogastric tube placement is explained in Additional Material S1.
Hemodynamic monitoring was performed by mathematical analysis of the arterial waveform analyzed through an arterial catheter placed in the radial, brachial, or femoral artery and recorded according to the PRAM (Pressure Recording Analytical Method) pressure analysis method (MostCare Vygon, Vytech, Italy). The PRAM is an algorithm that processes arterial pressure waveforms with high temporal resolution (1000 Hz) and does not require external calibration. This method derives cardiac index and other advanced hemodynamic parameters by evaluating the interplay between vascular resistance and arterial compliance in real time. It enables continuous and accurate monitoring of cardiovascular dynamics19. Transthoracic echocardiogram performed by two expert pediatric cardiologists completed the hemodynamic monitoring during the period study. The images of the transthoracic echocardiograms of all enrolled patients were recorded and stored on a dedicated computer.
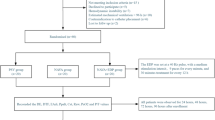
The randomization process consisted of a computer-generated random listing of treatment allocation using block sizes of 4 (Three Randomization Plan Generators. Available from URL: https://gdallal.pages.tufts.edu/random_block_size.htm). The randomization started when the enrolled patients started spontaneously triggering the mechanical ventilator. Patients were ventilated in pressure regulated volume control before starting assisted spontaneous breathing. To initiate weaning from MV, the patient had to mantain a PaO2/FiO2 ratio > 200 and SpO2 > 90% with FiO2 < 0.4 and PEEP 4–5 cm H2O. Each patient was randomized to a ventilation mode sequence (PSV/NAVA/PSV or NAVA/PSV/NAVA).
Each patient was studied for a duration of 2 h, divided in three trials of 40-minutes (Fig. 1). The first 30-minutes of each trial was considered for washout of the previous ventilatory mode. The results were recorded only in the following ten minutes of each trial.
Considering PSV trial: the initial PEEP was set on 4–5 cm H2O and PS level was set to reach tidal volume of 6–9 ml/kg, a reduction of respiratory rate to physiological values for age20, and absence of clinical signs of increased work of breathing (chest retractions, diaphragm paradox). The flow trigger was set to the maximum sensitivity level not causing autotriggering. The clinician set the expiratory cycling off to achieve the best synchronization according to the flow/pressure tracings, and the fastest pressure ramp time. During application of NAVA, Eadi trigger was at default value of 0.5 µV. To determine the corresponding NAVA level, able to achieve a similar inspiratory mean airway pressure to that obtained in PSV, a dedicated function called NAVA Preview was used. Periods of coughing/suctioning were excluded from the analysis. All measurements were performed if stable traces were correctly displayed. The measurements were recorded and analyzed after the patients were discharged from the PICU. Two blinded researchers (G.S. and R.C.) independently analyzed the recorded data. In case of disgreement between the two, a third blinded investigator (G.C.) reviewed the recordings and resolved the discrepancies.”
Two blinded pediatric cardiologists with more than ten years of experience in critically ill children performed an echocardiogram during the last ten minutes of each trial. Arterial line was connected to MostCareUp monitor values were recorded in the last ten minutes of each trial.
Analgesia was provided according to the PICU protocol for patient admitted after liver transplantation (morphine 10 mcg/kg/h after intravenous bolus of 50–100 mcg/kg).
Perioperative variables were collected as follows: gender, age, weight, body mass index, Status classification, Pediatric End Liver Disease (PELD), primary disease, cirrhosis, PICU admission before transplantation, Pediatric Index of Mortality 3 (PIM3), living liver donor, liver ischemia time, total volume of transfusion per weight, cumulative fluid balance (according to formula described by Goldstein et al.21, mesh, surgery duration, graft-to-recipient weight ratio (GRWR, graft weight in gram/recipient weight in kg × 10), if the patient needed noninvasive ventilation post transplantation (NIV), ventilator-free days, length of stay (LOS) in PICU and hospital, mortality at 28 days.
Patient-ventilator interaction parameters. To estimate the asynchrony rate, we calculated the asynchrony index (AI), which is the ratio between the number of asynchronous events and the total respiratory rate, expressed as percentage. An AI > 10% was considered a high rate of asynchrony11. The asynchronies observed and analysed were wasted efforts (defined as a patient inspiratory effort not assisted by the ventilator), auto-triggering (defined as a mechanical insufflation in absence of a patient inspiratory effort), late cycling (defined as a cycle with the mechanical inspiratory time greater than twice the patient’s neural time) and double triggering, defined as two mechanical breaths separated by a short expiratory time during the same inflection in Edi signal (< half of neural expiratory timing). Other variables collected were: tidal volume, peak airway pressure, mean airway pressure, oxygenation index, and PaO2/FiO2 ratio, inspiratory trigger delay, expiratory trigger delay, time of synchrony defined as the time during which patient’s inspiratory effort and ventilatory assistance are in phase, and time during which respiratory effort and ventilator assistance were synchronous. The amount of inspiratory effort was calculated as the Pressure Time Product of Edi per breath and per minute (PTPEdi/breath and PTPEdi/min) defined as the area under the Edi trace from the neural inspiration to the end of the neural expiration. The method of sampling patient-ventilator interaction parameters is described in Additional Material S2.
Echocardiographic evaluation. Variables were collected using Philips CX 50 echocardiography device with S5/S8 probes. Measurements: TAPSE (tricuspid annular plane systolic excursion), RVFAC (right ventricular fractional area change), IT (tricuspid valve insufficiency), TDI (Tissue Doppler Echocardiography to measure systolic velocities in right ventricle), LV EF (left ventricle ejection fraction), RV-PSV (right ventricle peak systolic velocity).
Hemodynamic parameters. Collected hemodynamic measurements were heart rate, systolic blood pressure, diastolic blood pressure, mean arterial pressure, cardiac index, systemic vascular resistance index, and central venous pressure.”
Primary outcomes
Primary outcomes were: (1) To compare the magnitude of patient ventilator asynchronies between PSV and NAVA in patients undergoing liver transplantation. Evaluate the variation in asynchronies (AI) between patient and ventilator during weaning from MV with the use of NAVA (compared with PSV); (2) To investigate the physiological effects of each ventilation mode on cardiac function.
Secondary outcomes
Evaluate the differences between the two ventilation modes in terms of pulmonary function (PaO2/FiO2, oxygenation index, mean airway pressure, tidal volume), patient ventilator interaction (auto triggering, late cycling, double triggering, wasted efforts, inspiratory and expiratory trigger delay, pressurization time, time of synchrony, PTPEadi/breath and PTPEadi/min), cardiac and hemodynamic parameters mentioned above.
Statistical plan
Collected data were presented as count and proportions (categorical data) or median and interquartile range (continuous data).
A bivariate quantile regression analysis was applied to estimate the effect of NAVA compared to PSV on each metabolic, ventilation and hemodynamic variable. Estimates of the outcome variables for change in a covariate were reported as medians and standard error.
An adjusted quantile regression model was applied to account for potential confounders to evaluate the effect of ventilation mode NAVA (PSV mode was the reference value) on asynchrony index, TAPSE and left ventricles strain. The determinants of AI and RVFAC for which the p-value was < 0.20 in the bivariate analysis were included in the initial multivariable logistic regression model. Subsequently, Patient baseline characteristics such as weight, graft/recipient weight ratio, cumulative fluid balance after surgery and cirrhosis with ascites (which are clinically and pathophysiologically relevant to respiratory mechanics and related to diaphragm and abdominal muscle function during spontaneous ventilation) were considered as potential confounders.
According to previous studies22, we estimated an AI of 50% in PSV and we expected NAVA to reduce it to 2%. Considering α-error equal to 0.05 and power equal to 80%, the study would have needed 16 patients to detect a 48% reduction in AI. Statistical software Stata 15.0 (StataCorp) was used for statistical analysis.
Results
From March 2021 to September 2022 thirty-seven pediatric patients were screened for inclusion. Twenty-four liver recipients were enrolled in the study, two showed hemodynamic instability after randomization, and one returned to the operating room (Fig. 2). All the remaining twenty-one patients completed measurements of ventilatory and invasive hemodynamic parameters during the three post-randomization phases. Only 12 patients were studied by echocardiography (6 for each ventilation sequence) due to the unavailability of the cardiologists overnight. Patient characteristics are shown in Table 1. No differences were detected by gender. The most frequent primary disease is biliary tract malformation (15/21, 71%). One third of the patients had cirrhosis with ascites (7/21, 33%). All patients received massive transfusions during transplantation (> 40 ml/kg in 6 h)23. Six patients had GRWR > 4% (6/21, 29%).
In a regression model with one covariate (Table 2 and Table S1), no significant changes in respiratory gas exchange or metabolism were observed during the application of NAVA and PSV. In terms of patient-ventilator interaction, NAVA was associated with significantly lower asynchrony index (1.5 versus 6.8%, p 0.016), inspiratory delay time (80 versus 150 milliseconds, p < 0.001), peak airway pressure (7.2 vs. 10.3 cmH2O, p < 0.001). The main asynchronies (late ciclying, double triggering, and wasted efforts) were not included in Table 2 due to collinearity among variables. In particular, the strong correlation between some asynchrony indices led to their automatic omission in the regression model. PSV is associated with lower pressurization time (415 versus 615 milliseconds, p < 0.001) and synchronization time (420 versus 615 milliseconds, p 0.046).
In the multivariable logistic model analysis (Table 3) NAVA is significantly associated with a reduction in patient-ventilator asynchronies (AI) compared to PSV (Coeff − 6.66, 95% CI −11.5 to −1.78, p 0.08). In contrast, NAVA did not have an effect on right/left ventricular function. These findings are confirmed in the logistic model, which accounted for covariates with a p-value < 0.2 in the bivariate analysis (Table 2s).
Discussion
In this trial involving pediatric liver recipients, the application of NAVA reduces asynchronies between patient and ventilator.
Improved synchrony during NAVA, as well as respiratory exchanges and reduced wasted efforts, have been shown in other studies in patients with PARDS, bronchiolitis and post-cardiac surgery24,25,26.
In the previous studies in which NAVA was compared to PSV, the median AI varied between 1.7 and 11% during the application of NAVA, in our population NAVA resulted in an AI value of 1.5%. It should be noted that even during the application of PSV, AI was lower in comparison with the above-mentioned previous studies (8.6 vs. 8.8–25%). The presence of Edi monitoring allowed to optimize the expiratory trigger.
In our study, we did not detect significant changes in respiratory exchanges. No studies have compared echocardiographic function during NAVA and PSV in pediatric patients undergoing major abdominal surgery. A study in adult population submitted to abdominal surgery showed greater efficacy of NAVA (vs. PSV) in respiratory exchange and Eadi but did not evaluate asynchronies and cardiac function27.
The advantage of NAVA, during weaning from MV, becomes more relevant when analyzing same specific aspects (abdominal and not) that characterize patients undergoing major abdominal surgery. During anesthesia and surgery, the insult on chest wall due to retractor and the reduction in muscle tone lead to a cephalic elevation of the diaphragm, which is prone to atelectasis development; in the post-operative period, pain and diaphragmatic dysfunction lead to a reduction in residual functional capacity and may consequently lead induce the persistence or development of new atelectasis28. It should be added that, unlike ascites, which is drained by tubes, pleural effusion can get worse because of pulmonary and diaphragmatic dysfunction.
Abdominal factors that may alter weaning and spontaneous/assisted ventilatory activity include abdominal surgery itself, the need for postoperative intravenous analgesia, graft-to-recipient weight ratio (GRWR), and the presence of abdominal mesh. Spontaneous ventilatory activity also relies on abdominal muscle action coordinated with the diaphragm; therefore, the bilateral transection of the abdominal muscles (with the lost of physiological continuity of abdominal wall), the presence of abdominal mesh and the painful stimulus already determine respiratory adjustment during postoperative period. Grimaldi et al. pointed out that a GRWR of > 4% increases the risk of abdominal distension and vascular complications of the graft29. It is understandable that abdominal mass may alter diaphragmatic excursion and influence venous return to the right atrium. In our population, mesh was used in three (3/21, 14%) of the 6 patients (6/21, 29%) with GRWR > 4%. In both cases (simultaneous presence of abdominal mesh and GRWR > 4%) we have patients with abdominal features that may affect the effectiveness of diaphragmatic activity during weaning from MV. Despite these issues, NAVA is superior in terms of patient-ventilator.
The effect of positive cumulative fluid balance and massive transfusion (> 40 ml/Kg in 6 h) are associated with prolonged invasive MV, difficulty weaning from MV and postoperative pulmonary complications. In this study, while in all patients we detected massive transfusions and postoperative fluid balance between 10 and 20%, the use of NAVA results in lower peak and mean airway pressure proving less stress of the lungs than PSV.
In a cross-over study with an A-B-A design, the return to phase A allows for the assessment of washout effects, ensuring that any residual influence from the previous treatment in phase B does not persist. This controls for carry-over effects, which could confound results by allowing treatment effects to persist into subsequent phases. Additionally, the design evaluates the reversibility of treatment effects, enhances statistical robustness with repeated baseline measurements, and minimizes inter-individual variability by using participants as their own controls.
There are several limitations to this study. Firstly, the study was conducted in a single center. Second, the number of patients, although higher than in previous studies, is still small. Thirdly, the potential role of echocardiography in assessing the effect of the two ventilation modes on asynchronies and cardiac function could not be fully explored in all patients studied. The absence of blinding of the investigators at the patient’s bedside (with the exception of the cardiologists and researchers who analysed the ventilatory curve recordings) may introduce bias by influencing themselves.
Conclusion
Diaphragmatic dysfunction, positive cumulative balance and massive transfusions may adversely affect weaning from invasive MV in children undergoing major abdominal surgery. Confirming data from previous studies31,32, NAVA significantly improves patient-ventilator interaction, as evidenced by a reduction in AI, compared to PSV mode. This study highlights a potential application in patients undergoing spine, thoracic and cardiac surgery, where the chest would combine the surgical factors and ventilatory management; additionally, the role of the diaphragm would be involved during the postoperative period after abdominal oncological surgery, where its function may also be altered. Incorporating diaphragmatic ultrasound may provide added value as a confirmatory tool for detecting diaphragmatic dysfunction, particularly in relation to duration of mechanical ventilation and type of surgery performed. Further multicenter studies are needed to evaluate the clinical effects of NAVA on weaning from MV in post-operative period and to assess the effects of NAVA on ventricular function.
Data availability
Data is provided within the manuscript or supplementary information files.
Abbreviations
- AI:
-
Asynchrony index
- Eadi:
-
Electrical diaphragm activity
- RV FAC:
-
Right ventricular fractional area change
- FiO2 :
-
Fraction of inspired oxygen
- GRWR:
-
Graft ratio weight recipient
- IT:
-
Tricuspid valve insufficiency
- LOS:
-
Length of stay
- LT:
-
Liver transplant
- LV EF:
-
Left ventricle ejection fraction
- MV:
-
Mechanical ventilation
- NAVA:
-
Neurally adjusted ventilatory assist
- NAVIGATE:
-
The Use of Neurally Adjusted Ventilator Assist versus Pressure Support Ventilation During Weaning from Mechanical Ventilation in Pediatric Patients After Liver Transplantation
- NIV:
-
Non invasive ventilation
- PaO2 :
-
Arterial partial pressure of oxygen
- PELD:
-
Pediatric end liver disease
- PICU:
-
Pediatric intensive care unit
- PIM3:
-
Pediatric index of mortality 3
- PSV:
-
Pressure support ventilation
- PTPEadi:
-
Pressure time product of Eadi
- RV:
-
Right ventricle
- RV-PSV:
-
Right ventricle peak systolic velocity
- RVFAC:
-
Right ventricular fractional area change
- TAPSE:
-
Tricuspid annular plane systolic excursion
- TDI:
-
Tissue doppler echocardiography
References
Esquivel, C. O. et al. Indications for pediatric liver transplantation. J. Pediatr. 111(6 Pt 2), 1039–1045 (1987).
Chiusolo, F. et al. CPAP by helmet for treatment of acute respiratory failure after pediatric liver transplantation. Pediatr. Transplant. 22(1). (2018).
Hauser, G. J., Kaufman, S. S., Matsumoto, C. S. & Fishbein, T. M. Pediatric intestinal and multivisceral transplantation: A new challenge for the pediatric intensivist. Intensive Care Med. 34(9), 1570–1579 (2008).
Avolio, A. W. et al. Postoperative respiratory failure in liver transplantation: Risk factors and effect on prognosis. PLoS One 14(2), e0211678 (2019).
Antonelli, M. et al. Noninvasive ventilation for treatment of acute respiratory failure in patients undergoing solid organ transplantation: A randomized trial. JAMA 283(2), 235–241 (2000).
Moulin, D. et al. Intensive care for children after orthotopic liver transplantation. Intens. Care Med. 15(Suppl 1), S71–S72 (1989).
Vieillard-Baron, A. et al. Cyclic changes in right ventricular output impedance during mechanical ventilation. J. Appl. Physiol. (1985) 87(5), 1644–1650 (1999).
Yuan, X. et al. Neurally adjusted ventilatory assist as a weaning mode for adults with invasive mechanical ventilation: A systematic review and meta-analysis. Crit. Care. 25(1), 222 (2021).
Brochard, L., Pluskwa, F. & Lemaire, F. Improved efficacy of spontaneous breathing with inspiratory pressure support. Am. Rev. Respir Dis. 136(2), 411–415 (1987).
Thille, A. W., Rodriguez, P., Cabello, B., Lellouche, F. & Brochard, L. Patient-ventilator asynchrony during assisted mechanical ventilation. Intens. Care Med. 32(10), 1515–1522 (2006).
Blanch, L. et al. Asynchronies during mechanical ventilation are associated with mortality. Intensive Care Med. 41(4), 633–641 (2015).
Chidini, G. et al. Early noninvasive neurally adjusted ventilatory assist versus noninvasive flow-triggered pressure support ventilation in pediatric acute respiratory failure: A physiologic randomized controlled trial. Pediatr. Crit. Care Med. 17 (11), e487–e95 (2016).
Piquilloud, L. et al. Neurally adjusted ventilatory assist improves patient-ventilator interaction. Intens. Care Med. 37(2), 263–271 (2011).
Schmidt, M. et al. Neurally adjusted ventilatory assist and proportional assist ventilation both improve patient-ventilator interaction. Crit. Care 19(1), 56 (2015).
Berger, D., Bloechlinger, S., Takala, J., Sinderby, C. & Brander, L. Heart-lung interactions during neurally adjusted ventilatory assist. Crit. Care 18(5), 499 (2014).
Vincent, J. L. et al. Perioperative cardiovascular monitoring of high-risk patients: A consensus of 12. Crit. Care 19(1), 224 (2015).
Scott, M. J. & Group, A. H. I. W. Perioperative patients with hemodynamic instability: Consensus recommendations of the anesthesia patient safety foundation. Anesth. Analg. (2023).
Lang, R. M. et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American society of echocardiography and the European association of cardiovascular imaging. Eur. Heart J. Cardiovasc. Imaging 16(3), 233–270 (2015).
Romagnoli, S., Franchi, F., Ricci, Z., Scolletta, S. & Payen, D. The pressure recording analytical method (PRAM): Technical concepts and literature review. J. Cardiothorac. Vasc Anesth. 31(4), 1460–1470 (2017).
Bonafide, C. P. et al. Development of heart and respiratory rate percentile curves for hospitalized children. Pediatrics 131(4), e1150–e1157 (2013).
Goldstein, S. L., Currier, H., Graf Cd, Cosio, C. C., Brewer, E. D. & Sachdeva, R. Outcome in children receiving continuous venovenous hemofiltration. Pediatrics 107(6), 1309–1312 (2001).
Beck, J., Emeriaud, G., Liu, Y. & Sinderby, C. Neurally-adjusted ventilatory assist (NAVA) in children: A systematic review. Minerva Anestesiol 82(8), 874–883 (2016).
Neff, L. P. et al. Clearly defining pediatric massive transfusion: Cutting through the fog and friction with combat data. J. Trauma. Acute Care Surg. 78(1), 22–28 (2015). discussion 8–9.
Spinazzola, G. et al. Pressure support ventilation (PSV) versus neurally adjusted ventilatory assist (NAVA) in difficult to wean pediatric ARDS patients: A physiologic crossover study. BMC Pediatr. 20(1), 334 (2020).
Bordessoule, A., Emeriaud, G., Morneau, S., Jouvet, P. & Beck, J. Neurally adjusted ventilatory assist improves patient–ventilator interaction in infants as compared with conventional ventilation. Pediatr. Res. 72(2), 194–202 (2012).
Bonacina, D. et al. Pressure support ventilation, sigh adjunct to pressure support ventilation, and neurally adjusted ventilatory assist in infants after cardiac surgery: A physiologic crossover randomized study. Pediatr. Pulmonol. 54(7), 1078–1086 (2019).
Coisel, Y. et al. Neurally adjusted ventilatory assist in critically ill postoperative patients: A crossover randomized study. Anesthesiology 113(4), 925–935 (2010).
Laghi, F. & Tobin, M. J. Indications for mechanical ventilation - postoperative respiratory failure. In: (ed Tobin, M. J.) Principles and Practice of Mechanical Ventilation. Second ed: McGraw-Hill; 142–144. (2006).
Grimaldi, C., Spada, M. & Maggiore, G. Liver transplantation in children: An overview of organ allocation and surgical management. Curr. Pediatr. Rev. 17(4), 245–252 (2021).
Schneider, M. et al. Echocardiographic assessment of right ventricular function: Current clinical practice. Int. J. Cardiovasc. Imaging 35(1), 49–56 (2019).
Yonis, H. et al. Patient-ventilator synchrony in neurally adjusted ventilatory assist (NAVA) and pressure support ventilation (PSV): A prospective observational study. BMC Anesthesiol 15, 117 (2015).
Treussart, C. et al. Patient-Ventilator synchrony in extremely premature neonates during non-invasive neurally adjusted ventilatory assist or synchronized intermittent positive airway pressure: A randomized crossover pilot trial. Neonatology 119(3), 386–393 (2022).
Acknowledgements
This work was supported by the Italian Ministry of Health with “Current Research funds”.
Author information
Authors and Affiliations
Contributions
F.C, G.C. were responsible for study conception and oversight. F.C, G.S., R.C., A.F., F.T., F.P., E.R., M.C., L.R., V.F., and G.C. were involved with study design and development of the data collection forms. L.R., V.F., G.S., R.C, G.C., and F.C. were the primary data analysts. F.C., G.C., V.F., G.S., and L.R. were responsible for the primary interpretation of the results. F.C., G.C., V.F., G.S., and M.C. provided additional data collection and interpretation of results. All authors have read, edited, and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participants
NAVIGATE protocol was approved by institutional research board at Bambino Gesù Children’s Hospital (document 1695_OPBG_2018, March 19, 2019). All research was performed in accordance with relevant guidelines/regulations, and written informed consent was obtained from parents/legal guardian. The study has been performed in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chiusolo, F., Spinazzola, G., Costa, R. et al. Effect of neurally adjusted ventilator assist versus pressure support ventilation on asynchronies and cardiac function in pediatric liver transplantation. Sci Rep 15, 7158 (2025). https://doi.org/10.1038/s41598-025-91590-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-91590-z