Abstract
Increasing studies have shown that the efficacy of Weizmannia coagulans in treating various cancers. We recently identified W. coagulans MZY531 with potent cell anti-proliferation and exhibiting apoptosis induction activities against the mouse H22 hepatocellular carcinoma cell line.However, the anti-cancer effect of W. coagulans MZY531 against liver cancer in vivo has not been verified. The objective of this study was to assess the anti-hepatoma effect of W. coagulans MZY531 on H22 tumor-bearing mice and the underlying mechanism. The results demonstrated that W. coagulans MZY531 reduced the weight and size of the tumor in comparison to the model group. The levels of serum pro-inflammatory cytokines, including IL-1β, IL-6, IL-2 and TNF-α were suppressed by W. coagulans MZY531 administration. Immunofluorescence and TUNEL analyses demonstrated that W. coagulans MZY531 significantly increased the number of cleaved caspase-3 cells and induced apoptosis in tumor tissues. Importantly, W. coagulans MZY531 activated the AMPK/mTOR autophagy-dependent apoptosis pathway, and regulated the TLR4/MyD88/TRAF-6/NF-κB and JAK2/STAT3 inflammatory signaling pathways through mechanisms. Additionally, Fecal analysis demonstrated the capacity of W. coagulans MZY531 to remodel the gut microbiota of hepatocellular carcinoma-infected mice. Collectively, this experimental finding suggested that W. coagulans MZY531 exhibited prominent anticancer activities in vivo at least partly via reducing inflammation, inducing autophagy-dependent apoptosis, and regulating gut microbiota in H22 tumor-bearing mice.
Similar content being viewed by others
Introduction
Probiotics are living microorganisms that can achieve health benefits to the host if administrated in adequate amounts1. The health-promoting effects of probiotic include regulation of intestinal flora, enhancement of immune system function, improving digestibility and metabolism, antimicrobial, and anti-inflammatory, etc2. Both in vitro and animal models studies have demonstrated that probiotics inhibit initiation or progression of various malignant tumors through multiple pathways, with the most prevalent being colorectal, cervical, breast, lung, and liver cancers3,4,5,6,7.The mechanisms of probiotics in anti-cancer activity may include inhibiting cancer cell proliferation8, regulating the gut microbiota9, inducing apoptosis10, reducing inflammation11, enhancing immune function12, and producing anti-cancer metabolites13. However, the potential mechanisms of probiotic anti-cancer activity remain unclear. These findings require further confirmation through in vitro studies, animal models, and clinical trials.
Weizmannia coagulans (formerly Bacillus coagulans) is a gram-positive bacteria that is both a spore-forming and lactic acid-producing novel probiotic. W. coagulans is a microorganism that has been classified as “Generally Recognized as Safe (GRAS)” for human and animal consumption. The strain exhibits unique advantages in stability in processing and storage, and is extensive used in the in food, medicine, and animal husbandry14,15,16. In recent years, the medical applications of W. coagulans have attracted considerable attention, as it has been demonstrated to promote host health through a range of mechanisms, including antioxidant, maintenance of normal flora in the digestive tract, prevention of intestinal inflammation, and modulation of the immune response14. Recent studies on W. coagulans have demonstrated strain-specific anti-tumor potential across diverse cancer models. In vitro studies report that specific W. coagulans strains inhibit proliferation of human colon cancer (HT-29), chronic myeloid leukemia (K562), and cervical cancer (HeLa) cells via apoptosis induction and cell cycle arrest17. The supernatant of W. coagulans culture significantly induced apoptosis in MCF-7 cancer cells18,19. Animal studies further reveal that W. coagulans mitigates colitis-associated colorectal cancer in AOM/DSS-treated mice by suppressing pro-inflammatory cytokines (e.g., IL-6, TNF-α) and restoring gut microbiota balance20.
The precise mechanisms by which W. coagulans inhibits cancer cell activity remain elusive, despite several proposed mechanisms suggesting its potential to inhibit tumor cell proliferation, induce apoptosis, enhance immunity, and modulate gut microbiota and its metabolism. However, significant gaps in knowledge persist. The strain-specificity of its anti-cancer effects remains poorly characterized, with limited comparative studies across different isolates. Additionally, most mechanistic insights are derived from in vitro experiments or colorectal cancer models, leaving its efficacy in hepatocellular carcinoma (HCC) largely unexplored. Furthermore, the clinical translational potential of W. coagulans is hindered by insufficient data on optimal dosing, safety in immunocompromised hosts, and interactions with conventional therapies. Our previous study found that an independently isolated and identified strain of W. coagulans MZY531 inhibited the proliferation of H22 hepatocellular carcinoma cells, affected the cell cycle, and induced apoptosis of tumor cells21. Building on this foundation, the current study systematically investigates the multi-modal anti-hepatoma mechanisms of strain MZY531 in tumor-bearing mice, focusing on apoptosis-related signaling pathways, inflammation modulation, and gut microbiota-metabolite crosstalk. These findings are expected to address critical gaps in the strain-specific mechanistic understanding of W. coagulans and provide foundational data for developing probiotic-adjuvant therapies against hepatoma.
Materials and methods
Bacteria strain and cultivation
W. coagulans MZY531 was supplied by Jilin Mingzhiyuan Biotechnology Co. Ltd. for this study. A single colony of activated W. coagulans MZY531on the LB solid plate was inoculated into GPY liquid broth, and incubated with shaking at 1800 rpm for 20 h at 50 ℃. Following centrifugation, the bacterial pellet was harvested (at 3000 rpm, 4 ℃, for 10 min), resuspended in a sterile saline solution, the bacterial concentration was adjusted to 1.0 × 109 CFU/ml, and preserved at 4 °C for subsequent use.
Cell line and cell culture
The murine hepatoma cell line H22 was procured from Wuhan Servicebio Technology Co., Ltd. (Wuhan, China) and cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum, 100 IU/mL penicillin, and 100 µg/mL streptomycin. Cells were cultured in a humidified environment of 5% carbon dioxide at 37 degrees Celsius and subcultured every 2–3 days.After reaching confluence, cells were detached using trypsin, harvested, washed, and counted using the trypan blue dye exclusion method. Following centrifugation, cells were resuspended in phosphate-buffered saline (PBS) and adjusted to a concentration of 1.0 × 107 cells/mL for subsequent syngeneic grafting experiments.
Animal model and treatment
Thirty 6-week-old female BALB/c mice weighing 18–22 g were procured from Experimental Animal Center of Jilin University First Hospital (Changchun, China) and allowed to acclimate for one week before experimentation. The study was carried out in compliance with the ARRIVE guidelines. The experimental design was approved by the Changchun University Animal care committee and the Research Ethics Committee (Approval no. 20220311 A) prior to performing the experiments.All animal housing and experimentswere conducted in strict accordance with the institutional quidelines for care and use of laboratory animals.Tumor cells, totaling 1 × 107 viable cells in 0.2 mL PBS, were subcutaneously inoculated into the right flanks of the mice.
Upon reaching approximately 0.5 cm in diameter22, the mice were randomly assigned to either the model group or the 5-fluorouracil (5-FU) group, and MZY531 group, with 10 mice in each group. Mice in the MZY531 group received daily W. coagulans MZY531 treatment at 0.2 mL/day. The 5-FU group received intraperitoneal injection of 5-FU (25 mg/kg/day), the model group administered oral gavage of physiological saline. Throughout the 28-day treatment period, measurements of tumor length (a) and width (b) were taken every 3 days to calculate tumor volume using the formula V = ab2/2. Following the final treatment, all mice were euthanized by injecting 200 mg/kg of sodium pentobarbital through the tail vein.The tumor was removed, weighed, and the tumor inhibition index (TIR) was calculated23. Tumor samples are preserved at -80 ℃ for subsequent analysis.
Biochemical analysis
After standing at 4 ℃ for 1 h, the plasma underwent centrifugation at 4000 rpm for 10 min, maintaining a temperature of 4 ℃. The supernatant obtained from this process was then collected and stored at -80 ℃ for further analysis. The concentrations of IL-1β, TNF-α, IL-6, and IL-2 in the serum were accurately measured utilizing enzyme-linked immunosorbent assay kits sourced from Jiangsu Enzyme Labeling Biotechnology and Biological Co.
Immunofluorescence staining of caspase-3
Tumor tissues were fixed in paraffin and stored at -20 °C before being sectioned into 20 μm thick coronal slices. These sections were initially incubated in PBS (pH = 7.4) containing 3% bovine serum albumin and 0.3% Triton X-100 for 1 h. They were then exposed to Anti-Cleaved-Caspase-3 antibody (Servicebio, GB11532, 1:500) for 12 h at 4 °C, followed by incubation with a secondary antibody (Servicebio, GB21301) for detection. Caspase-3 expression was evaluated using a light microscope, and the positive cell rate was quantified using Image J software. The caspase-3 antibody (Servicebio, GB11532, diluted to 1:300) was also incubated with the secondary antibody (Servicebio, GB21301, diluted to 1:500) for 1 h at room temperature, followed by incubation with diaminobenzidine for approximately 10 min.
TUNEL assay
Tumor tissue sections embedded in paraffin from each group were processed through deparaffinization using xylene, followed by rehydration with a series of graded ethanol solutions. After rinsing with PBS, the sections were dried and treated with proteinase K at room temperature for 20 min, then rinsed again with PBS. Next, a TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling) working solution (comprised of TdT enzyme, dUTP, and buffer in a 1:5:50 ratio) was applied to the sections, which were then covered with a preservative film and incubated in the dark at 37 °C for 2 h. Post-incubation, the slides were washed with PBS, dried, and treated with a DAPI solution. Fluorescence microscopy was used to observe apoptotic cells. Under UV light, the nuclei were stained blue by DAPI, while the apoptotic nuclei were stained red.
Western blot analysis
The Western blot workflow was in accordance with the recommendations of Shen et al.24. Tumor tissue (100 mg) was homogenized using RIPA lysis buffer (containing protease and phosphatase inhibitors) to extract total protein. The protein content was determined using the BCA method and standardized to the same concentration. Protein samples were prepared by boiling in denaturation buffer and then resolved on 8%, 10%, or 12% SDS-PAGE gels. Following electrophoresis, proteins were transferred to a nitrocellulose membrane (Millipore, MA, USA). The membrane was blocked with 3% BSA in Tris-buffered saline and Tween-20 (TBST) and then incubated with primary antibodies. The antibodies used included rabbit anti-β-actin (GeneTex, GTX629630), rabbit anti-STAT3 (GeneTex, GTX636400), rabbit anti-p-STAT3 (Bioss, bs-22386R), rabbit anti-p-JAK2 (GeneTex, GTX132784), rabbit anti-p-mTOR (GeneTex, GTX132803), rabbit anti-mTOR (Bioss, bsm-54471R), rabbit anti-JAK2 (Bioss, bs-0908R), rabbit anti-AMPK (Bioss, bs-10344R), and rabbit anti-p-AMPK (Cell Signaling, #2535). Following the incubation with the primary antibody, the membrane was subjected to treatment with a horseradish peroxidase-conjugated secondary antibody, conducted at 37 ℃ over the course of one hour. Beta-actin served as a loading control, and the grayscale value of the target protein was analyzed using Image Quant LAS 4000 (Fuji Film, Tokyo, Japan).
Gut microbiota analysis
Remove the fecal samples from − 80 °C storage and allow them to thaw. Total DNA was isolated and refined from fecal material using a QIAamp Fast DNA Fecal Mini Kit (QIAGEN, Germany). PCR amplification of bacterial 16 S rRNA genes targeting the V3-V4 region using primers 338 F (5’-ACTCCTACGGGGGGGGCAGCA-3’) and 806R (5’-GACTACHVGGGTWTCTAAT-3’)(Project No.PRJNA1131097 ). Assess the purity and concentration of the PCR products using agarose gel electrophoresis and a NanoDrop spectrophotometer. Analyze the diversity and abundance of gut microbiota using QIIME2 (https://qiime2.org/) and R version 3.2.0 (Vienna, Austria) at a 97% similarity level based on operational taxonomic units (OTUs). Compute α-diversity to compare species richness and diversity across different groups, and determine β-diversity to assess variations in microbial communities. Compare the abundance of taxa at the phylum and genus levels between samples using QIIME software.
Statistical analysis
All data are presented as mean ± standard deviation (SD). Statistical differences were determined using One-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. Additionally, data compilation and the construction of box-and-dot plots were performed using Origin 8.0 software. P < 0.05 was used to indicate a statistically significant difference.
Results
W. coagulans MZY531 inhibit the tumor growth in H22 tumor-bearing mice
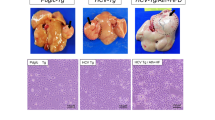
After 28 days of W. coagulans MZY531 administration, the body weights of mice in the MZY531 group were similar and lower than the weights of mice in the Model group, as displayed in Fig. 1A. The Model group had the fastest increase in tumor volume (Fig. 1B), while the tumor volumes of the MZY531 group were smaller in comparison. Both the MZY531 and 5-FU groups demonstrated tumor growth inhibition. The tumor inhibition rates of MZY531 group were 30.00%, suggesting that W. coagulans MZY531 has the ability to inhibit tumor growth, which is statistically significant compared to the model group (P < 0.05) (Table 1).
W. coagulans MZY531 suppress inflammatory cytokines production in the serum of H22 tumor-bearing mice
The anti-inflammatory effects of W. coagulans MZY531 were evaluated by measuring serum cytokine levels (Fig. 2). The model group exhibited significantly higher serum levels of pro-inflammatory cytokines compared to the MZY531 and 5-FU groups (P < 0.05). Administration of W. coagulans MZY531 resulted in reductions of IL-1β, IL-6, IL-2, and TNF-α levels by 11.2%, 7.8%, 21.6%, and 2.0% respectively, compared to the model group (p < 0.05).(Fig. 2A–D).
Effect of W. coagulans MZY531 on levels of pro-inflammatory cytokines in the serum of H22 tumor-bearing mice. Including (A) IL-1β content; (B) IL-6 content; (C) IL-2 content and (D) TNF- α content in H22 tumor-bearing mice. All data were expressed as the mean ± standard deviation (SD), n = 10. * p < 0.05, ** p < 0.01 vs. the model group.
Effect of W. coagulans MZY531 on the expression of cleaved-caspase-3 in H22 tumor-bearing mice
The rate of caspase-3 positive cells in mouse tumor tissues is presented in Fig. 3. The number of cleaved-caspase-3 cells in the tumor tissues of the 5-FU and MZY531 groups were significantly higher compared to the Model group (P < 0.01). Apoptosis was reduced, and the rate of cleaved-caspase-3 cells in the MZY531 group reached 38.41%, displaying a statistically significant difference (P < 0.01).
W. coagulans MZY531 induces caspase-3 expression in in H22 tumor-bearing mice. (A) Immunofluorescence staining of cleaved-caspase-3-positive cells in the tumor tissue of mice and (B) positivity of cleaved-caspase-3 cells in each group. Compared with the Model group, * P < 0.05, ** P < 0.01, mean ± SD (n = 10 for 5-FU, n = 10 for MZY531).
In vivo apoptosis induction by W. coagulans MZY531
The study utilized the TUNEL method, and the results revealed that the 5-FU group alone exhibited a positive cell rate of 80.4%. However, the MZY531 group showed a significant increase in positive cell numbers (P < 0.01). The group designated as “Model” displayed the lowest occurrence of apoptosis with a positive cell rate of 9.3% (Fig. 4). These findings confirm that W. coagulans MZY531 induces apoptosis, which subsequently results in substantial anti-cancer effects in mice afflicted with H22 hepatocellular carcinoma.
W. coagulans MZY531 on apoptosis rate of H22 tumor-bearing mice. (A) TUNEL immunofluorescence staining of tumor tissue in each group in 400 visual field. (B) Percentage of TUNEL-positive cells in tumor tissues in each group. Compared with the Model group, * P < 0.05, * * P < 0.01, mean ± SD (n = 10 for 5-FU, n = 10 for MZY531).
Effects of W. coagulans MZY531 on the AMPK/mTOR autophagy-dependent apoptosis pathway
In this study, the effect of administering W. coagulans MZY531 in H22 tumor-bearing mice was verified through the AMPK/mTOR signaling pathway. AMPK and mTOR protein levels were detected, and as demonstrated in Fig. 5, the Western blot analysis confirmed treatment with W. coagulans MZY531 upregulated p-AMPK and downregulated p-mTOR protein expression compared with the Model group (P < 0.05). Additionally, the administration of W. coagulans MZY531 treatment exhibited a therapeutic effect equivalent to that of the 5-FU drug. This indicates that its anti-tumor effect is achieved through the activation of AMPK signaling to inhibit mTOR signaling.
Effects of W. coagulans MZY531 on Inhibition of TLR4-MyD88- NF-κB p65 signaling
Figure 6 demonstrates that mice in the MZY531 group exhibited decreased levels of TLR4, MyD88, TRAF-6, and NF-κB p65 proteins in H22 tumor-bearing mice compared to the Model group (P < 0.05). Treatment with W. coagulans MZY531 resulted in reductions of TLR4, MyD88, TRAF-6, and NF-κB p65 protein levels by 12.57%, 22.31%, 13.41%, and 20.74%, respectively. The above findings indicate that administering W. coagulans MZY531 may possess anti-inflammatory effects through the TLR4 signaling pathway.
(A) Effect of W. coagulans MZY531 on the expression of TLR4/MyD88/TRAF-6/NF-κB p65 in H22 tumor-bearing mice. The TLR4 (B), MyD88 (C). TRAF-6 (D) and NF-κB (E) proteins were expressed by Westernblot. Data analysed by one-way ANOVA: *P < 0.05, **P < 0.01 vs. model group, mean ± SD. β-Actin was used as a standard control for analysis.
Effects of W. coagulans MZY531 on the expression of JAK2/STAT3 proteins
To further explore the potential anti-inflammatory mechanism of W. coagulans MZY531 on H22 tumor-bearing mice, the expression and phosphorylation degree of JAK2 and STAT3 in tumor tissues were detected. Figure 7 illustrates that the total expression of JAK2 and STAT3 was largely unchanged in the tumor tissues. However, W. coagulans MZY531 administration significantly reduced the levels of phosphorylated STAT3 and JAK2. The therapeutic effect of this treatment was similar to that of 5-FU, which further supports the potential of W. coagulans MZY531 administration treatment in inhibiting the growth of liver tumors through the JAK2/STAT3 pathway.
(A) Effect of W. coagulans MZY531 on the expression of JAK2 and STAT3. JAK2 (B) and STAT3 (C) Western blot analysis was used to detect protein expression. One-way ANOVA was used to analyse the data: *P < 0.05, **P < 0.01 vs. model group, mean ± SD. β-actin was used as a standard control for analysis.
Effects of W. coagulans MZY531 on the composition of gut microbiota in H22 tumor-bearing mice
Fecal analysis showed that administration of W. coagulans MZY531 significantly improved the richness as well as diversity of the gut microbiota (Fig. 8). When examining α-diversity (Fig. 8A, B), both Chao1 and Shannon indices of the W. coagulans MZY531 group showed significant increases in comparison to the model group (P < 0.01). At the phylum level (Fig. 8C), W. coagulans MZY531 treatment not only significantly increased the abundance of the Firmicutes phylum but also reduced the prevalence of potentially harmful bacteria (such as Bacteroidetes). The outcomes of PCoA analysis (Fig. 8D) revealed a noteworthy separation between the model group and the other two groups. Furthermore, in Fig. 8E, W. coagulans MZY531 intervention was found to decrease the levels of Enterococcus and Alistipes while significantly increasing the presence of Lactobacillus and Lactococcus compared to the other groups. Spearman analysis indicated a potential positive correlation between Lactobacillus and the levels of p-JAK2/JAK2, p-STAT3/STAT3, p-mTOR/mTOR, TLR4, MyD88, TRAF-6, NF-κB, IL-6, IL-2, IL-1β, TNF-α, but it exhibited a negative correlation with the levels of p-AMPK/AMPK factors (P < 0.01) (Fig. 8F) .These findings collectively suggested that W. coagulans MZY531 may potentially affect cancer-associated apoptosis by modulating factors such as Lactobacillus and Lachnospiraceae_NK4A136_group.
Effects of W. coagulans MZY531 on the diversity and composition of the gut microbiota. Chao1 (A) and Shannon (B) indices. (C) Species composition at the phylum level. (D) PCoA analysis based on weighted UniFrac phylogeny distances. (E) Heat map at the genus level. (F) Correlation between gut microbiota and apoptosis factors, where red indicates a positive correlation and blue indicates a negative correlation. *P < 0.05 and **P < 0.01 versus model group (n = 5).
Discussion
Autophagy and apoptosis are usually tumor suppressor pathways25. A study report confirmed that Lactobacillus plantarum metabolites exerted anticolorectal cancer effects by decreasing the expression of autophagy-associated proteins, such as Atg9, Atg5, Atg16L1, and Beclin-126. And the role of AMPK/mTOR-mediated autophagy in hepatocellular carcinoma therapy has been reported27. Therefore, we investigated this pathway. The results indicated that W. coagulans MZY531 treatment increased AMPK phosphorylation levels and decreased mTOR phosphorylation levels. As a downstream target of AMPK, mTOR regulates cell growth, survival, protein synthesis, and transcription24. mTOR also acts as a downstream target of PI3K/AKT and also regulates proliferation, apoptosis28. Based on the previous experiments, we confirmed that W. coagulans MZY531 could induce apoptosis and thus exert anti-hepatocellular carcinoma effects through PI3K/AKT/mTOR and Bax/Bcl-2/caspase-3 pathways in vitro7, which was consistent with the in vivo immunofluorescence results of the present study. In addition, a growing number of research reports have also confirmed that some probiotics can exert antitumor effects through apoptosis29,30. Taken together, W. coagulans MZY531 can exert anticancer effects through the AMPK/mTOR autophagy-dependent apoptosis pathway, but its mechanism needs further study.
Excessive production of pro-inflammatory cytokines is linked to the initiation and advancement of cancer31. Probiotics can reduce the secretion of pro-inflammatory cytokines and play a crucial role in the prevention of carcinogenesis. Previous studies have demonstrated that probiotics can inhibit tumor growth through anti-inflammatory effects32,33,34. In this study, our results indicate that W. coagulans MZY531 can reduce the release of pro-inflammatory cytokines IL-1β, IL-6, IL-2, and TNF-α. W. coagulans MZY531 can also inhibit the protein expression level of TLR4/MyD88/TRAF-6/NF-κB inflammatory signaling pathway. TLR4 is a key receptor involved in inflammatory response, which affects the expression of downstream signals, including MyD88, TRAF-6, and NF-κB, thereby affecting the release of inflammatory cytokines35,36. Previous studies have shown that Companilactobacillus crustorum MN047 can significantly reduce tumorigenesis and inflammation by inhibiting the TLR4/NF-κB pathway37. Bao et al. illustrated that Weissella cibaria FB069 inhibits colorectal cancer cell growth via the TLR4/MyD88/NF-κB signaling pathway38. Our findings support this conclusion. In contrast, SUN et al. showed that targeting TLR4 suppresses VEGF expression, influencing the PI3K/AKT pathway and pancreatic cancer angiogenesis39. Furthermore, Zhu et al. discovered that Lactobacillus casei and Lactobacillus reuteri reduce TLR4 and MyD88 expression in pancreatic cancer cells40. Thus, W. coagulans MZY531’s anti-hepatocellular carcinoma effect may be linked to TLR4-mediated inflammation inhibition.
The relationship between the JAK2/STAT3 signaling pathway and IL-6 is of significant pathological importance in the development and progression of hepatocellular carcinoma. IL-6, a key cytokine, activates the JAK2/STAT3 signaling pathway through its receptor. In the tumor microenvironment, elevated IL-6 levels often lead to hyperactivation of the JAK2/STAT3 pathway, which is associated with tumor cell proliferation, survival, invasion, and immune evasion41. This hyperactivation is typically linked to poor prognosis. In our study, we found that W. coagulans MZY531 significantly inhibits the proliferation of hepatocellular carcinoma cells, with this effect being closely related to the modulation of the JAK2/STAT3 signaling pathway. Specifically, treatment with W. coagulans MZY531 led to decreased expression of IL-6, which in turn resulted in reduced phosphorylation levels of JAK2 and STAT3 proteins. This modulation likely interrupts IL-6-mediated signaling, thereby blocking the activation of JAK2 and its downstream molecule STAT3, ultimately affecting cell proliferation and survival. Moreover, numerous studies have shown that probiotics can modulate the host’s immune response and inflammatory status by reducing the expression of pro-inflammatory cytokines, including IL-6. Our findings also demonstrated that W. coagulans MZY531 significantly lowered serum IL-6 levels and directly inhibited the phosphorylation of JAK2 and STAT3. This regulatory effect may be associated with apoptosis, autophagy, and the inhibition of solid tumor growth. These results are consistent with those reported by An et al.42, who found that the consumption of probiotic Kimchi significantly reduced the expression of IL-6 and its receptor, as well as the expression of the JAK2 and STAT3 genes. This suggests that W. coagulans MZY531 may exert its anticancer effects by modulating the IL-6/JAK2/STAT3 signaling pathway.
The gut flora comprises a complex and dynamic community of bacteria, fungi, protozoa, vibrios, and viruses43. The interaction between the gut microbiota and the liver constitutes the gut-liver axis. Through this enterohepatic axis, the intestinal flora modulates pro-inflammatory alterations in both the liver and intestines, thus influencing the progression of hepatitis, liver fibrosis, cirrhosis, and hepatocellular carcinoma44. The intestinal microbiota is comprised of nine major phyla, with the phylum Thick-walled Bacteria and Bacteroidetes being the most prevalent. The results of gut flora analysis indicate that W. coagulans MZY531 enhances the abundance of beneficial strains, such as lactobacilli, within the gut microbiota. Previous studies have demonstrated that Lactobacillus exerts antitumor effects45. Additionally, Zhang et al. created a rat liver cancer model using diethylnitramine, demonstrating that oral administration of the VSL#3 probiotic blend decreased the intestinal inflammatory response, preserved intestinal mucosal integrity, and inhibited tumor growth46. Similarly, the Prohep probiotic blend reduced Th17 cell counts in tumors, thereby hindering the development of hepatocellular carcinoma in a subcutaneous mouse model47. We also performed a Spearman analysis to investigate the potential relationship between gut microbiota and cytokines such as TLR4 and IL-6. Dapito et al. suggested that targeting gut flora and TLR4 could prevent the progression of hepatocellular carcinoma48, which aligns with our findings. However, some clinical trials have not demonstrated clinical benefits of probiotics in cancer treatment49.
Conclusion
In conclusion, our study demonstrates that W. coagulans MZY531 exerts significant anti-cancer effects in H22 hepatocellular carcinoma-bearing mice through multiple mechanisms. Specifically, it reduces the levels of pro-inflammatory cytokines and inhibits key components of the TLR4 signaling pathway, thereby mitigating inflammation. Additionally, W. coagulans MZY531 activates the AMPK/mTOR pathway, leading to autophagy-dependent apoptosis. Furthermore, this probiotic strain enhances the abundance of beneficial gut bacteria, such as Lactobacillus, which may contribute to its anti-cancer effects. These findings highlight the multifaceted mechanisms underlying the therapeutic potential of W. coagulans MZY531 in hepatocellular carcinoma.
Data availability
All data were included within this article.
References
Trush, E. A. et al. The evolution of human probiotics: challenges and prospects. PROBIOTICS ANTIMICRO. 12 (4), 1291–1299 (2020).
Kaur, I. P. Chopra K,Saini A, probiotics: potential pharmaceutical applications. Eur. J. Pharm. Sci. 15 (1), 1–9 (2002).
Ambalam, P., Raman, M., Purama, R. K. & Doble, M. Probiotics, prebiotics and colorectal cancer prevention. Best Pract. Res. Clin. Gastroenterol. 30 (1), 119–131 (2016).
Jahanshahi, M. et al. Anti-tumor activities of probiotics in cervical cancer. J. Ovarian Res. 13 (1), 68 (2020).
Thu, M. S. et al. Effect of probiotics in breast cancer: A systematic review and Meta-Analysis. Biology (Basel) 12(2). (2023).
Sharma, A., Viswanath, B. & Park, Y-S. Role of probiotics in the management of lung cancer and related diseases: an update. J. Funct. 40, 625–633 (2018).
Zhao, Z. et al. Anticancer potential of Bacillus coagulans MZY531 on mouse H22 hepatocellular carcinoma cells via anti-proliferation and apoptosis induction. BMC Complement. Med. Ther. 23 (1), 318 (2023).
Deng, X. et al. An update on the pivotal roles of probiotics, their components, and metabolites in preventing Colon cancer. Foods 12(19) (2023).
Zheng, D. W. et al. Prebiotics-Encapsulated probiotic spores regulate gut microbiota and suppress Colon cancer. Adv. Mater. 32 (45), e2004529 (2020).
Badgeley, A., Anwar, H., Modi, K., Murphy, P. & Lakshmikuttyamma, A. Effect of probiotics and gut microbiota on anti-cancer drugs: mechanistic perspectives. Biochim. Biophys. Acta Rev. Cancer. 1875 (1), 188494 (2021).
Pahumunto, N. & Teanpaisan, R. Anti-cancer properties of potential probiotics and their Cell-free supernatants for the prevention of colorectal cancer: an in vitro study. Probiotics Antimicrob. Proteins. 15 (5), 1137–1150 (2023).
Schwartz, D. J., Rebeck, O. N. & Dantas, G. Complex interactions between the Microbiome and cancer immune therapy. Crit. Rev. Clin. Lab. Sci. 56 (8), 567–585 (2019).
Aimaier, R. et al. The secondary metabolites of Bacillus subtilis strain Z15 induce apoptosis in hepatocellular carcinoma cells. Probiotics Antimicrob. Proteins 1–11. (2023).
Mu, Y. & Cong, Y. Bacillus coagulans and its applications in medicine. Benef Microbes. 10 (6), 679–688 (2019).
Konuray, G. & Erginkaya, Z. Potential Use of Bacillus coagulans in the Food Industry. Foods 7(6) (2018).
Zhou, Y. et al. Application of Bacillus coagulans in animal husbandry and its underlying mechanisms, animals (2020). (Basel) 10(3).
Madempudi, R. S., & Kalle, A. M. Antiproliferative effects of Bacillus coagulans unique IS2 in colon cancer cells. Nutr. Cancer. 69 (7), 1062–1068 (2017).
Dolati, M., Tafvizi, F., Salehipour, M., Movahed, T. K. & Jafari, P. Inhibitory effects of probiotic Bacillus coagulans against MCF7 breast cancer cells. Iran. J. Microbiol. 13 (6), 839 (2021).
Khaledizade, E., Tafvizi, F. & Jafari, P. Anti-breast cancer activity of biosynthesized selenium nanoparticles using Bacillus coagulans supernatant. J. Trace Elem. Med. Biol. 82, 127357 (2024).
Hiramoto, K. et al. Bacillus coagulans (species of lactic acid-forming Bacillus bacteria) ameliorates azoxymethane and dextran sodium sulfate-induced colon cancer in mice. J. Funct. 100, 105406 (2023).
Zhao, Z. et al. Anticancer potential of Bacillus coagulans MZY531 on mouse H22 hepatocellular carcinoma cells via anti-proliferation and apoptosis induction. BMC Complement. Med. 23 (1), 318 (2023).
Liu, C. Y. et al. Etoposide sensitizes CT26 colorectal adenocarcinoma to radiation therapy in BALB/c mice. World J. Gastroenterol. 11 (31), 4895–4898 (2005).
Wang, Q. et al. Antitumor effect of exopolysaccharide from Lactiplantibacillus plantarum WLPL09 on melanoma mice via regulating immunity and gut microbiota. Int. J. Biol. Macromol. 254 (Pt 1), 127624 (2024).
Shen, Y. et al. Transformation of Ginsenosides by Lactiplantibacillus plantarum MB11 Fermentation: Minor Ginsenosides Conversion and Enhancement of Anti-Colorectal Cancer Activity. Molecules 29(1) (2023).
Kouroumalis, E., Tsomidis, I. & Voumvouraki, A. Pathogenesis of Hepatocellular Carcinoma: The Interplay of Apoptosis and Autophagy. Biomedicines 11(4). (2023).
Jeong, S. et al. Lactobacillus plantarum Metabolites Elicit Anticancer Effects by Inhibiting Autophagy-Related Responses. olecules 28(4) (2023).
Wang, H. et al. The upstream pathway of mTOR-Mediated autophagy in liver diseases. Cells 8(12) (2019).
Castedo, M., Ferri, K. F. & Kroemer, G. Mammalian target of Rapamycin (mTOR): pro- and anti-apoptotic. Cell. Death Differ. 9 (2), 99–100 (2002).
Yan, F. & Polk, D. B. Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J. Biol. Chem. 277 (52), 50959–50965 (2002).
Behzadi, E., Mahmoodzadeh Hosseini, H. & Imani Fooladi, A. A. The inhibitory impacts of Lactobacillus rhamnosus GG-derived extracellular vesicles on the growth of hepatic cancer cells. Microb. Pathog. 110, 1–6 (2017).
Dinarello, C. A. The paradox of pro-inflammatory cytokines in cancer. Cancer Metastasis Rev. 25 (3), 307–313 (2006).
Han, K. J., Lee, N. K., Park, H. & Paik, H. D. Anticancer and Anti-Inflammatory activity of probiotic Lactococcus lactis NK34. J. Microbiol. Biotechnol. 25 (10), 1697–1701 (2015).
Chung, I. C. et al. Pretreatment with a Heat-Killed probiotic modulates the NLRP3 inflammasome and attenuates Colitis-Associated colorectal Cancer in mice. Nutrients 11(3) (2019).
Mendes, M. C. S. et al. Microbiota modification by probiotic supplementation reduces colitis associated colon cancer in mice. World J. Gastroenterol. 24 (18), 1995–2008 (2018).
O’Neill, S. et al. Heat shock protein 90 Inhibition abrogates TLR4-mediated NF-κB activity and reduces renal ischemia-reperfusion injury. Sci. Rep. 5, 12958 (2015).
Fusella, F., Seclì, L., Cannata, C. & Brancaccio, M. The one thousand and one chaperones of the NF-κB pathway. Cell. Mol. Life Sci. 77 (12), 2275–2288 (2020).
Wang, T. et al. Protective effects of Companilactobacillus crustorum MN047 against dextran sulfate Sodium-Induced ulcerative colitis: A fecal microbiota transplantation study. J. Agric. Food Chem. 70 (5), 1547–1561 (2022).
Le, B., Ngoc, A. P. T. & Yang, S. H. Synbiotic fermented soymilk with Weissella cibaria FB069 and xylooligosaccharides prevents proliferation in human colon cancer cells. J. Appl. Microbiol. 128(5), 1486–1496 (2020).
Sun, Y. et al. Toll-like receptor 4 promotes angiogenesis in pancreatic cancer via PI3K/AKT signaling. Exp. Cell. Res. 347(2), 274–282 (2016).
Zhu, Z. et al. Lactobacillus casei combined with Lactobacillus reuteri alleviate pancreatic cancer by inhibiting TLR4 to promote macrophage M1 polarization and regulate gut microbial homeostasis. BMC Cancer. 23 (1), 1044 (2023).
Huang, B., Lang, X. & Li, X. The role of IL-6/JAK2/STAT3 signaling pathway in cancers. Front. Oncol. 12, 1023177 (2022).
An, J. M. et al. Dietary intake of probiotic Kimchi ameliorated IL-6-driven cancer cachexia. J. Clin. Biochem. Nutr. 65 (2), 109–117 (2019).
Liu, S. & Yang, X. Intestinal flora plays a role in the progression of hepatitis-cirrhosis-liver cancer. Front. Cell. Infect. Microbiol. 131140126 (2023).
Woodhouse, C. A., Patel, V. C., Singanayagam, A. & Shawcross, D. L. Review Article: the gut Microbiome as a therapeutic target in the pathogenesis and treatment of chronic liver disease. Aliment. Pharmacol. Ther. 47(2), 192–202 (2018).
Lau, H. C. et al. Lactobacillus acidophilus suppresses non-alcoholic fatty liver disease-associated hepatocellular carcinoma through producing valeric acid. EBioMedicine100, 104952 (2024).
Zhang, H. L. et al. Profound impact of gut homeostasis on chemically-induced pro-tumorigenic inflammation and hepatocarcinogenesis in rats. J. Hepatol. 57 (4), 803–812 (2012).
Li, J. et al. Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc. Natl. Acad. Sci. U S A. 113 (9), E1306–E1315 (2016).
Dapito, D. H. et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 21 (4), 504–516 (2012).
Anderson, A. D., McNaught, C. E., Jain, P. K. & MacFie, J. Randomised clinical trial of synbiotic therapy in elective surgical patients. Gut 53 (2), 241–245 (2004).
Acknowledgements
This work was financially supported by Jilin Province Science and Technology Development Program (YDZJ202401038ZYTS).
Author information
Authors and Affiliations
Contributions
Chunhong Liu: Methodology, Investigation, Writing - original draft, Writing - review & editing, Visualization. Mengyao Tan: Methodology, Data curation, Writing - review & editing. Lijun Zhao: Project administration, Supervision, Resources. Meichen Gai: Software,Writing - review & editing.Tingting Zhou: Methodology, Supervision.Caixin Yu: Formal analysis. Zhongwei Zhao: Conceptualization, Project administration, Resources, Investigation, Writing - original draft, Writing - review & editing, Funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Institutional review board statement
The experiment adhered to the Animal Care Committee guidelines of Changchun University (Ethical reference number: 20220311 A).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, C., Tan, M., Zhao, L. et al. Anticancer activity of Weizmannia coagulans MZY531on H22 tumor-bearing mice by regulating inflammation, autophagy-dependent apoptosis, and gut microbiota. Sci Rep 15, 8250 (2025). https://doi.org/10.1038/s41598-025-92825-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-92825-9