Abstract
Chitinase 3-like protein 1(CHI3L1) has been found to be a biomarker for inflammatory diseases, but the diagnostic value of Kawasaki disease (KD) is not investigated. A total of 180 subjects, including 80 KD patients, 70 febrile controls and 30 healthy controls were recruited. Serum of CHI3L1 were measured with an enzyme-linked immunosorbent assay. The correlation between CHI3L1 and clinical parameters was assessed by Spearman correlation coefficient. Multiple logistic regression was employed to investigate the association between CHI3L1 and the incidence of KD. The diagnostic power was evaluated with the receiver operating characteristic curve analysis. Serum CHI3L1 levels in the KD group were significantly higher than those in the fever control group and healthy group. Compared with fever patients, both patients with incomplete KD and complete KD had higher serum CHI3L1 levels. Serum CHI3L1 levels were positively associated with white blood cell counts, neutrophils, platelet, erythrocyte sedimentation rate, C-reactive protein, alanine aminotransferase and the incidence of KD, and negatively associated with hemoglobin, aspartate aminotransferase and albumin. High CHI3L1 tertiles was significantly associated with the high incidence of KD in the unadjusted or adjusted models. Analysis of the Receiver operating characteristic curves, it was showed that the area under the curve was 0.908, with sensitivity of 0.838 and specificity of 0.8 for continuous CHI3L1, and was 0.884 for categorical CHI3L1, with sensitivity of 0.938 and specificity of 0.643 to distinguish all types of KD, respectively. CHI3L1 had the AUC of 0.901, with sensitivity of 0.826 and specificity of 0.8, and had the area under curve of 0.952, with sensitivity of 0.818 and specificity of 0.971 to discriminate complete KD and incomplete KD from febrile diseases, respectively. Serum of CHI3L1 may be a novel and reliable biomarker for the diagnosis of KD.
Similar content being viewed by others
Introduction
Kawasaki disease (KD) is an acute inflammatory disorder primarily affecting infants and young children, leading to the development of coronary artery lesions (CAL) and consequently being recognized as the most prevalent cause of acquired heart disease in children in developed nations. Recent research indicates a significant prevalence of incomplete KD (iKD) both domestically and internationally1. A nationwide survey in Japan conducted from 2013 to 2016 revealed that 22% of cases were classified as iKD. Additionally, studies suggest a rising incidence of iKD in China1. Children with iKD often experience delayed diagnosis, particularly in infants and older children over 5 years old, which can increase the risk of coronary artery lesions (CAL)2. Despite being identified over 60 years ago by Dr. Tomisaku Kawasaki, the diagnosis of KD continues to heavily rely on clinical symptoms due to the absence of definitive biomarkers, potentially resulting in KD cases being overlooked. Further exploration of potential laboratory diagnostic biomarkers is great importance for the timely diagnosis and therapy of KD.
Previous studies have extensively investigated potential markers for KD. While various inflammatory markers such as white blood cell (WBC) count, C-reactive protein (CRP)3, IL-64 and prokineticin 25 have shown potential for diagnosing KD either in combination with clinical symptoms/other inflammatory parameters or independently, they generally lack specificity for the disease. Chitinase 3-like protein 1 (CHI3L1), also known as YKL-40 in humans, is a secreted glycoprotein with multiple functions that is expressed in various cell types, including endothelial cells, activated macrophages, chondrocytes, neutrophils, fibroblasts, synovial and various tumor cells6. It has been established that CHI3L1 plays a significant role in the regulation of various essential biological processes, including inflammatory activation, oxidative damage, and apoptosis7, as well as in immune cell function6. Additionally, it has been implicated in inflammatory diseases7,8,9,10. Recent research has shown that CHI3L1 serves as a marker for several inflammatory conditions, including atherosclerosis, acute coronary syndrome or stable coronary artery disease11, rheumatoid arthritis12 and system lupus erythematosus13. Prior research has indicated an elevation of CHI3L1 in both serum and coronary arteries in a KD mouse model induced by lactobacillus casei cell wall extract14. Additionally, a study by KY et al. reported an increase in serum CHI3L1 levels in KD15. Nevertheless, the sample size was limited and the diagnostic potential of CHI3L1 in the acute phase of KD remains incompletely understood.
Building upon these findings, the current study seeks to validate the diagnostic utility of CHI3L1 in the acute phase of KD.
Materials and methods
Patients
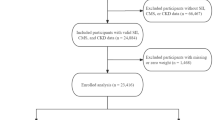
80 patients with acute phase of KD were recruited between Jun 2021 and December 2023 from the Affiliated Hospital of Southwest Medical University. Diagnosis of complete KD (cKD) and iKD met the standard of the AHA 201716. Exclusion criteria: other immune diseases; treatment with corticosteroid; treatment of IVIG or aspirin before hospital during the current course of disease; metabolic diseases; liver and kidney diseases. Simultaneously, 70 patients with common fever who were admitted to hospital were selected and 30 healthy children were recruited during the same time period. These common fever patients had infection and were diagnosed with upper or lower respiratory tract infections, encephalitis and sepsis. This study was approved by the Ethics Committee of the Affiliated Hospital of Southwest Medical University (KY2024193) and the written informed consent was obtained from each participant’s guardian. The informed consent for the collection of serum samples in our study were obtained from each participant’s guardian. All procedures were performed in accordance with the ethical standards of the 1964 Helsinki Declaration and its later amendments.
Clinical laboratory data for patients with KD were collected before initial infusion of intravenous immunoglobulin (IVIG), including demographic characteristics, WBC, neutrophils counts (NEU), lymphocytes counts (LYM), hemoglobin (Hb), platelet counts (PLT), CRP, erythrocyte sedimentation rate (ESR), aspartate aminotransferase (AST), albumin (ALB), alanine aminotransferase (ALT), and procalcitonin (PCT).
Sample collection and measurement of serum CHI3L1
Serum samples were collected from participants with KD before infusion of IVIG and serum samples of patients with common fever were obtained before treatment. All samples were centrifuged at 1000 rpm for 10 min, and then stored at -80℃ until testing. The concentration of CHI3L1(CUSABIO, NO.CSB-E13608h, USA) was measured with an enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions. According to the CHI3L1 levels, participants with KD and common fever were divided into three equal parts: T1 group (≤ 2849 pg/ml, n = 50), T2 group (2850–6021 pg/ml, n = 50) and T3 group (≥ 6022 pg/ml, n = 50).
Statistical analysis
Statistical analysis was performed with SPSS version 24.0 (IBM Corp., Armonk, NY, USA), GraphPad Prism 9 (GraphPad Software, Inc., San Diego, CA, USA) and Python software (statsmodels 0.11.1). Continuous variables are presented as median with interquartile range, and categorical variables are presented as number (%). Categorical variables were analyzed by chi-square tests, continuous variables were analyzed by the t test or Mann–Whitney U test or one-way analysis of variance, and correlation analysis was analyzed by the Spearman test. To investigate the relationship CHI3L1and KD prevalence, logistic regression analysis was employed. We established three models: Model 1, unadjusted; Model 2, adjusted for gender; and Model 3, adjusted for gender, WBC, NEU, Hb, PLT, ESR, CRP, ALT and ALB. The restricted cubic spline (RCS) was employed to explore the dose-response association of continuous CHI3L1 and the incidence of KD. Receiver operating characteristic (ROC) curve analysis was performed to assess the diagnostic ability of CHI3L1. Values of p < 0.05 were considered to indicate statistical significance.
Results
Clinical characteristics of patients with KD
In a cohort of 80 patients diagnosed with KD, 54 were male and 26 were female, with a median age of 35 months (range: 16.8–49.0 months). Of these patients, 69 had cKD and 11 had iKD. Compared to both healthy and fever control groups, the KD group exhibited significantly elevated WBC, NEU, PLT, CRP, ESR, PCT, and ALT, as well as decreased levels of Hb, ALB, and AST (all p < 0.05). There were no significant differences in BMI, age, gender, or LYM between the groups (all p > 0.05) (Table 1).
Serum levels of CHI3L1 in all participants
Serum of CHI3L1 levels were significantly higher in KD patients compared to both the fever control and healthy groups (P < 0.001), as illustrated in Fig. 1A. In comparison to the fever group, patients with iKD and cKD exhibited significantly elevated serum CHI3L1 levels (P < 0.001) (Fig. 1B).
Participant characteristic according to the tertiles of CHI3L1 among patients with KD or fever
The baseline characteristics of patients with KD or fever based on the tertiles of CHI3L1 levels were presented in Table 2. Among the three groups, individuals with higher CHI3L1 levels displayed elevated WBC, NEU, PLT, ESR, CRP, and ALT, as well as decreased levels of Hb and ALB, and were more likely to be male (all p < 0.05). As depicted in Fig. 2, there was a significant association between higher serum CHI3L1 levels and increased incidence of KD (p < 0.01).
Correlation of serum CHI3L1 levels with the laboratory parameters
The results presented in Fig. 3 illustrate a visual correlation heat map indicating that serum levels of CHI3L1 were positively correlated with male (r = 0.21, p = 0.011), WBC (r = 0.23, p = 0.004), NEU (r = 0.21, p = 0.009), PLT (r = 0.28, p < 0.001), ESR (r = 0.52, p < 0.001), CRP (r = 0.22, p < 0.001), and ALT (r = 0.31, p = 0.001). Conversely, serum levels of CHI3L1 were found to be negatively correlated with Hb levels (r=-0.21, p = 0.01), AST levels (r=-0.19, p = 0.018), and ALB levels (r=-0.46, p < 0.001).
The association between CHI3L1 and KD
A univariate logistic regression analysis was initially conducted to identify potential factors associated with KD in order to avoid the effect of overfitting events. Variables with a significance level of P < 0.1 in the univariate analysis were subsequently included in a multivariate logistic regression model. Model 1, which was unadjusted, revealed a positive correlation between serum CHI3L1 levels and the presence of KD (OR = 216.0, 95% CI = 39.88-1170.03, p for trend < 0.001). Model 2 (Model 1 with adjusted for gender) (OR = 205.07, 95% CI = 37.76-1113.75, p for trend < 0.001) and model 3 (Model 2 with adjusted for WBC, NEU, Hb, PLT, ESR, CRP, ALT and ALB) (OR = 123.5, 95% CI = 10.13,1505.09, p for trend < 0.001) also showed a positive association of serum of CHI3L1 levels with the presence of KD (Table 3). As shown in Fig. 4, the RCS demonstrated a linear association between serum of CHI3L1 levels and the incidence of KD after adjustment for all confounders (p for non-linearity = 0.098).
Potential diagnostic value of serum of CHI3L1 levels for KD
ROC curve analysis was utilized to assess the discriminatory ability of CHI3L1 in distinguishing KD from febrile illness (Fig. 5). The results indicated that continues CHI3L1 had an area under the curve (AUC) of 0.908 (with a cut-off value of 3720.0 pg/ml, sensitivity of 0.838, and specificity of 0.8) for differentiating all forms of KD(Fig. 5A). The tertiles of CHI3L1 exhibited significant predictive value for all types of KD, as evidenced by ROC curve areas of 0.884, a sensitivity of 0.938, and a specificity of 0.643 (Fig. 5B). Furthermore, continues CHI3L1 exhibited an AUC of 0.901, sensitivity of 0.826 and specificity of 0.8 for discriminating cKD at a cut-off of 3775.0 pg/ml (Fig. 5C), and an AUC of 0.952, sensitivity of 0.818 and specificity of 0.971 for discriminating iKD at a cut-off of 5773.8 pg/ml from febrile illness (Fig. 5D).
Discussion
This study represents the first investigation into the diagnostic utility of serum CHI3L1 in KD. Our findings indicate elevated levels of CHI3L1 in both complete and incomplete Kawasaki disease, with a strong positive correlation between CHI3L1 levels and various inflammatory markers. These results suggest that serum CHI3L1 may serve as a promising novel biomarker for KD.
Early identification of KD in febrile illnesses is of paramount importance. Various KD scoring systems utilizing laboratory tests, clinical manifestations, or a combination of both have been developed to differentiate KD from other febrile diseases3,17. Furthermore, serological markers have also been explored as potential indicators for distinguishing KD from febrile illnesses18,19. Regrettably, a definitive and consistently reliable laboratory test for distinguishing KD from other febrile conditions has yet to be established. In this study, elevated serum of CHI3L1 levels were found to be significantly higher in patients with KD compared to both healthy controls and fever controls, consistent with previous findings from a study with a limited sample size15. Our investigation further demonstrated a positive correlation between serum of CHI3L1 levels and the presence of KD, which remained significant even after controlling confounders. RCS also showed a linear association between serum of CHI3L1 levels and the incidence of KD. Furthermore, the diagnostic accuracy of CHI3L1 as determined by the ROC curve indicated that both continuous and categorical CHI3L1 exhibited high sensitivity and specificity in distinguishing KD from other febrile illnesses. Similar findings were observed in neurodegenerative diseases20, liver fibrosis21, cardiovascular disease11, inflammatory diseases22,23. Previous studies have confirmed the diagnostic value of several biomarkers for KD, such as N-terminal pro-BNP24, serum ferritin25 and platelet-derived growth factor CC (PDGF-CC)18. N-terminal pro-BNP demonstrated a sensitivity of 0.89 and a specificity of 0.72 for the diagnosis of KD24. In contrast, PDGF-CC exhibited a sensitivity of 0.75 and a specificity of 0.76 for diagnosing KD18. Additionally, serum ferritin showed a sensitivity of 0.75 and a specificity of 0.83 in the diagnosis of KD25. Compared with these biomarkers, serum CHI3L1 demonstrates high sensitivity and specificity in both cKD (sensitivity: 0.826 and specificity: 0.8 and iKD (sensitivity: 0.818 and specificity: 0.971) in present study. This finding supports the notion that CHI3L1 may serve as a promising biomarker for KD. Pediatricians commonly encounter challenges in diagnosing iKD due to the absence of clear diagnostic criteria. Current findings demonstrate a significant elevation in serum CHI3L1 levels in both cKD and iKD patients compared to those with febrile illnesses. Additionally, in a mouse model of KD-like vasculitis, serum CHI3L1 levels were also found to be elevated14, which is in line with current results. CHI3L1 demonstrates satisfactory performance in distinguishing iKD and cKD from febrile diseases based on the ROC curve analysis. This data provides additional support for the diagnostic utility of CHI3L1 in KD.
Elevated levels of inflammatory cytokines such as interleukin-6 (IL-6), IL-20, interferon-γ (IFN-γ), and tumor necrosis factor-α (TNF-α)26,27, along with an increase in monocytes, macrophages, neutrophils28,29, and M1 macrophages30, have been implicated in the pathogenesis of KD. CHI3L1, a 40-kDa proinflammatory glycoprotein, plays a role in the pathophysiology of various conditions including asthma, inflammatory diseases31,32, cancers33,34, and cardiovascular diseases32. CHI3L1 has the ability to activate immune cells such as macrophages and neutrophils, leading to the production of proinflammatory cytokines such as IL-6, IL-8, and TGF-β32,35,36. Inhibition of CHI3L1 has been demonstrated to attenuate inflammation37. These findings suggest that increased levels of CHI3L1 may contribute to the release of inflammatory cytokines and activation of immune cells, potentially playing a role in the development of KD. Actually, our study showed that a positive association of serum CHI3L1 levels with inflammatory factors (i.e.,WBC, NEU, ESR, CRP), and negative association of serum CHI3L1 levels with Hb, AST and ALB in KD patients. Additionally, high CHI3L1 have the high incidence of KD, and high WBC, NEU, PLT, ESR, CRP and ALT, as well as lower Hb and ALB according to the tertiles of CHI3L1. Moreover, it has been established in prior studies that the upregulation of CHI3L1 can be induced by TNF-α, IL-6, and M1 macrophage conditioned media38,39. These results suggest that the interplay between CHI3L1 inflammatory factors and macrophages may play a role in the pathogenesis of KD. However, further investigation is required to validate this hypothesis.
It is important to note some limitations in our study, including its single-center design and the relatively small sample size, despite being larger than previous studies. Furthermore, the levels of CHI3L1 serum at various time points during the acute phase of KD were not taken into consideration. Finally, it is imperative to further investigate the potential mechanism of CHI3L1 in the pathogenesis of KD.
Conclusion
Serum of CHI3L1 may be a novel and reliable biomarker for the diagnosis of KD.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Jiao, F. Y. et al. [Diagnosis and treatment of incomplete Kawasaki disease in children]. Zhongguo Dang Dai Er Ke Za Zhi. 25(3), 238–243. https://doi.org/10.7499/j.issn.1008-8830.2209127 (2023).
Kuo, H. C. Diagnosis, progress, and treatment update of Kawasaki disease. Int. J. Mol. Sci. 24(18). (2023).
Tsai, C. M. et al. A novel score system of blood tests for differentiating Kawasaki disease from febrile children. PLoS One. 16(1), e0244721 (2021).
Nandi, A., Pal, P. & Basu, S. A comparison of serum IL6 and CRP levels with respect to coronary changes and treatment response in Kawasaki disease patients: a prospective study. Rheumatol. Int. 39(10), 1797–1801 (2019).
Zeng, L. et al. Prokineticin 2 as a potential biomarker for the diagnosis of Kawasaki disease. Clin. Exp. Med. 23(7), 3443–3451 (2023).
Connolly, K. et al. Potential role of chitinase-3-like protein 1 (CHI3L1/YKL-40) in neurodegeneration and Alzheimer’s disease. Alzheimers Dement. 19(1), 9–24 (2023).
Li, F., Liu, A., Zhao, M. & Luo, L. Astrocytic Chitinase-3-like protein 1 in neurological diseases: potential roles and future perspectives. J. Neurochem. 165(6), 772–790 (2023).
Yeo, I. J., Lee, C. K., Han, S. B., Yun, J. & Hong, J. T. Roles of chitinase 3-like 1 in the development of cancer, neurodegenerative diseases, and inflammatory diseases. Pharmacol. Ther. 203, 107394 (2019).
Floro, S. et al. Role of chitinase 3-like 1 as a biomarker in multiple sclerosis: A systematic review and Meta-analysis. Neurol. Neuroimmunol. Neuroinflamm 9(4). (2022).
Dichev, V. et al. The lncRNAs/miR-30e/CHI3L1 Axis is dysregulated in systemic sclerosis. Biomedicines 10(2) (2022).
Wang, Y. et al. YKL-40 a new biomarker in patients with acute coronary syndrome or stable coronary artery disease. Scand. Cardiovasc. J. 42(5), 295–302 (2008).
Matsumoto, T. & Tsurumoto, T. Serum, YKL-40 levels in rheumatoid arthritis: correlations between clinical and laborarory parameters. Clin. Exp. Rheumatol. 19(6), 655–660 (2001).
Vos, K. et al. Raised human cartilage glycoprotein-39 plasma levels in patients with rheumatoid arthritis and other inflammatory conditions. Ann. Rheum. Dis. 59(7), 544–548 (2000).
Cao, Y. et al. [Role and mechanisms of CHI3L1 in coronary artery lesions in a mouse model of Kawasaki disease-like vasculitis]. Zhongguo Dang Dai Er Ke Za Zhi. 25(12), 1227–1233 (2023).
Kim, K. Y., Ahn, Y., Kim, D. Y., Kim, H. S. & Kim, D. S. Elevated serum YKL-40 levels in patients with Kawasaki disease. Biomarkers 22(3–4), 326–330 (2017).
McCrindle, B. W. et al. Diagnosis, treatment, and Long-Term management of Kawasaki disease: A scientific statement for health professionals from the American heart association. Circulation 135(17), e927–927e999 (2017).
Ling, X. B. et al. Point-of-care differentiation of Kawasaki disease from other febrile illnesses. J. Pediatr. 162(1), 183–8e3 (2013).
Zhang, J. et al. Serum levels of PDGF-CC as a potential biomarker for the diagnosis of Kawasaki disease. Ital. J. Pediatr. 50(1), 16 (2024).
Cai, X. et al. Plasma interleukin-41 serves as a potential diagnostic biomarker for Kawasaki disease. Microvasc Res. 147, 104478 (2023).
Baldacci, F. et al. The neuroinflammatory biomarker YKL-40 for neurodegenerative diseases: advances in development. Expert Rev. Proteom. 16(7), 593–600 (2019).
Bao, J. et al. Serum CHI3L1 as a biomarker for Non-invasive diagnosis of liver fibrosis. Discov Med. 33(168), 41–49 (2022).
Blazevic, N. et al. YKL-40 as a biomarker in various inflammatory diseases: A review. Biochem. Med. (Zagreb). 34(1), 010502 (2024).
Yu, R. et al. Serum CHI3L1 as a biomarker of interstitial lung disease in rheumatoid arthritis. Front. Immunol. 14, 1211790 (2023).
Lin, K. H. et al. Usefulness of natriuretic peptide for the diagnosis of Kawasaki disease: a systematic review and meta-analysis. BMJ Open. 5(4), e006703 (2015).
Kim, S. H. et al. Serum ferritin as a diagnostic biomarker for Kawasaki disease. Ann. Lab. Med. 41(3), 318–322 (2021).
Takahashi, K., Oharaseki, T. & Yokouchi, Y. Pathogenesis of Kawasaki disease. Clin. Exp. Immunol. 164(Suppl 1(Suppl 1), 20–22 (2011).
Wang, Y. et al. Evaluation of intravenous Immunoglobulin resistance and coronary artery lesions in relation to Th1/Th2 cytokine profiles in patients with Kawasaki disease. Arthritis Rheum. 65(3), 805–814 (2013).
Takahashi, K., Oharaseki, T., Yokouchi, Y., Hiruta, N. & Naoe, S. Kawasaki disease as a systemic vasculitis in childhood. Ann. Vasc Dis. 3(3), 173–181 (2010).
Takahashi, K., Oharaseki, T. & Yokouchi, Y. Histopathological aspects of cardiovascular lesions in Kawasaki disease. Int. J. Rheum. Dis. 21(1), 31–35 (2018).
Ohashi, R. et al. M1 macrophage is the predominant phenotype in coronary artery lesions following Kawasaki disease. Vasc Med. 24(6), 484–492 (2019).
Vos, K. et al. Cellular immune response to human cartilage glycoprotein-39 (HC gp-39)-derived peptides in rheumatoid arthritis and other inflammatory conditions. Rheumatol. (Oxford). 39(12), 1326–1331 (2000).
Yu, J. E. et al. Significance of chitinase-3-like protein 1 in the pathogenesis of inflammatory diseases and cancer. Exp. Mol. Med. 56(1), 1–18 (2024).
Shantha Kumara, H. M. et al. Plasma chitinase 3-like 1 is persistently elevated during first month after minimally invasive colorectal cancer resection. World J. Gastrointest. Oncol. 8(8), 607–614 (2016).
Wang, S. et al. Diagnostic and prognostic value of serum chitinase 3-like protein 1 in hepatocellular carcinoma. J. Clin. Lab. Anal. 36(2), e24234 (2022).
He, C. H. et al. Chitinase 3-like 1 regulates cellular and tissue responses via IL-13 receptor Α2. Cell. Rep. 4(4), 830–841 (2013).
Lee, C. G. et al. Role of breast regression protein 39 (BRP-39)/chitinase 3-like-1 in Th2 and IL-13-induced tissue responses and apoptosis. J. Exp. Med. 206(5), 1149–1166 (2009).
Chen, X. et al. CHI3L1 regulation of inflammation and the effects on osteogenesis in a Staphylococcus aureus-induced murine model of osteomyelitis. FEBS J. 284(11), 1738–1747 (2017).
Bhardwaj, R. et al. RelB/p50 complexes regulate cytokine-induced YKL-40 expression. J. Immunol. 194(6), 2862–2870 (2015).
Bonneh-Barkay, D. et al. Astrocyte and macrophage regulation of YKL-40 expression and cellular response in neuroinflammation. Brain Pathol. 22(4), 530–546 (2012).
Funding
This study was supported by the Sichuan Science and Technology Program (NO. 2022YFS0627), Luzhou Municipal People’s Government-Southwest Medical University Science and Technology strategic cooperation (NO. 2023LZXNYDJ042) and Clinical Medicine Program of Southwest Medical University (NO.2024LCYXZX48).
Author information
Authors and Affiliations
Contributions
Gang Li and Jian Zhao conceived the idea of the study and screened it. Bin Liu, Yan Duan and Dinghua Luo supervised the analyses. Jialin Zou, Wenjuan Li and Xu He collected the data. Jialin Zou drafted the manuscript. Jun Jiang and Gang Li critically reviewed and revised the manuscript; Dinghua Luo revised the manuscript. All authors have contributed to the manuscript and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zou, J., He, X., Li, W. et al. The association of serum CHI3L1 levels with the presence of Kawasaki disease. Sci Rep 15, 7693 (2025). https://doi.org/10.1038/s41598-025-91935-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-91935-8