Abstract
Knee osteoarthritis (OA) and atopic diseases are both characterized by chronic inflammation, yet their potential relationship remains unexplored. This study investigates whether atopic diseases are associated with an increased risk of knee OA in a large nationwide cohort. We conducted a nationwide cohort study using data from the Korean National Health Insurance Service (NHIS), including 880,300 individuals aged ≥ 50 years. Atopic disease was defined as ≥ 3 outpatient visits for asthma, allergic rhinitis, or atopic dermatitis. Knee OA incidence was identified using ICD-10 codes, and hazard ratios (HRs) were estimated using Cox proportional hazards models. Individuals with atopic diseases had a 36% higher risk of developing knee OA compared to those without (HR = 1.36, 95% CI: 1.35–1.37). A dose-response relationship was observed, with risk increasing progressively in individuals with multiple atopic conditions (HR = 1.44 for two conditions; HR = 1.51 for all three conditions). Subgroup analyses indicated that this association was strongest in younger individuals (50–59 years) and males. The results indicate a significant association between atopic diseases and an increased risk of knee OA, which was strongest in younger individuals. Further research is needed to understand the potential role of atopic-specific inflammation on OA development, and any potential implications for targeted therapies.
Similar content being viewed by others
Introduction
Knee osteoarthritis (OA) stands as a formidable challenge in global healthcare, affecting approximately 250 million people worldwide and projected to double in prevalence by 20401,2. Traditionally viewed as wear-and-tear arthritis, emerging evidence suggests that inflammation plays a pivotal role in knee OA’s onset and progression3,4. This paradigm shift opens new avenues for exploring potential connections with other inflammatory conditions, particularly atopic diseases, which include asthma, eczema, and allergic rhinitis.
Atopic diseases are experiencing a global surge, with prevalence rates increasing significantly, as evidenced by the notable rise in allergic diseases and asthma over the past five decades, particularly in developed countries5. These conditions share common inflammatory pathways with knee OA, including the activation of NF-κB and MAPK signaling cascades, which contribute to sustained inflammation and tissue degradation6,7,8. The chronic inflammation driven by pro-inflammatory cytokines in both knee OA and atopic diseases suggests a potential link between these conditions9,10,11, a connection that has not been thoroughly explored in the context of knee OA.
Although existing evidence indicates a general link between atopic diseases and various forms of arthritis, the specific connection to knee OA remains under-investigated9,12. Immune system dysregulation, particularly the overactivation of Th2 cells, and increased oxidative stress are common features in both conditions, further amplifying the potential link13,14. Additionally, some hypothesize that activity limitations due to atopic diseases could lead to weight gain or reduced physical activity, indirectly raising the risk of knee OA15. This study is one of the first to systematically explore this link on a large population scale, offering new insights into how atopic multimorbidity may contribute to knee OA. By addressing this gap, our research challenges traditional OA paradigms and introduces a new perspective on the role of systemic inflammation in knee OA development.
Our nationwide cohort study examines the relationship between single and multiple atopic diseases and the risk of incident knee OA over nine years. By analyzing a large cohort of 1,138,904 individuals aged 50 years and above, we aim to provide robust evidence on this potential association. This research not only reassesses traditional OA narratives by highlighting inflammation as a common thread between these conditions but also has the potential to reshape integrated care strategies.
The primary objectives of this study are:
-
1.
To explore the association between atopic diseases (asthma, atopic dermatitis, and allergic rhinitis) and the risk of developing knee OA.
-
2.
To understand the impact of multiple atopic conditions on the emergence of knee OA.
-
3.
To investigate potential variations in this association across different demographic and clinical subgroups.
By elucidating the interplay between atopic diseases and knee OA, our findings could inform more holistic approaches to patient care, potentially leading to earlier interventions, targeted therapies, and improved quality of life for individuals suffering from these chronic ailments.
Results
Baseline characteristics
The study cohort included 880,300 individuals, with 140,399 (15.9%) having one or more atopic diseases (Table 1). Significant differences were noted across several demographic and clinical variables between atopic and nonatopic groups.
Individuals with atopic diseases were generally older (mean age 59.9 ± 8.0 years vs. 58.9 ± 7.7 years in the nonatopic group) and had a lower proportion of males (53.7% vs. 60.6%). Among those with asthma, 20.42% were in their seventies, compared to 10.08% of those without asthma.
Comorbidities such as hypertension, diabetes mellitus, and dyslipidemia were more prevalent in the atopic group. For instance, hypertension was present in 47.0% of individuals with asthma compared to 39.6% without asthma. Conversely, the nonatopic group had higher proportions of smokers and drinkers.
In the atopic group, allergic rhinitis was the most prevalent condition (89.3%, n = 125,409), followed by asthma (22.7%, n = 31,936) and atopic dermatitis (2.4%, n = 3,375).
Primary outcome: risk for knee OA
During a mean follow-up of 7.7 ± 3.5 years, knee OA was newly diagnosed in 350,995 participants. The incidence rate was higher in the atopic group (69,362 cases; 70.50 per 1000 person-years) compared to the nonatopic group (281,633 cases; 48.58 per 1000 person-years). After adjusting for confounding variables, the atopic group exhibited a significantly increased risk of knee OA compared to the nonatopic group (Hazard Ratio [HR] = 1.36; 95% Confidence Interval [CI]: 1.35–1.37; P < 0.001) (Table 2). Kaplan-Meier analysis confirmed that the atopic group had a significantly lower knee OA-free survival rate (log-rank test, p < 0.001) (Fig. 1).
Kaplan-Meier Curve for Knee Osteoarthritis Incidence by Atopic Disease Status. The Kaplan-Meier curves show the cumulative incidence probability of knee osteoarthritis (OA) over the 9-year follow-up period, stratified by atopic disease status. The black line represents individuals without atopic diseases, while the red line represents those with one or more atopic conditions (asthma, atopic dermatitis, or allergic rhinitis).
A dose-response relationship was observed, where having multiple atopic diseases further elevated the risk of knee OA. Compared to the nonatopic group, the HR for knee OA was:
-
1.
1.35 (95% CI: 1.34–1.36) in individuals with a single atopic disease;
-
2.
1.44 (95% CI: 1.41–1.47) in those with two atopic diseases;
-
3.
1.51 (95% CI: 1.28–1.78) in those with all three atopic diseases.
This trend was statistically significant (P for trend < 0.001) and is illustrated in Fig. 2. The relationship is visually represented in Fig. 2 through both bar graphs showing incidence rates and line graphs depicting hazard ratios.
Incidence rates and hazard ratios for knee osteoarthritis by atopic disease presence. This figure displays the incidence rates (IR) per 1,000 person-years and hazard ratios (HR) for knee osteoarthritis across different groups of atopic disease presence. The bars represent the incidence rates, while the lines represent the hazard ratios with 95% confidence intervals. Data are adjusted for age, sex, income, smoking, drinking, physical activity, diabetes mellitus, dyslipidemia, and hypertension. Atopic diseases include asthma, allergic rhinitis, and atopic dermatitis. CI, confidence interval.
Among the individual atopic diseases, allergic rhinitis posed the highest risk for knee OA (HR = 1.36; 95% CI: 1.34–1.37). Furthermore, participants with three concurrent atopic conditions had the highest hazard rate for knee OA development (HR = 1.50; 95% CI: 1.28–1.78).
Subgroup analysis
Subgroup analyses revealed that the presence of atopic diseases was associated with an increased risk of knee OA across all examined categories (Table 3). Adjusted HRs with 95% CIs were calculated for various demographic and clinical subgroups.
Significant interactions were observed for age (p for interaction < 0.0001), sex (p < 0.0001), and obesity status (p < 0.0001). The association between atopic diseases and knee OA risk was strongest in the 50–59 age group (HR = 1.40; 95% CI: 1.38–1.41) and slightly attenuated in older age groups. Males showed a higher risk (HR = 1.41; 95% CI: 1.39–1.42) compared to females (HR = 1.33; 95% CI: 1.31–1.34). Non-obese individuals had a higher HR (1.39; 95% CI: 1.37–1.40) than obese individuals (HR = 1.31; 95% CI: 1.29–1.33).
No significant interaction was found between atopic diseases and diabetes mellitus (p for interaction = 0.2154). Detailed HRs for all subgroups are presented in Table 3.
Discussion
This nationwide cohort study provides robust evidence of a significant association between atopic diseases and an increased risk of developing knee OA. Our results demonstrate that individuals with at least one atopic condition—asthma, atopic dermatitis, or allergic rhinitis—face a higher incidence rate of knee OA compared to those without any atopic diseases. Notably, we observed a dose-response relationship, with the risk escalating progressively in the presence of multiple atopic diseases (HR: 1.36, 1.44, and 1.51 for one, two, and three atopic conditions, respectively).
These findings challenge the traditional view of knee OA as primarily a wear-and-tear condition16,17 and contribute to a more nuanced understanding of its etiology12,18. Our results indicate that individuals with atopic diseases have an increased risk of knee OA, suggesting that shared factors between atopic conditions and OA warrant further investigation19,20. This aligns with emerging literature positioning inflammation as a central element in OA development and progression12,21,22.
Our research extends previous observations on the link between atopic diseases and arthritis12,23. Chang et al. (2021) emphasized the role of immune cells in OA flares24, which parallels our observations of exacerbated joint pathology in atopic individuals. Moreover, our study uniquely quantifies the impact of different atopic conditions on knee OA risk, addressing a gap in existing research.
The chronic inflammatory state induced by atopic diseases may accelerate joint degradation through mechanisms similar to those observed in age-related OA progression25,26. This parallelism with the concept of “inflammaging” suggests that atopic diseases could potentially exacerbate age-related changes in joint tissues. Inflammaging, a term combining “inflammation” and “aging,” describes the chronic, low-grade inflammation that typically develops as individuals age and is associated with various age-related diseases.
Our study suggests that atopic diseases may predispose individuals to knee OA through systemic inflammation and immune dysregulation. Although our observational study design limits direct causal inference, our findings align with recent research. Koo et al. (2021) and Kim et al. (2024) have identified increased OA incidence in patients with atopic conditions, suggesting that chronic inflammatory states associated with these conditions could detrimentally impact joint health27,28. Yao et al. (2023) further emphasize the need to explore shared inflammatory pathways as potential contributors to joint disease in atopic dermatitis patients29.
These results highlight the need to consider atopic status when assessing OA risk. Further research is necessary to determine whether inflammatory pathways play a direct role in OA development in individuals with atopic diseases. Our findings emphasize the need for an integrated treatment approach for patients with both atopic diseases and OA, potentially involving coordinated care between rheumatologists, allergists, and primary care physicians.
Furthermore, our study opens up new possibilities for novel treatment strategies. Biologics used in severe atopic diseases that target specific inflammatory pathways (e.g., IL-4, IL-13 inhibitors) could be investigated for their potential benefits in preventing or slowing OA progression30.
This nationwide cohort study’s key strengths lie in its large, representative sample of the Korean population and its longitudinal design spanning nine years. This approach provides robust statistical power and allows for observing the development of knee osteoarthritis over a significant period, crucial for understanding the temporal relationships between atopic diseases and knee OA onset. Our statistical analysis, using the Cox proportional hazards regression model, adjusted for a wide range of confounders, enhancing the reliability of our findings by minimizing the influence of external factors.
Despite its strengths, our study has several limitations. First, surveillance bias may have influenced our findings, as individuals with atopic diseases tend to have more frequent healthcare visits, increasing the likelihood of OA diagnosis. However, this does not fully explain our results, given the dose-response relationship and adjustment for comorbidities. Second, we adjusted for obesity status (obese vs. non-obese) to mitigate collinearity, but BMI as a continuous variable could not be incorporated due to data access limitations. Future research with more granular BMI data could help refine these findings. Third, our definition of atopic diseases required ≥ 3 outpatient visits per year, which ensured sustained disease activity but may have excluded milder or episodic cases, limiting generalizability. Further studies should explore associations across different disease severities. Fourth, we lacked medication data, making it unclear whether anti-inflammatory treatments for atopic diseases influence OA risk. Given the distinct immune pathways of atopic diseases (Th2-driven) and OA (Th1/Th17-driven), IL-4/IL-13 blockade has been explored in inflammatory diseases31,32, warranting further investigation into its potential effects on OA progression. Finally, misclassification bias is possible due to reliance on ICD-10 codes, which may not fully capture disease severity. However, our use of a large, nationally representative cohort and rigorous case definitions helps mitigate this concern.
Despite these limitations, our study provides valuable insights into the link between atopic diseases and OA, underscoring the need for integrated OA risk assessment. Future research incorporating prospective designs, biomarker analyses, and genetic data could further clarify underlying mechanisms.
Future research should prioritize longitudinal cohort studies to track knee OA progression in individuals with atopic diseases, strengthening evidence for a causal relationship. Parallel experimental studies targeting shared inflammatory pathways could provide insights into underlying mechanisms. Identifying and validating specific biomarkers common to both conditions could enhance early diagnosis and inform targeted treatments. Finally, clinical trials assessing the impact of managing atopic inflammation on knee OA outcomes are essential for translating these findings into practice.
Conclusion
Our nationwide cohort study demonstrates a significant association between atopic diseases and an increased risk of knee OA, with the strongest effect observed in younger individuals. While these findings highlight atopic diseases as a potential risk factor, further research is needed to clarify the underlying mechanisms and the role of atopic-specific inflammation in OA development.
Methods
Study design and population
We conducted a population-based retrospective cohort study using data from the National Health Insurance Services (NHIS) database of Korea, which covers approximately 97% of the Korean population. Demographic, socioeconomic, and clinical data, including sex, age, height, weight, BMI, smoking status, alcohol consumption, exercise habits, income level, blood glucose, cholesterol levels, blood pressure, and eGFR, were obtained from the NHIS database. Height and weight were measured during routine health screenings conducted by trained medical personnel following standardized protocols, and BMI was calculated as weight (kg) divided by height squared (m²). Our study focused on individuals aged 50 years and above who participated in health screenings during 200933.
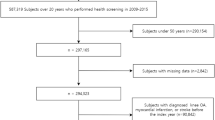
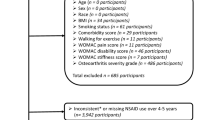
From an initial population of 4,234,412 individuals, we refined our cohort through a multi-step selection process (Fig. S1). Exclusion criteria included: age below 50 years, pre-existing knee OA or related interventions, insufficient follow-up data, and limited claims (1 or 2 within the year prior to screening) for atopic diseases. The final study cohort consisted of 880,300 individuals.
We followed up each participant starting one year after their health screening date in 2009 until they received a knee OA diagnosis, died, or until December 31, 2020, whichever came first.
Data sources and ethical considerations
This study utilized the National Health Insurance Services (NHIS) database of Korea, a comprehensive resource covering approximately 97% of the Korean population. Established by the Korean government, the NHIS database provides extensive healthcare service claims and screening data. The reliability of NHIS cohorts has been validated in previous studies, ensuring a robust foundation for our research34,35.
We conducted the study in accordance with the Declaration of Helsinki, and the Institutional Review Board (IRB) of the Catholic University of Korea approved it (protocol number VC24ZISI0188). Given the retrospective nature of the study and the use of de-identified data, the IRB of the Catholic University of Korea waived the requirement for individual informed consent. This approach ensured ethical rigor while facilitating access to a large-scale, representative dataset for population-based analysis.
Definition of atopic diseases
We defined atopic diseases in this study as asthma, atopic dermatitis, and allergic rhinitis, using the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) codes: asthma (J45-46), atopic dermatitis (L20), and allergic rhinitis (J301-304)33. We classified participants as having an atopic disease if they had at least one confirmed diagnosis of any component of the atopic triad. To ensure diagnostic accuracy, we required a minimum of three documented clinical visits per year for each condition. This threshold has been previously applied in NHIS-based studies to identify clinically significant atopic disease cases36,37. Individuals without any atopic triad diagnosis were categorized as nonatopic.
Primary outcome
The primary endpoint was the new onset of knee OA. We identified knee OA using ICD-10 codes specific to knee OA (M17) or general OA (M15 for polyarthrosis, M19 for other forms of arthrosis), along with a procedure code for knee X-ray within the same medical claim. This methodology is consistent with validated approaches from previous research38. The follow-up period commenced one year after the initial health screening and continued until knee OA diagnosis, death, or December 31, 2020, whichever occurred first.
Assessment of health behaviors and comorbidities
We assessed lifestyle factors and the presence of comorbid conditions through a comprehensive review of patient-reported outcomes and clinical data. Lifestyle behaviors were self-reported via standardized questionnaires. Socioeconomic status was determined through income tiers, with the lowest tier representing the bottom 25% of the population by income. Smoking habits were categorized into three distinct groups: non-smokers, former smokers, and active smokers. Alcohol consumption was classified based on daily intake: non-drinkers, moderate consumption (less than 30 g per day), and significant consumption (30 g or more per day). Physical activity levels were delineated based on type and frequency: non-exercisers, moderate exercisers (over 30 min of moderate activity at least once a week), and regular exercisers (over 30 min of moderate activity at least five times a week or over 20 min of vigorous activity at least three times a week).
Comorbid health conditions such as hypertension, diabetes mellitus, and dyslipidemia were identified using a combination of ICD-10-CM diagnostic codes, prescribed medication records, and biometric measures from health examinations. The criteria for these conditions were consistent with previously established and validated protocols39. In our health screenings, fasting blood tests were conducted to measure serum glucose and lipid levels, following at least eight hours of fasting, to ensure the accuracy of these diagnostic markers. Further information on the operational definitions used comorbid conditions can be found in the supplementary material of this study, delineated in table S1.
Statistical evaluation methods
Our data analysis commenced with the delineation of baseline characteristics, where we reported continuous variables as mean values with their corresponding standard deviations and categorized variables in frequencies and proportions. Comparative analysis of continuous data was executed via the application of the t test or non-parametric alternatives when appropriate. Chi-square testing facilitated the comparison of categorized variables.
We determined the incidence rates of knee OA by tallying new cases and dividing by the accumulated person-years, expressing the result as the number of events per 1,000 person-years. The risk estimation for the occurrence of knee OA was performed using the Cox proportional hazards regression model, yielding hazard ratios and 95% confidence intervals40. Our model adjustments progressed sequentially: the primary model accounted for age and gender, the secondary model additionally considered lifestyle factors and comorbidities, and the tertiary model incorporated further adjustments for chronic inflammatory conditions.
The strength of association was quantified using Cohen’s d, calculated from the natural logarithm of the hazard ratio and standardized41. We conducted a retrospective power calculation to validate the robustness of our findings. Subgroup analyses were stratified based on demographics, lifestyle factors, and comorbidity status, with the Bonferroni method employed to adjust for multiple testing.
The Kaplan-Meier estimator was used to plot survival curves, with the log-rank test comparing the curves. The Cox model also supported the survival analysis, and interactions were tested to identify significant differences between subgroups. We established significance for all tests at a p-value of less than 0.05 and conducted two-tailed analyses.
All statistical computations were performed with SAS (version 9.4, SAS Institute, Inc, Cary, NC, 2013) and R program (version 3.2.4, R Core Team, Vienna, Austria, 2017), ensuring rigorous data handling and analysis integrity.
Data availability
The datasets used and/or analyzed during the current study were provided by the Korean National Health Insurance Service (NHIS) and are part of a customized, de-identified database to ensure privacy. Due to NHIS data access restrictions, the raw data cannot be shared. Further information on accessing NHIS data is available at https://nhiss.nhis.or.kr. The datasets used in this study are available from the corresponding author on reasonable request, subject to NHIS approval and data access policies.
References
Cui, A. et al. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine 29–30. https://doi.org/10.1016/j.eclinm.2020.100587 (2020).
O’Brien, K. M. et al. Telephone-based weight loss support for patients with knee osteoarthritis: a pragmatic randomised controlled trial. Osteoarthr. Cartil. 26, 485–494. https://doi.org/10.1016/j.joca.2018.01.003 (2018).
van den Bosch, M. H. J. Inflammation in osteoarthritis: is it time to dampen the alarm(in) in this debilitating disease? Clin. Exp. Immunol. 195, 153–166. https://doi.org/10.1111/cei.13237 (2019).
Majeed, M., Majeed, S., Narayanan, N. K. & Nagabhushanam, K. A pilot, randomized, double-blind, placebo-controlled trial to assess the safety and efficacy of a novel Boswellia serrata extract in the management of osteoarthritis of the knee. Phytother Res. 33, 1457–1468. https://doi.org/10.1002/ptr.6338 (2019).
Holm, M. et al. Quantitative glycoproteomics of human milk and association with atopic disease. PLoS One 17, e0267967. https://doi.org/10.1371/journal.pone.0267967 (2022).
Tai, S. K., Lin, Y. H., Lin, C. H. & Lin, M. C. Antibiotic exposure during pregnancy increases risk for childhood atopic diseases: a nationwide cohort study. Eur. J. Med. Res. 29, 189. https://doi.org/10.1186/s40001-024-01793-9 (2024).
Rhew, K., Brown, J. D. & Oh, J. M. Atopic disease and Anemia in Korean patients: Cross-Sectional study with propensity score analysis. Int. J. Environ. Res. Public. Health 17. https://doi.org/10.3390/ijerph17061978 (2020).
Lugović-Mihić, L. et al. Atopic dermatitis: disease features, therapeutic options, and a multidisciplinary approach. Life (Basel) 13. https://doi.org/10.3390/life13061419 (2023).
Ren, G. et al. Serum and synovial fluid cytokine profiling in hip osteoarthritis: distinct from knee osteoarthritis and correlated with pain. BMC Musculoskelet. Disord. 19, 39. https://doi.org/10.1186/s12891-018-1955-4 (2018).
Molnar, V. et al. Cytokines and chemokines involved in osteoarthritis pathogenesis. Int. J. Mol. Sci. 22. https://doi.org/10.3390/ijms22179208 (2021).
Riggs, K. C. & Sankar, U. Inflammatory mechanisms in post-traumatic osteoarthritis: a role for CaMKK2. Immunometabolism (Cobham) 5, e00031. https://doi.org/10.1097/in9.0000000000000031 (2023).
Wang, Q. et al. IgE-mediated mast cell activation promotes inflammation and cartilage destruction in osteoarthritis. Elife 8. https://doi.org/10.7554/eLife.39905 (2019).
Liu, B., Zhang, M., Zhao, J., Zheng, M. & Yang, H. Imbalance of M1/M2 macrophages is linked to severity level of knee osteoarthritis. Exp. Ther. Med. 16, 5009–5014. https://doi.org/10.3892/etm.2018.6852 (2018).
Chen, F., Su, W., Bedenbaugh, A. V. & Oruc, A. Health care resource utilization and burden of disease in a U.S. Medicare population with a principal diagnosis of osteoarthritis of the knee. J. Med. Econ. 23, 1151–1158. https://doi.org/10.1080/13696998.2020.1801453 (2020).
Mariano, A. et al. The nutraceuticals as modern key to achieve erythrocyte oxidative stress fighting in osteoarthritis. Curr. Issues Mol. Biol. 44, 3481–3495. https://doi.org/10.3390/cimb44080240 (2022).
Jang, S., Lee, K. & Ju, J. H. Recent updates of diagnosis, pathophysiology, and treatment on osteoarthritis of the knee. Int. J. Mol. Sci. 22. https://doi.org/10.3390/ijms22052619 (2021).
Zeng, C. Y., Zhang, Z. R., Tang, Z. M. & Hua, F. Z. Benefits and mechanisms of exercise training for knee osteoarthritis. Front. Physiol. 12, 794062. https://doi.org/10.3389/fphys.2021.794062 (2021).
Park, S. & Choi, N. K. Association between serum Immunoglobulin E levels and knee osteoarthritis in Korean adults. Osteoarthr. Cartil. 28, 462–467. https://doi.org/10.1016/j.joca.2020.02.830 (2020).
Cudejko, T. et al. Proprioception mediates the association between systemic inflammation and muscle weakness in patients with knee osteoarthritis: results from the Amsterdam osteoarthritis cohort. J. Rehabil Med. 50, 67–72. https://doi.org/10.2340/16501977-2272 (2018).
Li, Y. et al. Relative efficacy and safety of Anti-Inflammatory biologic agents for osteoarthritis: A conventional and network Meta-Analysis. J. Clin. Med. 11. https://doi.org/10.3390/jcm11143958 (2022).
Baker, M. C. et al. Increased risk of osteoarthritis in patients with atopic disease. Ann. Rheum. Dis. 82, 866–872. https://doi.org/10.1136/ard-2022-223640 (2023).
Liu, S., Mi, J., Liu, W., Xiao, S. & Gao, C. Blocking of checkpoint receptor PD-L1 aggravates osteoarthritis in macrophage-dependent manner in the mice model. Int. J. Immunopathol. Pharmacol. 33, 2058738418820760. https://doi.org/10.1177/2058738418820760 (2019).
Valdes, A. M. et al. Omega-6 Oxylipins generated by soluble epoxide hydrolase are associated with knee osteoarthritis. J. Lipid Res. 59, 1763–1770. https://doi.org/10.1194/jlr.P085118 (2018).
Chang, M. H. et al. Arthritis flares mediated by tissue-resident memory T cells in the joint. Cell. Rep. 37, 109902. https://doi.org/10.1016/j.celrep.2021.109902 (2021).
Rezuș, E. et al. The link between inflammaging and degenerative joint diseases. Int. J. Mol. Sci. 20. https://doi.org/10.3390/ijms20030614 (2019).
Liao, Y. C. et al. NOX2-Deficient neutrophils facilitate joint inflammation through higher Pro-Inflammatory and weakened immune checkpoint activities. Front. Immunol. 12, 743030. https://doi.org/10.3389/fimmu.2021.743030 (2021).
Koo, H. K., Song, P. & Lee, J. H. Novel association between asthma and osteoarthritis: a nationwide health and nutrition examination survey. BMC Pulm Med. 21, 59. https://doi.org/10.1186/s12890-021-01425-6 (2021).
Sunmi, K. Association between Allergic Rhinitis and Osteoarthritis in the Korean Adult Population-Based on the 3rd to 8th Korea National Health and Nutrition Examination Survey. www.h 24, 11–19 (2024).
Yao, C., Ye, W. & Chen, M. Inhibition of mast cell degranulation in atopic dermatitis by Celastrol through suppressing MRGPRX2. Dis. Markers. 2023 (9049256). https://doi.org/10.1155/2023/9049256 (2023).
Defois, A. et al. Osteoarthritic chondrocytes undergo a glycolysis-related metabolic switch upon exposure to IL-1b or TNF. Cell. Commun. Signal. 21, 137. https://doi.org/10.1186/s12964-023-01150-z (2023).
Schrom, K. P., Kobs, A. & Nedorost, S. Clinical psoriasiform dermatitis following dupilumab use for autoeczematization secondary to chronic stasis dermatitis. Cureus 12 (4), e7561 (2020).
Jia, F., Liu, Y., Wang, X. & Zhang, T. Dupilumab: advances in the off-label usage of IL-4/IL-13 antagonist in dermatoses. Dermatol. Ther. 35 (12), e15924 (2022).
Choi, Y. J. et al. Increased risk of atrial fibrillation in patients with atopic triad: A nationwide Population-Based study. J. Allergy Clin. Immunol. Pract. 9, 3422–3430e3425. https://doi.org/10.1016/j.jaip.2021.04.056 (2021).
Lee, S. R., Choi, E. K., Han, K. D., Cha, M. J. & Oh, S. Trends in the incidence and prevalence of atrial fibrillation and estimated thromboembolic risk using the CHA(2)DS(2)-VASc score in the entire Korean population. Int. J. Cardiol. 236, 226–231. https://doi.org/10.1016/j.ijcard.2017.02.039 (2017).
Kwon, H., Han, K. D. & Park, C. Y. Weight change is significantly associated with risk of thyroid cancer: A nationwide population-based cohort study. Sci. Rep. 9, 1546. https://doi.org/10.1038/s41598-018-38203-0 (2019).
Han, J. H. et al. The risk of psoriasis in patients with allergic diseases: a nationwide population-based cohort study. Allergy Asthma Immunol. Res. 13 (4), 638. https://doi.org/10.4168/aair.2021.13.4.638 (2021).
Choi, Y. J. et al. Increased risk of atrial fibrillation in patients with atopic triad: a nationwide population-based study. J. Allergy Clin. Immunol. Pract. 9 (9), 3422–3430. https://doi.org/10.1016/j.jaip.2021.04.056 (2021).
Park, H. R. et al. Validation of algorithms to identify knee osteoarthritis patients in the claims database. Int. J. Rheum. Dis. 22, 890–896. https://doi.org/10.1111/1756-185x.13470 (2019).
Park, D. et al. Association of general and central obesity, and their changes with risk of knee osteoarthritis: a nationwide population-based cohort study. Sci. Rep. 13, 3796. https://doi.org/10.1038/s41598-023-30727-4 (2023).
Hsieh, F. Y. & Lavori, P. W. Sample-size calculations for the Cox proportional hazards regression model with nonbinary covariates. Control Clin. Trials. 21, 552–560. https://doi.org/10.1016/s0197-2456(00)00104-5 (2000).
Cohen, J. Statistical Power Analysis for the Behavioral Sciences. Academic Press. Revised. (Academic Press, 2013).
Acknowledgements
DP, HSK and KH conceived the presented idea. KH collected the study data and did the statistical analyses. DP wrote the initial draft of the paper. All authors reviewed the manuscript. HSK, YHC, and KH supervised the manuscript. All authors approved the paper. The authors thank the participants of the Korean National Health Insurance Service-Health Screening program.
Funding
The authors wishes to acknowledge the support of the St. Vincent’s Hospital, The Catholic University of Korea, Research Institute of Medical Science Foundation (VC24ZISI0188), and the finacial support of the Catholic Medical Center Research Foundation made in the program year of 2024.
Author information
Authors and Affiliations
Contributions
DP, HSK and KH conceived the presented idea. KH collected the study data and did the statistical analyses. DP wrote the initial draft of the paper. All authors reviewed the manuscript. HSK, YHC, and KH supervised the manuscript. All authors approved the paper. The authors thank the participants of the Korean National Health Insurance Service-Health Screening program.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical statement
Approval of the research protocol: This study was approved the IRB of the Catholic University of Korea (VC24ZISI0188).
Informed consent
N/A (the need for informed consent was waived by the IRB of the Catholic University of Korea due to the retrospective nature of the study and the anonymized nature of the data).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Park, D., Choi, YH., Han, K. et al. Risk of knee osteoarthritis in patients with multiple atopic conditions: a nationwide study. Sci Rep 15, 15293 (2025). https://doi.org/10.1038/s41598-025-92247-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-92247-7