Abstract
Leptomeningeal metastasis (LM) is the most devastating complication of non-small cell lung cancer (NSCLC), and its incidence is increasing. We investigated the survival outcomes of patients with NSCLC who received combined antiangiogenic and systemic therapies before and after LM progression and explored survival-associated factors. Patients with epidermal growth factor receptor (EGFR)-mutant or wild-type NSCLC-LM receiving systemic therapy were included. Survival outcomes were analyzed separately for patients who received different therapies before and after LM progression. The primary outcomes were the median time from NSCLC diagnosis to LM (LM-free survival [mLFS]) and overall survival (mOS). The mLFS and mOS of the 77 enrolled patients after receiving EGFR-tyrosine kinase inhibitor (TKI) plus antiangiogenic drugs were 19.0 and 21.9 months, respectively, which were significantly longer than those of the patients in the EGFR-TKI monotherapy group (14.0 and 8.3 months, respectively; P values for mLFS and mOS were 0.035 and 0.038, respectively). In patients receiving platinum-based chemotherapy, significantly longer mLFS and mOS were not dependent on antiangiogenic therapy. Metastatic counts at more than three sites were associated with a shorter LFS, and liver metastasis was an independent predictor of worse OS. Combining antiangiogenic and systemic therapies, particularly EGFR-TKIs, may prolong LFS and OS in NSCLC-LM, whereas metastatic counts at more than three sites and liver metastasis may be adverse prognostic factors.
Similar content being viewed by others
Introduction
Leptomeningeal metastasis (LM) is a devastating complication of advanced tumors, caused by the transfer of tumor cells to the pia mater and arachnoid, subarachnoid, and other cerebrospinal fluid (CSF) spaces via various routes1,2. Patients with non-small cell lung cancer (NSCLC) are particularly prone to LM, which negatively affects their quality of life and overall survival (OS). The incidence of LM in advanced NSCLC is increasing and is currently approximately 3–5%3. The incidence is particularly high in patients with mutations in the epidermal growth factor receptor (EGFR)4, in whom the incidence rate of LM reaches 9–16%. Nearly 30% of these patients develop central nervous system (CNS) metastasis after receiving EGFR tyrosine kinase inhibitors (TKIs)5. Approximately 13% of patients with NSCLC who experience clinical benefits from EGFR-TKIs experience CNS failure, of whom 18% develop LM6.
The survival period of patients with NSCLC-LM has increased from 1 to 3 months to 3–11 months because of multidisciplinary combination therapies, including chemotherapy, radiation, intrathecal treatment, targeted therapy, and immunotherapy1,7,8. However, no standard treatment is currently available for LM, highlighting the significant unmet need to improve prevention and treatment efficacy.
Antiangiogenic therapy is an important link in the therapeutic field of NSCLC. Antiangiogenic therapy targeting the vascular endothelial growth factor (VEGF) signaling pathway has shown efficacy when combined with platinum-based doublet chemotherapy in advanced NSCLC without driver alterations9 and when combined with EGFR-TKIs in advanced EGFR-mutated NSCLC10,11,12. Therefore, antiangiogenic agents may reinforce and enhance the effects of subsequent therapies but the role of antiangiogenic therapy in NSCLC-LM remains unclear. Moreover, the risk factors for LM metastasis in patients without baseline CNS lesions are not fully understood.
In this study, we explored the efficacy of combination therapy with antiangiogenic agents in patients with NSCLC both before and after progression to LM in a real-world setting and the factors associated with patient survival. Our findings may help physicians to develop individualized surveillance strategies and precise treatment choices.
Materials and methods
Patients and data collection
This was a retrospective study conducted at the Affiliated Tumor Hospital of Xinjiang Medical University, China, using electronic medical database records from January 2015 to March 2023. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology guidelines. The study protocol was approved by the Institutional Review Board of the Affiliated Tumor Hospital of the Xinjiang Medical University Research Ethics Committee.
The inclusion criteria were as follows: (i) age ≥ 18 years with histologically or cytologically confirmed locally advanced, metastatic, or recurrent NSCLC; (ii) diagnosis of LM positivity using CSF cytology (including malignant cells) or typical findings (including leptomeningeal enhancement or ventricle broadening) upon imaging, as confirmed by qualified neuroradiologists at the hospital; (iii) EGFR-mutated or wild-type tumors; and (iv) administration of at least two doses of systemic therapy. The exclusion criteria were as follows: (i) other types of malignant lung tumors, including, but not limited to, small cell lung cancer and pulmonary sarcoma; (ii) other driver gene mutations, including, but not limited to, anaplastic lymphoma kinase and ROS proto-oncogene 1 fusion; and (iii) loss to follow-up or no treatment.
Patient demographics, diagnostic and genomic testing information, LM symptoms, and treatment details were obtained from the electronic medical records. Data on patient demographics included sex, age, race, smoking status, and history of cancer in the patients and families. Diagnostic information included the tumor histology type, time of NSCLC diagnosis, time of LM diagnosis, site, and number of extracranial metastases. The treatment details collected included the date of treatment initiation, progress, line of therapy, and treatment modalities.
Comprehensive treatment
For antiangiogenic treatment, bevacizumab 7.5–15 mg/kg was administered intravenously on d 1 of each 3-week cycle; recombinant human endostatin 210 mg was administered as a continuous intravenous microinfusion (d1–d3, every 3 weeks), or anlotinib was administered orally 10/12 mg once daily (d1–d14, every 3 weeks). Platinum-based chemotherapy was administered intravenously (d1–d3, every 3 weeks). Oral gefitinib, erlotinib, osimertinib, furmonertinib, and aumolertinib were administered as EGFR-TKIs. All treatments were administered at the discretion of the physicians during clinical practice.
Therapeutic modalities were classified as platinum-based chemotherapy (chemo), platinum-based chemotherapy plus antiangiogenic agents (chemo + A), EGFR-TKIs, and EGFR-TKIs plus antiangiogenic agents (EGFR-TKI + A). Extracranial disease progression was defined using the Response Evaluation Criteria in Solid Tumours version 1.1.
Statistical analysis
Categorical clinical and pathological variables were summarized as frequency counts and percentages. Continuous variables were summarized as medians and quartiles and are represented by M (P25, P75). Kruskal–Wallis tests and the Fisher exact or chi-square tests were used to compare quantitative and categorical variables, respectively, among the treatment groups. LM OS was defined as the time from LM diagnosis to death from any cause or last follow-up. LM-free survival (LFS) was defined as the time from NSCLC diagnosis to progression to LM. The Kaplan–Meier method was used to plot the LFS and OS curves for the different therapy patterns. The 1- and 2-year survival rates and estimated median survival for each treatment cohort are reported. The log-rank test was used to compare LFS and OS between the groups. Univariate and multivariate analyses were performed to determine the prognostic factors using a Cox proportional hazards model. All factors that may be relevant for predicting LFS or OS were evaluated using univariate analysis with a log-rank test. After univariate analysis, the risk factors (P < 0.05) were included in the multivariate analysis. The optimal cutoff values for age, programmed death ligand 1 expression and metastasis count were determined using the X-tile program (https://medicine.yale.edu/lab/rimm/research/software/). Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated. Statistical significance was set at P < 0.05. All analyses were performed using the R Statistical Software version 4.1.0 (https://www.r-project.org/).
Results
Patient characteristics
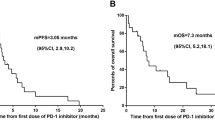
Between 2015 and 2023, 97 patients with lung cancer and LM were identified, of whom 77 with NSCLC-LM were included. Follow-up was completed for all 77 patients by May 20, 2023. The median follow-up time from diagnosis of NSCLC to study completion was 19.5 months (range, 1.8–83.3 months). Seventy (90.9%) patients died, and seven (9.1%) were alive at the end of the study. Of the 77 patients, 55 (71.4%) had EGFR mutations, and 22 (28.6%) had no driver mutations. Twenty-seven patients (35.1%) were diagnosed with LM at the initial diagnosis of NSCLC. Fifty patients (64.9%) were diagnosed with LM after NSCLC progression. The median LFS (mLFS) was 5.0 months (95% CI 2.2–7.8 months). The 1-year LFS rate was 33% (95% CI 24–45%). The 2-year LFS rate was 12% (95% CI 6–22%). Forty-five patients received systemic therapy before LM diagnosis. These included 11.1% (5/45) in the chemo group, 24.4% (11/45) in the chemo + A (endostatin for four and bevacizumab for seven patients), 42.2% (19/45) in the EGFR-TKI group, and 22.2% (10/45) in the EGFR-TKI + A (endostatin and bevacizumab for five patients each). After LM diagnosis, the median LM OS (mOS) was 8.9 months (95% CI 7.4–10.4 months). The 1-year mOS rate was 39% (95% CI 29–52%), and the 2-year mOS rate was 12% (95% CI 6–22%).
Altogether, 94.8% (73/77) of the patients received systemic treatment after LM diagnosis. Of these, 15.1% (11/73) were in the chemo group, 20.5% (15/73) in the chemo + A group (endostatin for one, bevacizumab for 10, and anlotinib for four patients), 49.3% (36/73) in the EGFR-TKI group, and 15.1% (11/73) in the EGFR-TKI + A group (endostatin for two and bevacizumab for nine patients; Fig. 1).
Flow diagram of the current study. ALK: anaplastic lymphoma kinase; Chemo: platinum-based chemotherapy; Chemo + A: platinum-based chemotherapy plus antiangiogenic therapy; EGFR-TKI: epidermal growth factor receptor tyrosine kinase inhibitor; EGFR-TKI + A: epidermal growth factor receptor tyrosine kinase inhibitor plus antiangiogenic therapy; LC: lung cancer; LM: leptomeningeal metastasis; NSCLC: non-small cell lung cancer; ROS1: ROS proto-oncogene 1.
Tables 1 and 2 present the clinical characteristics of the study cohort. Sex, age, smoking status, disease history, family history of cancer, brain parenchymal metastasis (BM), and extracranial disease status did not differ among the four treatment groups. In contrast, the percentage of patients from ethnic minorities with NSCLC treated before progression to LM was significantly different between the groups (chemo 80.0% vs. chemo + A 45.5% vs. EGFR-TKI 10.5% vs. EGFR-TKI + A 10.0%; P = 0.004).
LFS
Table 3 presents the LFS as well as the 1-year and 2-year LFS rates among different groups. There was no significant difference in mLFS between the chemo and the chemo + A group. Kaplan–Meier curves for LFS are shown in Fig. 2a. In the chemo + A group, the 1- and 2-year mLFS rates were higher than in chemo group. Compared with EGFR-TKI alone, mLFS was prolonged with EGFR-TKI + A. Kaplan–Meier curves for LFS are shown in Fig. 2b. In the EGFR–TKI + A cohort, both the 1-year and 2-year mLFS rates are higher than those in the EGFR-TKI cohort.
Kaplan–Meier curves for leptomeningeal metastasis-free survival. (a) Results of chemotherapy versus chemotherapy plus antiangiogenic therapy. (b) Outcomes of EGFR-TKI versus EGFR-TKI plus antiangiogenic therapy. Chemo: platinum-based chemotherapy; Chemo + A: platinum-based chemotherapy plus antiangiogenic; EGFR-TKI: epidermal growth factor receptor tyrosine kinase inhibitor; EGFR-TKI + A: epidermal growth factor receptor tyrosine kinase inhibitor plus antiangiogenic.
OS
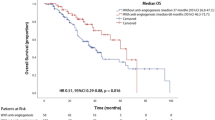
The detailed data regarding OS as well as 1-year and 2-year mOS rates are presented in Table 4. After progression to LM, the chemo + A group had a longer median overall survival (mOS) than the chemo group, though the difference was not statistically significant. In contrast, the EGFR–TKI + A group had a significantly longer mOS compared to the EGFR–TKI group. The Kaplan–Meier curves for OS are shown in Fig. 3.
Kaplan–Meier curves for the overall survival of leptomeningeal metastases. (a) Results of chemotherapy versus chemotherapy plus antiangiogenic therapy. (b) Outcomes of EGFR-TKI versus EGFR-TKI plus antiangiogenic therapy. Chemo platinum-based chemotherapy, Chemo + A platinum-based chemotherapy plus antiangiogenic, EGFR-TKI epidermal growth factor receptor tyrosine kinase inhibitor, EGFR-TKI + A epidermal growth factor receptor tyrosine kinase inhibitor plus antiangiogenic.
LFS prognostic factors
The results of the univariate and multifactorial analyses of LFS are listed in Table 5. Correlations in the univariate analysis were significant for age, family history of cancer, metastasis counts, BM, presence of EGFR mutation, and targeted therapy + A. These factors were included in the multivariate analysis.
In the multivariate analysis, presence of metastasis counts at more than three sites was associated with worse LFS (HR 4.02; 95% CI 1.00–16.08; P = 0.049). LFS also differed significantly depending on whether EGFR-TKI administration was supplemented with antiangiogenic therapy, with adjustment for metastasis counts (HR 0.37; 95% CI 0.14–0.95; P = 0.039).
LM OS prognostic factors
The results of the univariate and multifactorial analyses of OS are presented in Table 6. Liver metastasis (HR 1.65; 95% CI 0.96–2.81; P = 0.069) and EGFR-TKI + A levels (HR 0.46; 95% CI 0.22–0.97; P = 0.041) were included in the multifactorial regression analysis.
The multivariate analysis indicated that liver metastasis was a poor prognostic factor for OS (HR 2.31; 95% CI 1.28–4.19; P = 0.017). OS differed significantly between the groups treated with EGFR-TKI with or without antiangiogenic therapy, with adjustment for liver metastasis (HR 0.37; 95% CI 0.17–0.81; P = 0.012).
Discussion
One of the hallmarks of cancer pathophysiology is angiogenesis, a physiological process that involves the formation of new blood vessels from preexisting blood vessels. Various strategies targeting this process have been proposed and are currently being used in clinical practice. Antiangiogenic agents are used as adjuvants in standard therapies for many different cancer types and have led to improvements in patient prognosis. In the present study, we evaluated the efficacy of adding antiangiogenic agents to other treatments in patients with NSCLC without baseline LM and after LM development at a single institution. The observed mLFS and mOS after EGFR-TKI + A (19.0 and 21.9 months, respectively) were significantly longer than those in the EGFR-TKI monotherapy group (14.0 and 8.3 months, respectively). Similarly, mLFS and mOS were longer, although not significantly, in patients who received chemotherapy plus antiangiogenic treatment than in those who received chemotherapy alone. The multivariate analysis revealed that metastatic counts at more than three sites were associated with shorter LFS, whereas liver metastasis was an independent predictor of worse OS.
Patients harboring oncogene driver mutations are more likely to develop meningeal metastases, particularly those carrying EGFR mutations (9.4% vs. 1.7%; P < 0.001)8. Patients with NSCLC and EGFR mutations have a 9–10% probability of developing LM13. In another retrospective analysis reported that patients harboring the L858R mutation in EGFR had a higher risk of developing LM than those harboring the 19del mutation (10.7% vs. 3.4%; P = 0.006)14. In our retrospective study, patients with EGFR mutations accounted for 71.4% (55/77) of all patients with LM, with the L858R mutation identified in 56.4% (31/55) of all the patients with EGFR mutations. This further validates that, compared with the wild-type, EGFR mutations, particularly L858R, increase susceptibility to LM development. This finding will help physicians tailor surveillance strategies and therapeutic choices for patients with EGFR mutations and advanced NSCLC, as more rigorous brain imaging may need to be performed when L858R mutations are detected.
In the current study, 27 patients were initially diagnosed with LM, and 50 progressed to LM during follow-up. According to the different treatment modalities, the overall LFS time was prolonged in the EGFR-TKI group compared with the chemo group (14.0 months vs. 6.0 months; P = 0.008). EGFR-TKIs are effective in preventing or delaying CNS metastasis in patients who are initially metastasis-free. For example, compared with chemotherapy alone, gefitinib delayed BM in the ADJUVANT trial15. However, the Kaplan–Meier curves of the cumulative incidence of CNS metastasis in each treatment group were separated at 12–15 months but converged at 33–36 months. The same phenomenon was reported in another study that compared the cumulative incidence of CNS metastasis in patients treated with osimertinib and first-generation EGFRs16. These findings suggest that EGFR-TKIs may not be curative but may provide clinical benefits by delaying CNS metastasis. The plateaus of the CNS metastasis curves also indicate that CNS metastasis may originate owing to intrinsic biological properties of the primary tumor rather than from EGFR-TKI treatment; otherwise, CNS metastatic events would gradually increase during EGFR-TKI treatment. Studies exploring the molecular mechanisms involved in the development of CNS metastasis are expected to help optimize surveillance and therapeutic strategies.
The potency of LM prevention or delaying effects also differs between EGFR-TKIs and chemotherapy when combined with antiangiogenic agents. We found that EGFR-TKI combined with antiangiogenic therapy significantly delayed the onset of LM when compared with EGFR-TKI alone (19.0 months vs. 14.0 months; P = 0.035) but did not significantly prolong the time to LM in the chemotherapy group. By analyzing the results of the AVAiL study17, we concluded that the rate of BM as the first site of recurrence was significantly lower in the bevacizumab combined with standard platinum-based chemotherapy doublet arm than in the control chemotherapy arm (2.6% vs. 5.8%; P = 0.01); additionally, the risk of development of brain metastases over time was lower (HR 0.36; P = 0.001). The ECOG-ACRIN E1505 trial and subsequent post hoc analysis18, yielded similar results, and the addition of bevacizumab to chemotherapy was associated with a reduction in all-brain recurrence (HR 0.64; P = 0.02) and isolated brain recurrence (HR 0.62; P = 0.032). However, as none of these studies was designed primarily to investigate CNS prevention, these results need to be interpreted cautiously. To the best of our knowledge, to date, no clinical trials have been designed to investigate, or have investigated, the feasibility of using EGFR-TKI plus antiangiogenic agents for LM prophylaxis. The prominent performance of the antiangiogenic combination of EGFR-TKIs in the present study may provide new ideas for preventing LM in patients with NSCLC and EGFR mutations.
Generally, patients with CNS metastases, particularly LM, are excluded from clinical studies. Therefore, the clinical benefits of this combination therapy in patients with LM remain unclear. In animal experiments, VEGF has been shown to move from the circulation into CSF to promote the proliferation of tumor cells19,20. VEGF levels in the CSF are high and negatively correlated with survival time in patients with LM21,22. A preclinical experiment indicated that the combination of osimertinib and bevacizumab may exhibit a synergistic effect in an EGFR-mutant LM model, possibly by modulating E-cadherin levels23. These preclinical studies demonstrate the feasibility of using antiangiogenic agents for LM treatment.
EGFR-TKIs (erlotinib) and anti-VEGFR (bevacizumab) (A + T) achieve superior progression-free survival (PFS) and acceptable safety in patients with NSCLC with intracranial metastases24. A series of studies have shown that A + T therapy improves survival25. The JO2556710and NEJ02611 trials reported prolonged OS and PFS after A + T therapy and recommended the combination of erlotinib and bevacizumab as a first-line regimen in EGFR mutation-positive NSCLC. In contrast, other studies have shown that TKIs combined with angiogenesis inhibitors do not improve PFS in EGFR-mutant NSCLC26. WJOG8517L reported that, although the overall response rate was better with osimertinib plus bevacizumab than with osimertinib alone (68% vs. 54%), median PFS was not prolonged (9.4 months vs. 13.5 months), and median OS showed no significant difference (not reached vs. 11.2 months)27. Consistent with the studies mentioned above, our retrospective analysis indicated that EGFR-TKI plus antiangiogenic agents may improve the survival of patients with LM and EGFR-mutant NSCLC. The aforementioned clinical studies used the antiangiogenic drug bevacizumab, while in the present study, recombinant human endostatin and anlotinib were also used.
We enrolled patients with and without baseline BM and found no significant difference in the risk of developing LM (P = 0.166; Table 5) or LM OS (P = 0.781; Table 6) depending on initial BM. Similar results were obtained by Zhou16, who reported that baseline neuroimaging findings were not associated with the risk of developing CNS metastasis or OS. In a retrospective study on multidisciplinary management of NSCLC-LM28, a distinct conclusion was drawn—that presence of BM was associated with shorter LM survival (5.4 vs. 11.6 months; P = 0.019). Further confirmation is required in prospective studies with a larger sample size.
The present study had some limitations. First, assessment of PFS in patients with LM is challenging. The current belief is that a comprehensive assessment should be based on neurological examination, radiological evaluation, and CSF cytology. The main challenge is defining measurable and non-measurable (target) damage, allowing evaluation of response changes, and performing CSF cytology for assessment because lumbar puncture is an invasive test that most patients refuse to undergo. Therefore, LM PFS was not evaluated in this study. Second, we analyzed the efficacy of three antiangiogenic drugs: recombinant human endostatin, bevacizumab, and anlotinib. The results showed no significant differences in their efficacy. Due to the small sample size, the reliability of these results may be affected. The detailed analysis is presented in Supplementary Tables 2 and 3. Third, this being a retrospective study, our comparison was limited to the efficacy between patients receiving and not receiving anti—angiogenic treatment. Selection bias may arise from two aspects. Firstly, in the non-treatment group, there might have been patients who were ineligible for anti—angiogenic therapy. Secondly, as all the enrolled patients were from a single center, this could also contribute to selection bias. Thus, multi—center studies are required to validate our findings.
Conclusions
This study explored the use of antiangiogenic drugs to prevent or delay LM in patients with advanced NSCLC and evaluated the treatment outcomes after progression to LM. Our results suggest that, compared with EGFR-TKI monotherapy, administering a combination of antiangiogenic agents may delay the development of LM and extend OS in patients with NSCLC-LM. Additionally, a metastasis count at more than three sites could independently predict an increased risk of progression of NSCLC to LM. Lastly, the presence of liver metastasis predicted worse OS in patients with NSCLC-LM. Further prospective studies might entail conducting multicenter prospective investigations with diverse patient cohorts to enhance the generalizability of the findings. These studies could incorporate extended follow-up periods to comprehensively assess the long-term efficacy of antiangiogenic drugs in preventing LM and improving OS. Additionally, research efforts could focus on determining the optimal combination regimens and dosing schedules of antiangiogenic agents with other treatment modalities. Moreover, in-depth molecular analyses of tumors could facilitate the identification of specific patient subgroups that are more likely to benefit from antiangiogenic therapy and experience a delay in LM development.
Data availability
The datasets generated and/or analysed during the current study are available in the supplementary files of this submission.
References
Cheng, H. & Perez-Soler, R. Leptomeningeal metastases in non-small-cell lung cancer. Lancet Oncol. 19, e43–e55. https://doi.org/10.1016/s1470-2045(17)30689-7 (2018).
Remon, J., Le Rhun, E. & Besse, B. Leptomeningeal carcinomatosis in non-small cell lung cancer patients: A continuing challenge in the personalized treatment era. Cancer Treat Rev. 53, 128–137. https://doi.org/10.1016/j.ctrv.2016.12.006 (2017).
Wang, Y., Yang, X., Li, N. J. & Xue, J. X. Leptomeningeal metastases in non-small cell lung cancer: Diagnosis and treatment. Lung Cancer 174, 1–13. https://doi.org/10.1016/j.lungcan.2022.09.013 (2022).
Liao, B. C. et al. Epidermal growth factor receptor tyrosine kinase inhibitors for non-small-cell lung cancer patients with leptomeningeal carcinomatosis. J Thorac Oncol. 10, 1754–17561. https://doi.org/10.1097/jto.0000000000000669 (2015).
Yang, J. et al. Osimertinib in patients with epidermal growth factor receptor mutation-positive non-small-cell lung cancer and leptomeningeal metastases: The BLOOM study. J. Clin. Oncol. 38, 538–547. https://doi.org/10.1200/jco.19.00457 (2020).
Lee, Y. J. et al. Frequent central nervous system failure after clinical benefit with epidermal growth factor receptor tyrosine kinase inhibitors in Korean patients with nonsmall-cell lung cancer. Cancer-Am. Cancer Soc. 116, 1336–1343. https://doi.org/10.1002/cncr.24877 (2010).
Le Rhun, E. et al. Prognostic validation and clinical implications of the EANO ESMO classification of leptomeningeal metastasis from solid tumors. Neuro Oncol. 23, 1100–1112. https://doi.org/10.1093/neuonc/noaa298 (2021).
Li, Y. S. et al. Leptomeningeal metastases in patients with NSCLC with EGFR mutations. J. Thorac Oncol. 11, 1962–1969. https://doi.org/10.1016/j.jtho.2016.06.029 (2016).
Zhou, C. et al. BEYOND: A randomized, double-blind, placebo-controlled, multicenter, phase iii study of first-line carboplatin/paclitaxel plus bevacizumab or placebo in Chinese patients with advanced or recurrent nonsquamous non-small-cell lung cancer. J. Clin. Oncol. 33, 2197–2204. https://doi.org/10.1200/jco.2014.59.4424 (2015).
Hosomi, Y. et al. Erlotinib with or without bevacizumab as a first-line therapy for patients with advanced nonsquamous epidermal growth factor receptor-positive non-small cell lung cancer: Exploratory subgroup analyses from the phase II JO25567 study. Thorac. Cancer 13, 2192–2200. https://doi.org/10.1111/1759-7714.14541 (2022).
Saito, H. et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): Interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol. 20, 625–635. https://doi.org/10.1016/s1470-2045(19)30035-x (2019).
Zhou, Q. et al. Bevacizumab plus erlotinib in Chinese patients with untreated, EGFR-mutated, advanced NSCLC (ARTEMIS-CTONG1509): A multicenter phase 3 study. Cancer Cell. 39, 1279-1291.e3. https://doi.org/10.1016/j.ccell.2021.07.005 (2021).
Merkhofer, C. M. et al. Systemic treatment patterns and outcomes in patients with EGFR mutated non-small cell lung cancer and leptomeningeal disease. Clin. Lung Cancer 23, 446–455. https://doi.org/10.1016/j.cllc.2022.03.013 (2022).
Wu, Y. L. et al. Leptomeningeal metastasis after effective first-generation EGFR TKI treatment of advanced non-small cell lung cancer. Lung Cancer 127, 1–5. https://doi.org/10.1016/j.lungcan.2018.11.022 (2019).
Xu, S. T. et al. The unique spatial-temporal treatment failure patterns of adjuvant gefitinib therapy: A post hoc analysis of the ADJUVANT trial (CTONG 1104). J. Thorac. Oncol. 14, 503–512. https://doi.org/10.1016/j.jtho.2018.11.020 (2019).
Zhou, Y. et al. Survival outcomes and symptomatic central nervous system (CNS) metastasis in EGFR-mutant advanced non-small cell lung cancer without baseline CNS metastasis: Osimertinib vs. first-generation EGFR tyrosine kinase inhibitors. Lung Cancer. 150, 178–185. https://doi.org/10.1016/j.lungcan.2020.10.018 (2020).
Reck, M. et al. Overall survival with cisplatin-gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: Results from a randomised phase III trial (AVAiL). Ann. Oncol. 21, 1804–1809. https://doi.org/10.1093/annonc/mdq020 (2010).
Varlotto, J. M. et al. Bevacizumab’s association with a decreased risk of brain metastases in ECOG-ACRIN E1505, a phase 3 randomized trial of adjuvant chemotherapy with or without bevacizumab in surgically resected NSCLC. JTO Clin. Res. Rep. 3, 100274. https://doi.org/10.1016/j.jtocrr.2021.100274 (2022).
Boire, A. et al. Complement component 3 adapts the cerebrospinal fluid for leptomeningeal metastasis. Cell. 168, 1101-1113.e13 (2017).
Smalley, I. et al. Proteomic analysis of CSF from patients with leptomeningeal melanoma metastases identifies signatures associated with disease progression and therapeutic resistance. Clin. Cancer Res. 26, 2163–2175. https://doi.org/10.1158/1078-0432.ccr-19-2840 (2020).
Groves, M. D. et al. Biomarkers of disease: Cerebrospinal fluid vascular endothelial growth factor (VEGF) and stromal cell derived factor (SDF)-1 levels in patients with neoplastic meningitis (NM) due to breast cancer, lung cancer and melanoma. J. Neurooncol. 94, 229–234. https://doi.org/10.1007/s11060-009-9819-2 (2009).
Wu, P. F. et al. A pilot study of bevacizumab combined with etoposide and cisplatin in breast cancer patients with leptomeningeal carcinomatosis. BMC Cancer. 15, 299. https://doi.org/10.1186/s12885-015-1290-1 (2015).
Yi, Y. et al. Potential benefit of osismertinib plus bevacizumab in leptomeningeal metastasis with EGFR mutant non-small-cell lung cancer. J. Transl. Med. 20, 122. https://doi.org/10.1186/s12967-022-03331-9 (2022).
Zhao, B. et al. Efficacy and safety of therapies for EGFR-mutant non-small cell lung cancer with brain metastasis: An evidence-based Bayesian network pooled study of multivariable survival analyses. Aging (Albany NY) 12, 14244–14270 (2020).
Le, X. et al. Dual EGFR-VEGF pathway inhibition: A promising strategy for patients with EGFR-mutant NSCLC. J. Thorac. Oncol. 16, 205–215. https://doi.org/10.1016/j.jtho.2020.10.006 (2021).
Stinchcombe, T. E. et al. Effect of erlotinib plus bevacizumab vs erlotinib alone on progression-free survival in patients with advanced EGFR-mutant non-small cell lung cancer: A phase 2 randomized clinical trial. JAMA Oncol. 5, 1448–1455. (2019).
Akamatsu, H. et al. Efficacy of osimertinib plus bevacizumab vs osimertinib in patients with EGFR T790M-mutated non-small cell lung cancer previously treated with epidermal growth factor receptor-tyrosine kinase inhibitor: West Japan oncology group 8715l phase 2 randomized clinical trial. JAMA Oncol. 7, 386–394. https://doi.org/10.1001/jamaoncol.2020.6758 (2021).
Chen, K. Y., Wu, S. G., Lai, D. M., Kuo, L. T. & Huang, A. P. Multidisciplinary management of patients with non-small cell lung cancer with leptomeningeal metastasis in the tyrosine kinase inhibitor era. J. Neurosurg. 7, 1–9. https://doi.org/10.3171/2022.8.jns221175 (2022).
Funding
This work was supported by the Innovative Team for Research Related to the Immune Response Mechanism of Lung Cancer and Screening of Superior Population for Immunotherapy and Prediction of Prognosis in Xinjiang, Department of Science and Technology of Xinjiang Uygur Autonomous Region (grant number: 2022D14010), a study on the correlation between co-mutations of KRAS/STK11 genes in NSCLC and CD4+ and CD8+ T cells, Treg cells, and MDSC cells in the immune microenvironment, and the Natural Science Foundation of Xinjiang Uygur Autonomous Region Youth Science Fund Project (Grant Number: 2022D01C794).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. C.L. and G.S. proposed the topic and provided resources. R.A., N.L., M.A., and Y.S. prepared the material and collected and analyzed the data. X.Z. prepared the first draft of the manuscript, and all authors commented on previous versions of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The study was performed in line with the Declaration of Helsinki. Approval was granted by the Ethics Committee of the Affiliated Cancer Hospital of Xinjiang Medical University (date of approval: Dec. 18, 2022/protocol code K-2022040).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhu, Xd., Amanjiaoer, Rht., Shen, Yl. et al. Combination of antiangiogenic and systemic therapy in advanced non-small cell lung cancer: before and after progression to leptomeningeal metastasis. Sci Rep 15, 11901 (2025). https://doi.org/10.1038/s41598-025-91922-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-91922-z