Abstract
Although studies have reported various patterns of atherosclerotic aortic plaques (AAPs) detected by non-obstructive aortic angioscopy (NOA), the long-term outcomes associated with AAPs such as puff-chandelier rupture atheromatous plaque (PCR), remain unclear. This study investigated the long-term prognostic significance of AAPs detected by NOA in patients who underwent percutaneous coronary intervention (PCI). This retrospective multicenter cohort study included 167 patients who underwent PCI and NOA. The association between AAPs and the incidence of major adverse cardiac events (MACE) were assessed. MACE was categorized into: MACE1, including cardiac death, myocardial infarction, stroke, and ischemia-driven unplanned revascularization; and MACE2, including cardiac death, myocardial infarction, and stroke. There were no NOA-related complications. Of all AAPs, only PCR showed a significant prognostic value during the follow-up period (mean follow-up period: 6.3 years [range 5.9–6.6]). In multivariable Cox proportional hazards analysis, PCR was an independent predictor of MACE (MACE1; HR 1.91, 95% CI 1.04–3.49, P = 0.04, MACE2; hazard ratio [HR] 4.52, 95% confidence interval [CI] 1.23–16.57, P = 0.02). Kaplan–Meier analysis revealed that PCR was significantly associated with MACE. NOA during PCI is safe and feasible. Detecting PCR by NOA may provide reliable information for identifying patients at high risk of subsequent long-term adverse events after PCI.
Similar content being viewed by others
Introduction
Atherosclerosis is a disease driven by lipoproteins and characterized by a loss of elasticity owing to arterial wall thickening and stiffening, leading to plaque formation and progression owing to intimal inflammation, thickening, fibrosis, necrosis, and calcification1,2,3. Atherosclerosis can affect arteries of any size, including those in the coronary, carotid, cerebral, renal, peripheral, and aortic regions4. Numerous studies have reported a correlation between the advancement of aortic atherosclerosis and an elevated likelihood of subsequent morbidity and mortality attributable to vascular events, including acute coronary syndrome, stroke, and acute limb ischemia4,5,6,7,8,9,10,11,12. Atheromatous embolization is characterized by the release of debris from atherosclerotic aortic plaques (AAPs), resulting in showers of numerous microemboli, including cholesterol crystals, circulation from a proximal large artery to mid- and small-sized distal vessels. This causes occlusion and an inflammatory response, eventually leading to end-organ damage13,14. In clinical practice, the evaluation of patients with suspected or established atherosclerosis often relies on imaging modalities, including computed tomography angiography (CTA), magnetic resonance imaging (MRI), and positron emission tomography (PET)15,16,17. Furthermore, non-obstructive aortic angioscopy (NOA) has been reported as a valuable modality for direct visualization of coronary artery and aortic wall surfaces, as well as for detecting vulnerable AAPs, which might be most prone to atheromatous embolization at different stages of development within the aorta in vivo18,19,20. In a previously published study21, we reported that among all types of NOA-detected AAPs, the presence of puff-chandelier rupture atheromatous plaque (PCR) (Fig. 1, Supplementary Video 1) was significantly associated with an increased risk of future major adverse cardiac events (MACE). This was primarily driven by revascularization for recurrent ischemia, in patients who underwent percutaneous coronary intervention (PCI). However, our previous study had limitations, including a relatively short observation period (mean 2.9 years [range 2.1–3.8]) and the predominance of revascularization for recurrent ischemia as the primary MACE. To date, no studies have investigated the long-term outcomes associated with AAPs or the clinical significance of the presence of NOA-detected AAPs, particularly PCR, which may be associated with or cause vascular events. Therefore, this study aimed to investigate the long-term prognostic significance of AAPs or specific types of AAP detected using NOA, in patients who underwent PCI.
Representative images of NOA. (a) Representative images of PCR, material that scatters in a puff-like appearance and reflects against the light of NOA. (b) Representative images of normal intima without atherosclerotic changes. PCR, Puff-chandelier rupture atheromatous plaque. NOA, non-obstructive aortic angioscopy.
Methods
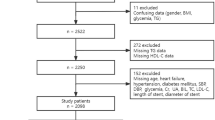
Study design and patient population
This retrospective multicenter registry study enrolled patients who underwent PCI and NOA examinations at Tsuchiura Kyodo General Hospital, Yokosuka Kyosai Hospital, and Yokohama Minami Kyosai Hospital between August 2014 and August 2018. The study design and short-term clinical outcomes have been described previously21. We included patients diagnosed with chronic coronary syndrome (CCS), unstable angina pectoris, non-ST-elevation myocardial infarction (NSTEMI), or ST-elevation myocardial infarction (STEMI) who underwent PCI and intra-aortic NOA scans at the time of PCI. The final decision to perform intra-aortic scans with NOA at the time of PCI was made at the discretion of the physician. In total, the data of 188 patients who underwent PCI and intra-aortic scanning along with NOA were included in the analysis. Thereafter, we excluded patients with a history of coronary artery bypass surgery (n = 3) or hemodialysis (n = 13). Therefore, the eligible dataset for this study included 172 NOA images obtained from 172 patients. Of the 172 patients initially enrolled who underwent PCI and intra-aortic scans with NOA, five were excluded from the final analysis due to suboptimal NOA image quality. Thus, 167 patients were studied in the final analysis. The definition of CCS followed the guidelines of the European Society of Cardiology15. All patients with CCS had positive noninvasive tests or fractional flow reserve values equal or less than 0.80. STEMI was defined as the presence of continuous chest pain, ST-segment elevation of ≥ 1 mm in two or more contiguous leads, and elevated cardiac biomarkers. NSTEMI was defined by ischemic symptoms without ST-segment elevation on electrocardiography but with elevated cardiac biomarkers. Unstable angina pectoris was defined by ischemic symptoms without ST-segment elevation on electrocardiography and with non-elevated cardiac biomarkers. Baseline patient characteristics and follow-up data were collected by reviewing the patient’s medical charts and conducting telephonic interviews. Prompt guideline-directed medical therapy was initiated in all patients after PCI.
Ethics statement
This study was approved by the institutional ethics committee on human research of Tsuchiura Kyodo General Hospital (No.755) and conformed to the Declaration of Helsinki statement on research involving human subjects. Informed consent was waived by the Tsuchiura Kyodo General Hospital Ethics Committee due to the retrospective nature of the study. The institutional PCI/NOA database was anonymized prior to analysis. All procedures were performed in accordance with the relevant guidelines.
Catheterization protocol and angiographic system
Catheterization was performed according to a standard institutional protocol. Coronary angiography and PCI procedures were performed via the radial or femoral artery using the 6-French system. Quantitative coronary angiography was performed using the CMS-MEDIS system (Medis Medical Imaging Systems, Leiden, Netherlands) to measure lesion length, minimum lumen diameter, reference lumen diameter, and percent diameter stenosis of the target lesion. Angiographic lesion morphology was classified according to the American Heart Association/American College of Cardiology lesion classification. The SYNTAX score was calculated using the SYNTAX score calculator (available at http://www.syntaxscore.com) to determine the relationship between the presence of AAP detected by NOA and the severity of coronary artery disease. After PCI, intracoronary scans with NOA were performed.
NOA image acquisition and analysis
The aortic wall was observed using NOA through a guiding catheter inserted via the radial or femoral artery with the 6-French system used for PCI. Coronary and aortic scans were performed using an angioscopy system (VISIBLE Fiber, Fiber Tech Co., Ltd., Tokyo, Japan) and a Fiber Imaging System (Fiber Tech Co., Ltd.) with a proprietary console (Intertec Medicals, Osaka, Japan). The angioscopy system was rotated to examine the aortic wall and was then slowly withdrawn from the ascending aorta to the abdominal aorta or from the abdominal aorta to the ascending aorta, depending on the access site. The entire aortic wall, from the ascending aorta to the abdominal aorta (and vice versa when using the radial approach), was continuously observed to evaluate the presence or absence of AAPs. AAPs detected by NOA were classified according to previous reports18,21: PCR was defined as a material that appears as a puff-like scatter and reflects light under NOA; strawberry jam appearance was defined as red thrombi that adhered to the surface of the aorta, resembling strawberry jam, which cannot be washed away by the infusion of low-molecular weight dextran; cotton candy appearance was defined as a solid white or red material resembling cotton candy but immovable; fissure was defined as a material that elongated a cleft or tear; and ulcer was defined as an ulcerative lesion deeper than erosion.
Study end points
In this study, MACE1 was defined as the cumulative composite incidence of cardiac death, non-fatal myocardial infarction, disabling stroke, and ischemia-driven remote unplanned revascularization (> 3 months after index PCI)22. MACE2 was defined as the cumulative composite incidence of cardiac death, non-fatal myocardial infarction, and disabling stroke. Scheduled revascularization for non-culprit lesions identified during the index coronary angiography was not considered a MACE.
Data collection and clinical follow-up
All clinical events were adjudicated by an independent clinical event committee blinded to baseline patient characteristics and NOA findings. Clinical follow-up was conducted via regular outpatient visits, telephone calls, and communication with referral medical institutions to ensure complete follow-ups for all patients.
Statistical analysis
Patient demographics are presented as n (%), where appropriate. Categorical data are expressed as numbers and percentages, and they were compared using the χ2 test or Fisher exact test, as appropriate. Continuous variables were expressed as mean ± standard deviation for normally distributed variables or as median (25th–75th percentile) for non-normally distributed variables, and these variables were compared using the Student t-test and Mann–Whitney U test, respectively. The predictors of MACE were determined using the Cox proportional hazard regression model. The variables used in multivariate analyses were selected if P < 0.01 in the univariate analysis, and then the forced-entry method was used. Survival curves for the presence of PCRs were created using the Kaplan–Meier method, and the data were compared using the log-rank test. A P value < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS, version 23.0 (SPSS, Inc., Chicago, IL, USA).
Results
Baseline patient characteristics, angiographic data, and NOA findings
The baseline patient characteristics and angiographic data are summarized in Table 1. In this cohort, most patients (73%) presented with CCS and showed type B2/C lesions on coronary angiography, as classified by the ACC/AHA. The NOA findings are summarized in Table 2. AAPs were detected in 126 patients (75%). PCRs were detected in 70 patients (42%), strawberry jam appearance was observed in 54 patients (32%), and cotton candy appearance was observed in 18 patients (11%). Findings of AAPs identified by NOA were concordant with the guidelines18. The procedure was completed in approximately 10–15 min, and there were no major or specific complications related to the catheterization procedures for angioscopy in this cohort.
Association between puff-chandelier rupture and clinical factors
As previously described21, patients with PCR have several coronary risk factors. They were significantly older and had a higher prevalence of hypertension, diabetes, dyslipidemia, and CKD compared with those without PCR. There were no statistically significant differences in clinical presentation or medications at baseline between the two groups. Regarding angiographic findings, ACC/AHA type B2/C lesions and multivessel disease were more frequently observed, and the SYNTAX score was higher in patients with PCR compared with those without it (Table 1). In multivariable analysis, patients’ age (odd ratio [OR]: 1.05 [1.01–1.10], P = 0.004), dyslipidemia (OR: 2.17 [1.01–4.68], P = 0.047), and multivessel diseases (OR: 2.36 [1.15–4.86], P = 0.021) were independent determinants of NOA-detected PCR (Supplementary Table 1).
Clinical outcomes at the long-term follow-up
Long-term follow-up was completed with no patients lost to follow-up. During a median follow-up of 6.3 years (range 5.9–6.6 years), MACE1 occurred in 47 patients (28.1%). Cardiac death, non-fatal myocardial infarction, disabling stroke, and ischemia-driven remote unplanned revascularization occurred in four (2.4%), four (2.4%), five patients (3.0%), and 34 patients (20.3%), respectively. Regarding MACE2, 14 patients (8.3%) experienced events. Specifically, cardiac death, non-fatal myocardial infarction, and disabling stroke occurred in five (3.0%), four (2.4%), and five patients (3.0%), respectively (Table 3).
The characteristics of the patients with and without MACE are shown in Supplementary Table 2. Regarding MACE1, patients with subsequent MACE1 had a higher prevalence of dyslipidemia and were more frequently prescribed statins at baseline than those without MACE1. There were no statistically significant differences in the clinical presentation or angiographic findings at baseline between the two groups. PCR was more frequently observed in patients with MACE1 compared with in those without MACE1, whereas no significant differences were observed in other NOA findings between the two groups (Table 2). Patients with subsequent MACE2 had a higher prevalence of dyslipidemia at baseline than those without MACE2. There were no statistically significant differences in the clinical presentation, medications, or angiographic findings at baseline between the two groups. Regarding NOA findings, PCR was more frequently observed in patients with MACE2 than in those without MACE2, whereas no significant difference was observed in other NOA findings between the two groups (Table 2).
Predictors of MACE
The predictors of MACE were determined using Cox proportional hazards models (Table 4). For MACE1, the univariable analysis identified NOA-detected PCR, NOA-detected cotton candy appearance, age, dyslipidemia, chronic kidney disease (CKD), white blood cells, statin medications, and SYNTAX scores as significant predictors. In multivariable analysis, NOA-detected PCR, CKD, and statin medications were independent predictors of MACE1. Kaplan–Meier analysis of survival from MACE1 also indicated significantly worse clinical outcomes in patients who underwent PCR compared with in those who did not undergo PCR (Fig. 2). Regarding MACE2, in univariable analysis, NOA-detected PCR, β-blocker medications, total cholesterol, and C-reactive protein were significant predictors of MACE2. In multivariable analysis, NOA-detected PCR and C-reactive protein levels emerged as independent predictors of MACE1. Kaplan–Meier analysis of survival from MACE2 showed that patients with PCR had significantly worse clinical outcomes compared to those without PCR (Fig. 2).
Kaplan–Meier analyses for a major adverse cardiac event according to the presence of PCR. (a) MACE1, cardiac death, non-fatal myocardial infarction, disabling stroke, and ischemia-driven remote unplanned revascularization (>3 months after index PCI) (b) MACE2, cardiac death, non-fatal myocardial infarction, and disabling stroke; Curves were compared using the log-rank test. PCR, Puff-chandelier rupture atheromatous plaque.
Discussion
The main findings of this study are as follows: (1) the cumulative incidence of MACE1 and MACE2 during a median follow-up of 6.3 years was 28.1% and 8.3%, respectively. (2) There were no specific complications related to NOA examinations. (3) Among patients who underwent PCI and NOA, the frequency of the presence of PCR, a type of AAP detected by NOA, was 42%, indicating that it was not uncommon. Additionally, both MACE1 and MACE2-free survival rates were significantly lower in patients with PCR during a median follow-up of 6.3 years compared with in those without PCR.
To the best of our knowledge, this is the first study to demonstrate the long-term clinical significance of PCR, a type of AAP detected using NOA. Atherosclerosis is a chronic inflammatory disease characterized by a dysfunctional interplay between immune response and lipids23,24. Cholesterol crystals, potential biomarkers of atherosclerosis, are abundant in atherosclerotic plaques and are associated with various diseases25,26. These cholesterol crystals, which are the main components of the necrotic core in atherosclerotic plaques, can be released into the circulation, leading to embolization in various organs27,28,29,30. The embolization of cholesterol crystals triggers both local and systemic inflammation, resulting in vascular fibrosis and obstruction, which can cause subsequent adverse events29,30,31. In previous NOA studies20,32,33, Komatsu et al. reported that in humans, cholesterol crystals in spontaneously ruptured aortic plaques were recognized as the main trigger of innate inflammation. They found that PCR exhibited a significantly higher number of cholesterol crystals, suggesting a potential correlation with inflammatory levels. A previous imaging study reported that vulnerable AAPs detected using transesophageal echocardiography, MRI, PET, and CTA were closely associated with coronary artery disease and were strong predictors of future adverse events5,11,16,17. The findings of our study highlight a significant association between NOA-detected PCR and the extended clinical significance for subsequent MACE during long-term follow-up. In contrast, patients without NOA-positive PCR had a favorable long-term prognosis (Fig. 2), consistent with the findings from previous imaging studies5,11,16,17. This study uniquely conducted long-term observations to evaluate whether NOA-detected PCR influences MACE1 and MACE2. While MACE2 is underpowered, it was retained to highlight the significance of PCR on hard endpoints over the long term. Our findings suggest that, while the process of PCR formation and development is not fully understood, PCR may represent one of the most unstable characteristics among AAPs. There is no doubt that the stage of atherosclerosis has advanced considerably when PCR is detected. Noninvasive tools like CT, MRI, and echocardiography are useful for assessing morphology and anatomy, but they cannot detect or differentiate AAPs as effectively as NOA, which provides high-resolution, true-color in vivo evaluation. NOA techniques, which enable direct aortic wall visualization and the analysis of AAPs, are currently the only methods, that provide us with reliable diagnostic information regarding the presence or absence of PCR, although a specific treatment targeting PCR has not yet been established. Continued future studies comparing noninvasive modalities with NOA findings may help predict the presence of AAPs and PCR detected by NOA using noninvasive modalities. For patients with PCR, aggressive risk-reduction therapy should be considered, although further large-scale prospective studies are needed to test our findings and develop a targeted therapeutic strategy.
This study had some limitations. First, the present study evaluated only 167 patients for the final analysis and carried the inherent limitations due to its small sample size, small event, and observational nature; therefore, precluded extensive subgroups analyses and a selection bias can not be canceled. It was conducted across three centers without a dedicated core laboratory for NOA analysis. Although the definition of AAPs and their documentation have been previously reported18, which formed the basis of our findings, further large-scale multicenter prospective trials are needed to validate the prognostic capabilities of NOA findings, including the presence of PCR, in patients who have undergone PCI and to confirm the findings of this study. Second, the decision to perform intra-aortic scans with NOA was made at the discretion of the physician, which may introduce selection bias and influence the generalizable findings. Third, anatomical complexities, such as a tortuous aortic wall, may limit complete circumferential imaging. Forth, the number of AAP findings was not described from each center, thus we could not assess its relationship with outcomes. Furthermore, in this study, none of the combinations of AAPs emerged as independent prognostic factors for MACE1 in the multivariate Cox regression analysis. This suggests that individual AAP features may be more prognostically significant than their combinations. Future work may explore a more granular analysis of the AAP burden. Finally, angioscopy is an invasive tool and operators must undergo a learning curve for its effective daily practical application. Despite these challenges, the safety shown in this study suggests that the clinical benefits of performing NOA are significant.
Conclusions
In patients who underwent PCI and NOA examinations, the presence of NOA-defined PCR was significantly associated with an increased risk of future MACE during the long-term follow-up. Detection of the presence of PCR in the aorta using NOA may help identify patients at high risk for subsequent adverse events after PCI, who may benefit from adjunctive aggressive management.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Bentzon, J. F., Otsuka, F., Virmani, R. & Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 114, 1852–1866. https://doi.org/10.1161/CIRCRESAHA.114.302721 (2014).
Frostegard, J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 11, 117. https://doi.org/10.1186/1741-7015-11-117 (2013).
Wolf, D. & Ley, K. Immunity and Inflammation in atherosclerosis. Circ. Res. 124, 315–327. https://doi.org/10.1161/CIRCRESAHA.118.313591 (2019).
Montgomery, D. H. et al. Natural history of severe atheromatous disease of the thoracic aorta: A transesophageal echocardiographic study. J. Am. Coll. Cardiol. 27, 95–101. https://doi.org/10.1016/0735-1097(95)00431-9 (1996).
Cohen, A. Atherosclerosis of the thoracic aorta further characterization for higher risk of vascular events. J. Am. Coll. Cardiol. 52, 862–864. https://doi.org/10.1016/j.jacc.2008.04.063 (2008).
Meissner, I. et al. Atherosclerosis of the aorta: Risk factor, risk marker, or innocent bystander? A prospective population-based transesophageal echocardiography study. J. Am. Coll. Cardiol. 44, 1018–1024. https://doi.org/10.1016/j.jacc.2004.05.075 (2004).
French Study of Aortic Plaques in Stroke Group et al. Atherosclerotic disease of the aortic arch as a risk factor for recurrent ischemic stroke. N. Engl. J. Med. 334, 1216–1221. https://doi.org/10.1056/NEJM199605093341902 (1996).
Ross, R. Atherosclerosis–an inflammatory disease. N. Engl. J. Med. 340, 115–126. https://doi.org/10.1056/NEJM199901143400207 (1999).
Fuster, V., Badimon, L., Badimon, J. J. & Chesebro, J. H. The pathogenesis of coronary artery disease and the acute coronary syndromes (1). N. Engl. J. Med. 326, 242–250. https://doi.org/10.1056/NEJM199201233260406 (1992).
Tunick, P. A. et al. High risk for vascular events in patients with protruding aortic atheromas: A prospective study. J. Am. Coll. Cardiol. 23, 1085–1090. https://doi.org/10.1016/0735-1097(94)90595-9 (1994).
Kalsch, H. et al. Aortic calcification onset and progression: Association with the development of coronary atherosclerosis. J. Am. Heart Assoc. 6. https://doi.org/10.1161/JAHA.116.005093 (2017).
Flory, C. M. Arterial occlusions produced by emboli from eroded aortic atheromatous plaques. Am. J. Pathol. 21, 549–565 (1945).
Kronzon, I. & Saric, M. Cholesterol embolization syndrome. Circulation 122, 631–641. https://doi.org/10.1161/CIRCULATIONAHA.109.886465 (2010).
Cross, S. S. How common is cholesterol embolism?. J. Clin. Pathol. 44, 859–861. https://doi.org/10.1136/jcp.44.10.859 (1991).
Knuuti, J. et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 41, 407–477. https://doi.org/10.1093/eurheartj/ehz425 (2020).
Guimaraes, J., de Almeida, J., Mendes, P. L., Ferreira, M. J. & Goncalves, L. Advancements in non-invasive imaging of atherosclerosis: Future perspectives. J. Clin. Lipidol. https://doi.org/10.1016/j.jacl.2023.11.008 (2023).
Blomberg, B. A. et al. Thoracic aorta calcification but not inflammation is associated with increased cardiovascular disease risk: Results of the CAMONA study. Eur. J. Nucl. Med. Mol. Imaging 44, 249–258. https://doi.org/10.1007/s00259-016-3552-9 (2017).
Hiro, T. et al. Consensus standards for acquisition, measurement, and reporting of non-obstructive aortic angioscopy studies: A report from the working group of Japan vascular imaging research organization for standardization of non-obstructive aortic angioscopy (Version 2017). Angioscopy 4, 1–11. https://doi.org/10.15791/angioscopy.re.17.0018 (2018).
Komatsu, S. et al. Early detection of vulnerable atherosclerotic plaque for risk reduction of acute aortic rupture and thromboemboli and atheroemboli using non-obstructive angioscopy. Circ J 79, 742–750. https://doi.org/10.1253/circj.CJ-15-0126 (2015).
Komatsu, S. et al. Angioscopic evaluation of spontaneously ruptured aortic plaques. J. Am. Coll. Cardiol. 71, 2893–2902. https://doi.org/10.1016/j.jacc.2018.03.539 (2018).
Yamaguchi, M. et al. Clinical significance of the presence of puff-chandelier ruptures detected by nonobstructive aortic angioscopy. Catheter. Cardiovasc. Interv. 96, 784–792. https://doi.org/10.1002/ccd.28574 (2020).
Nissen, S. E. et al. Bempedoic acid and cardiovascular outcomes in statin-intolerant patients. N. Engl. J. Med. 388, 1353–1364. https://doi.org/10.1056/NEJMoa2215024 (2023).
Herrero-Fernandez, B., Gomez-Bris, R., Somovilla-Crespo, B. & Gonzalez-Granado, J. M. Immunobiology of atherosclerosis: A complex net of interactions. Int. J. Mol. Sci. 20. https://doi.org/10.3390/ijms20215293 (2019).
Libby, P. Inflammation in atherosclerosis. Nature 420, 868–874. https://doi.org/10.1038/nature01323 (2002).
Li, X., Bayliss, G. & Zhuang, S. cholesterol crystal embolism and chronic kidney disease. Int. J. Mol. Sci. 18. https://doi.org/10.3390/ijms18061120 (2017).
Baumer, Y., McCurdy, S. G. & Boisvert, W. A. Formation and cellular impact of cholesterol crystals in health and disease. Adv. Biol. 5, e2100638. https://doi.org/10.1002/adbi.202100638 (2021).
Ghanem, F. et al. Cholesterol crystal embolization following plaque rupture: A systemic disease with unusual features. J. Biomed. Res. 31, 82–94. https://doi.org/10.7555/JBR.31.20160100 (2017).
Thazhathveettil, J., Kumawat, A. K., Demirel, I., Sirsjo, A. & Paramel, G. V. Vascular smooth muscle cells in response to cholesterol crystals modulates inflammatory cytokines release and promotes neutrophil extracellular trap formation. Mol. Med. 30, 42. https://doi.org/10.1186/s10020-024-00809-8 (2024).
Nidorf, S. M., Fiolet, A. & Abela, G. S. Viewing atherosclerosis through a crystal lens: How the evolving structure of cholesterol crystals in atherosclerotic plaque alters its stability. J. Clin. Lipidol. 14, 619–630. https://doi.org/10.1016/j.jacl.2020.07.003 (2020).
John Chapman, M. & Preston Mason, R. Cholesterol crystals and atherosclerotic plaque instability: Therapeutic potential of eicosapentaenoic acid. Pharmacol. Ther. 240, 108237. https://doi.org/10.1016/j.pharmthera.2022.108237 (2022).
Nelles, G. et al. Cholesterol crystals at the culprit lesion in patients with acute coronary syndrome are associated with worse cardiovascular outcomes at two years follow up—results from the translational OPTICO-ACS study program. Int. J. Cardiol. 399, 131665. https://doi.org/10.1016/j.ijcard.2023.131665 (2024).
Komatsu, S. et al. Cholesterol crystals as the main trigger of interleukin-6 production through innate inflammatory response in human spontaneously ruptured aortic plaques. J. Atheroscler. Thromb. 30, 1715–1726. https://doi.org/10.5551/jat.64098 (2023).
Komatsu, S. et al. Different characteristics and interleukin-6 ratios of scattering-type aortic plaques. Cureus 16, e52949. https://doi.org/10.7759/cureus.52949 (2024).
Author information
Authors and Affiliations
Contributions
M.Y., conception, design, analysis of data, and drafting the manuscript; H.F., design and critical revision of the manuscript; H.S., data collection and critical revision of the manuscript; M.S., data collection and critical revision of the manuscript; M.H., data collection and critical revision of the manuscript; E.U., data collection and critical revision of the manuscript; Y.K., data collection and critical revision of the manuscript; A.I., data collection and critical revision of the manuscript; K.H., data collection and critical revision of the manuscript; T.M., data collection and critical revision of the manuscript; H.H., data collection and critical revision of the manuscript; T.Y., data collection and critical revision of the manuscript; M.S., data collection and critical revision of the manuscript; T.S., data collection and critical revision of the manuscript; T.K., analysis of data, critical revision of the manuscript, and final approval of submission. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yamaguchi, M., Fujii, H., Suzuki, H. et al. Long-term clinical significance of the presence of puff-chandelier ruptures detected by non-obstructive aortic angioscopy. Sci Rep 15, 8530 (2025). https://doi.org/10.1038/s41598-025-92062-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-92062-0