Abstract
Male sex is a well-known predictor of short-term prognosis in patients with coronavirus disease (COVID-19). Data, however, on long-term outcomes are scarce. We aimed to assess the differences in mortality between sexes and find other important predictors of survival from a long-term perspective. Data from all patients retrieved from a database of COVID-19 patients hospitalized at University Hospital in Krakow, Poland, between February 13, 2020, and May 10, 2021, were analyzed for clinical in-hospital data and after a 42 months follow-up period. Of the 4071 COVID-19 patients hospitalized, 2183 were men (53.6%). Males were on average younger and more likely to have concomitant chronic obstructive pulmonary disease, heart failure, coronary artery disease (including acute and chronic coronary syndrome) compared to women. In terms of laboratory findings, more advanced inflammatory markers and troponin I were predominantly observed in male patients than in female patients. Males were found to have a greater predisposition for relevant cardiovascular comorbidities and were more likely to have died during the 42 months follow-up. Additionally, higher levels of troponin I, N-terminal pro B-type natriuretic peptide and D-dimer were associated with a greater risk of death. Kaplan–Meier survival analyses revealed a worse 42 months survival for men up to the age of 65 years. Cardiovascular comorbidities, male sex and older age, as well as higher concentrations of markers indicating a thrombotic state and myocardial injury, were associated with poorer long-term prognosis in patients with COVID-19.
Similar content being viewed by others
Introduction
Coronavirus disease (COVID-19) has emerged as a significant health concern from both short- and long-term perspectives. Individuals with multiple comorbidities exhibit increased susceptibility to complications and experience a more severe course of infection. Patients with cardiac conditions represent a special group1,2,3,4, albeit COVID-19 did not increase the CV risk calculated for primary prevention5. The progression of COVID-19 is more pronounced in these individuals1,6, and even isolated infections, in the absence of multiple comorbidities, markedly influence cardiovascular treatment outcomes. Furthermore, an increased prevalence of cardiovascular disease among COVID-19 patients is evident 12 months following infection3,7.
Based on the available data, men had a greater risk of a more severe course of COVID-19 at the acute stage, a greater risk of hospitalization, admission to the intensive care unit (ICU) and death1,8. The mortality rate among male COVID-19 patients was approximately double that of female patients, even after adjustment for comorbidities, including arterial hypertension, heart failure (HF), and diabetes mellitus (DM), over a few weeks of observation9,10. The estimated 3 months population risk of mortality due to COVID-19 was greater among men with comorbidities, including cardiovascular or pulmonary problems, DM, neoplasms or impaired kidney function11. An elevated risk of in-hospital mortality, ICU admission, requirement for mechanical ventilation, and vasopressor therapy alongside a higher susceptibility to acute cardiac injury and thromboembolism, was observed in men during a 3 months follow-up12.
Currently, the challenges associated with in-hospital care for COVID-19 patients may be less severe compared to those encountered at the peak during the initial pandemic phase in 2020. This is attributed to the accrued knowledge and experience gained during the initial pandemic period, along with a decreased frequency of infections necessitating hospitalization. However, despite this observation, data concerning the prognosis of patients following COVID-19 infection remain insufficient, and there is a significant need for further understanding of risk factors and predictors of mortality. Such knowledge is crucial for the triage of these breakthrough patients to ensure they receive more frequent follow-up care, thereby reducing unexpected mortality. Recent observations of patients admitted to ICU centers have shown that 90 days mortality rates were significantly higher among male patients during a 180 days observation period13. Similar findings have been reported in patients suffering from heart failure or arrhythmias14.
The underlying reasons for this phenomenon remain incompletely elucidated. A prevailing hypothesis suggests that females exhibit a more robust immune response, potentially mitigating the severity of infectious diseases. This conjecture is supported by observations that female patients are more susceptible to autoimmune conditions throughout their lives, indicating that the immune system in females, absent immunosuppression therapy, may be better prepared to mount an effective initial defense15. This observation aligns with the finding that male patients tend to experience COVID-19 infections more frequently.
Moreover, the relationships between gender and various cardiovascular comorbidities, as well as long-term survival following COVID-19 infection, remain inadequately comprehended. Given the substantial number of individuals worldwide impacted by mild to moderate COVID-19 infections, numerous patients may not be effectively assessed to identify pertinent risk factors that could affect mortality outcomes. This study was conceived to address this knowledge gap over a 42 months period (approximately a 3.5 years perspective) of patients post-COVID-19 infection.
Methods
We conducted a retrospective analysis of the medical records of all sequential patients admitted due to SARS-CoV-2 infection to the University Hospital in Krakow from February 13, 2020, to May 10, 2021. The diagnosis of COVID-19 in patients was established in accordance with the WHO and national Polish guidelines, utilizing the RT-PCR method (rhino-oropharyngeal swab positivity for SARS-CoV-2 RNA)16,17,18. The treatment protocol for COVID-19 adhered to the recommendations provided by the Polish Association of Epidemiologists and Infectiologists16,17. Patient data were sourced from the electronic medical records maintained by the University Hospital of Krakow. Cardiovascular risk factors and diseases were ascertained through an examination of antecedent medical history and/or characteristic prior treatment, or diagnoses made during hospitalization, and were defined in accordance with the prevailing guidelines set forth by the European Society of Cardiology19.
Clinical data pertaining to demographics, medical history, inpatient clinical course, laboratory findings, treatment interventions, and in-hospital outcomes were sourced from the electronic medical records utilized by the University Hospital of Krakow. All comorbidities analyzed were diagnosed either prior to hospitalization (as per historical data) or during the hospitalization period. The incidence of mortality within a 42 months timeframe following the initial hospitalization date was evaluated using data procured from the National Electronic Population Registration System in Poland, with the cause of mortality remaining indeterminate. Cases in which patients died during hospitalization or where information regarding death remained inaccessible were excluded from the analysis (Fig. 1). This study constitutes a retrospective analysis of the medical records of patients who had been discharged from the hospital setting. Consequently, it was deemed unnecessary to convey information regarding the study to potential participants. Given that the study’s methodology did not compromise patient safety nor induce potential complications, the acquisition of consent from study participants was considered non-essential and therefore not obtained. The study adhered to the principles of the Declaration of Helsinki, and the aforementioned methodological approach received approval from the Bioethics Committee of Jagiellonian University (decision number 1072.6120.278.2020).
Statistical analysis
Categorical variables are reported as numbers and percentages, while continuous variables are detailed through means and standard deviations (SDs) or medians and interquartile ranges (IQRs). Normality was examined using the Shapiro–Wilk test. The study population was stratified into groups according to sex and survival status. Inter-group differences were assessed through the Student’s or Welch’s t-test based on the equality of variances for normally distributed variables. The Mann–Whitney U test was applied for non-normally distributed continuous variables. Receiver operating characteristic (ROC) curve analysis was conducted to define single cutoff points for troponin I, N-terminal pro B-type natriuretic peptide (NT-proBNP), D-dimer, C-reactive protein (CRP), and interleukin-6 (IL-6) as predictors of 42 months mortality. Multivariate Cox proportional hazard analyses were employed to discern independent predictors of mortality. The variables incorporated in the initial analysis were selected as potential risk factors based on their clinical relevance to death at 42 months. These encompass demographic and clinical variables, including sex and age over 65 years, hypertension, hypercholesterolemia, DM, atrial fibrillation (AF), coronary artery disease (CAD), HF, chronic kidney disease (CKD), stroke, and chronic obstructive pulmonary disease (COPD)20,21. The second multivariable Cox model incorporated laboratory markers. The outcomes are reported as hazard ratios (HRs) with 95% confidence intervals (CIs). Additionally, in order to evaluate event-free survival at the 42 months follow-up post hospital admission, Kaplan‒Meier curves were constructed according to sex, focusing on pertinent comorbidities such as hypertension, DM, AF, CAD, HF and CKD in patients discharged from the hospital. In all statistical analyses, a P value of 0.05 or less was deemed statistically significant. Data analysis was conducted using Statistica (data analysis software system), TIBCO Software Inc., version 13 (2017).
Results
A cohort of 4071 COVID-19 patients hospitalized at the University Hospital in Krakow was analyzed during the aforementioned period. Of these, 2183 were male, accounting for 53.6% of the cohort. The demographic and clinical characteristics of the study groups, stratified by sex and survival status, are detailed in Table 1. The mean age of the participants was 59.7 years with a SD of 16.2. The most prevalent comorbidities observed in the entire population included hypertension, DM, hypercholesterolemia, and CAD, as presented in Table 2. Among cardiovascular medications, beta-blockers were the most frequently prescribed, and over 25% of patients received dexamethasone during their hospitalization for COVID-19, as noted in Table 3.
In comparison to females, males were younger and presented with more frequent cases of CAD, previous myocardial infarction, HF, and COPD (Tables 1 and 2). Additionally, males required dexamethasone, noninvasive oxygen therapy, and dialysis more frequently during hospitalization for COVID-19 (Table 3). Among both sexes, non-survivors tended to be older. Males demonstrated a lower body mass index (BMI) among non-survivors compared to survivors, a difference not observed in females (Table 1). Most of the analyzed comorbidities were more prevalent in the nonsurvivor group regardless of sex (Table 2).
Angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), statins, and beta-blockers were more prevalently administered during hospitalization among nonsurvivors of both sexes. Noninvasive ventilation was implemented more frequently among survivors irrespective of sex. No significant differences were observed in the administration of dexamethasone or mechanical ventilation between the survival cohorts of both sexes. Vasopressor therapy during hospitalization was more commonly administered to male patients who died during the postdischarge period (Table 3).
Within the male group, no differences in systolic blood pressure were observed between survivors and non-survivors on admission. In contrast to non-survivors, male survivors had lower respiratory rates and higher heart rates on admission (Table 1). Individuals in the non-survivor group, regardless of sex, had longer hospital stays than survivors, possibly indicating a more severe course of COVID-19 (Table 3).
Higher levels of CRP were observed among nonsurvived women compared to those who survived, while elevated levels of D-dimer and IL-6 were noted among nonsurvivors in comparison to survivors, irrespective of sex. Similarly, irrespective of sex, nonsurvivors characterized more pronounced myocardial injury, evidenced by increased levels of NT-proBNP and troponin I, than survivors (Table 4). Additionally, the nonsurvivor group was distinguished by lower eGFR values and a more frequent requirement for dialysis in comparison to the survivor group (Tables 3 and 4).
An analysis of laboratory parameters following stratification by sex disclosed that males had higher CRP and IL-6 concentrations compared to females. In contrast to females, males also demonstrated higher troponin I levels yet lower NT-proBNP concentrations (Table 4).
In the multivariate Cox regression analysis, the presence of comorbidities such as DM, HF, CAD, AF, stroke, CKD, COPD, along with male sex and age above 65 years, were correlated with an increased 42 months mortality rate. Hypercholesterolemia alone was linked with a reduced mortality risk (Fig. 2).
Male sex was associated with a diminished survival rate among COVID-19 patients younger than 65 years, although this was not observed in the entire study cohort or among those older than 65 years (Fig. 3a–c).
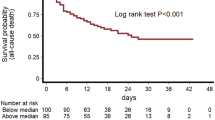
Utilizing the ROC curve, cutoff thresholds for markers indicative of myocardial injury (troponin I, NT-proBNP), thrombotic state (D-dimer), and inflammation (CRP, IL-6) were established as predictors of 42 months mortality (Fig. 4). The multivariate Cox regression analysis conducted on the entire cohort identified that elevated levels of troponin I, NT-proBNP, and D-dimer beyond these thresholds correlated with an increased risk of mortality (Fig. 5). Furthermore, Kaplan–Meier survival analysis demonstrated heightened mortality rates in patients exhibiting levels of troponin I, NT-proBNP, D-dimer, CRP, and IL-6 exceeding the established thresholds, compared to those with levels below these cutoff points (Fig. 6a–e).
Kaplan‒Meier curves displaying proportional survival rate at the 42 months follow-up stratified by the levels of troponin I (a), NT-proBNP (b), D-dimer (c), CRP (d), and interleukin-6 (e). Abbreviations: CRP, C-reactive protein; NT-proBNP, N-terminal pro B-type natriuretic peptide; IL-6, interleukin-6; TnI, troponin I.
Discussion
The principal results of this investigation are as follows:
-
(1)
Among patients diagnosed with COVID-19, the independent risk factors associated with mortality at 42 months include age exceeding 65 years, male sex, and the presence of cardiovascular comorbidities;
-
(2)
For individuals with COVID-19, elevated maximal levels of troponin I > 11.15 ng/l, NT-proBNP > 1087 pg/ml, D-dimer > 1.09 mg/l, CRP > 82.6 mg/l, and IL-6 > 23.24 pg/ml during hospitalization were correlated with inferior 42 months survival outcomes. It is noteworthy that male sex was linked to a reduced survival rate exclusively in COVID-19 patients under the age of 65;
-
(3)
During the period of hospitalization, male patients with COVID-19 exhibited higher levels of CRP, IL-6, and troponin I compared to their female counterparts, despite a younger age profile.
Individuals with cardiovascular disorders appear to have increased susceptibility to infectious diseases. The prognosis for these individuals is generally poorer from the onset of the infectious disease22,23,24,25. Notably, infections of the lower respiratory tract are inclined to manifest with greater severity in patients with a history of cardiac disease22. This phenomenon was also recently observed during pandemic infections attributed to SARS-CoV-21,2,3. Moreover, COVID-19 presents additional non-pulmonary complications, including thromboembolic events and myocarditis26.
In a meta-analysis conducted by Puri et al., it was demonstrated that a higher prevalence of hypertension and cardiovascular disease was observed among patients with severe COVID-19 as compared to those with nonsevere manifestations of the disease27. These conclusions are consistent with the findings of our study, wherein cardiovascular comorbidities were more frequently encountered among nonsurvivors than among survivors.
In large-scale epidemiological studies, males afflicted with COVID-19 exhibited elevated morbidity and mortality rates compared to females28. Such disparities may be attributed to sex-specific responses to viral infections29, a phenomenon observed prior to the COVID-19 era. The expression of genes integral to immunological responses varies between males and females. Several potential factors may account for this disparity. Firstly, the X and Y chromosomes encompass numerous genes pertinent to immune response regulation, which can be impacted by the mosaic loss of the Y chromosome in leukocytes. Conversely, in females, there exists the possibility of escape from inactivation in some cells owing to the presence of two copies of specific genes on the X chromosome30,31. Secondly, males have been observed to express higher levels of angiotensin-converting enzyme 2 (ACE2), including its soluble forms. The coronavirus gains cell entry more readily by binding with ACE2, a receptor present on the surface of the upper respiratory tract. Typically, the ACE2 receptor facilitates anti-inflammatory processes; however, when blocked by the virus, this function is lost30,32. This may elucidate why males have a higher likelihood of encountering cytokine storms than females. This observation is supported by elevated IL-6 levels in males compared to females, a trend also noted in other viral infections30. Consistent findings of elevated IL-6 levels were observed in males over females in our study. Consequently, the significance of estrogenic steroids ought to be considered. Estrogens enhance the expression of toll-like receptor 7 in immune cells, consequently accelerating the elimination of the SARS-CoV-2 virus. Moreover, an increased estradiol concentration, resulting in the rising ratio of CD4 + to CD8 + lymphocytes, is linked with a more robust early response against COVID-19. These hormones, through modulating their effects, also tend to augment the production of IFN alpha. Given the unique characteristics of immune responses between sexes, it is also pertinent to consider findings indicating that females, following a half-dose influenza vaccination, produce comparable antibody levels to males who have received a full dose. Furthermore, antibody levels are correlated with estradiol concentrations33. These mechanisms collectively may elucidate sex-related differences in COVID-19 outcomes.
The potential involvement of estrogen steroids in the course of COVID-19 can also be suspected because sex-dependent differences in mortality were observed in our study only in individuals younger than 65 years of age.
From a short-term perspective, certain studies have demonstrated that elevated levels of CRP, IL-6, D-dimer, and ferritin are associated with increased mortality among both male and female patients34,35,36,37. However, contradictory findings have been reported concerning CRP, ferritin, and D-dimer38. There is a paucity of data regarding the long-term implications of these parameters. Our research identified that males exhibited higher levels of inflammatory markers than females; similarly, nonsurvivors within each sex group showed elevated levels compared to survivors. Nonetheless, results suggests that myocardial damage and prothrombotic processes may be more critical determinants of mortality risk in COVID-19 patients over a 42 months period than inflammation.
An intensified inflammatory state has been identified as a probable mechanism contributing to myocardial injury, facilitating thrombotic processes, including the development of microthrombi that compromise cardiac tissue integrity and lead to vascular dysfunction. ACE2 expression is believed to enhance cardiac involvement by infection, while virus-mediated downregulation of ACE2 might obstruct ACE2-dependent compensatory protective mechanisms36. Myocardial injury is characterized by elevated troponin levels, particularly in men and the elderly population. Among patients with COVID-19, increased levels of both troponin and NT-proBNP possess prognostic importance in both short-term and in-hospital evaluations36,38,39,40,41,42,43,44, as well as in 1 year follow-up assessmets45. Prior investigations have not explored cardiac injury secondary to COVID-19 infection over such an extended follow-up period. Our study validated notably elevated levels of troponin I and NT-proBNP in nonsurvivors, encompassing both sexes. Despite the heightened prevalence of heart failure in males, our study found that the concentration of NT-proBNP was increased in females, aligning with physiological conditions46. Furthermore, within the entire group, levels of troponin I and NT-proBNP were associated with an elevated risk of mortality in long-term follow-up. Patients experiencing more significant myocardial injury also exhibited poorer survival outcomes over a 42 months period.
In COVID-19 patients, acute cardiac injury, both preexisting and newly developed cardiovascular complications, as well as acute kidney injury, have been identified as predictors of mortality in short-term follow-up2,47. Comparable correlations were documented in a cohort of U.S. female veterans with comorbid cardiovascular disease, CKD, COPD, and DM, who required anticoagulation and tested positive for COVID-19 within 60 days48. Conversely, a Danish study demonstrated that, at the 30 days follow-up, only female patients with coexisting AF or HF experienced a more severe progression of COVID-19 and higher mortality, a finding restricted to early-phase COVID-19 cases (prior to 01/05/2020). Among later cases, HF or AF had no impact on outcomes49. No data is available concerning the influence of comorbidities on long-term outcomes in COVID-19 patients with cardiovascular burdens. According to our multivariate analysis, hypercholesterolemia, DM, HF, CAD, AF, stroke, CKD, and COPD were associated with mortality at 42 months. Notably, hypercholesterolemia was observed to exert a protective effect on COVID-19 outcomes. This protective effect may be attributable to the pleiotropic effects, including anti-inflammatory properties, of statins, which are prevalently utilized by patients with hypercholesterolemia50.
A study conducted in Japan identified male sex as a significant predictor of in-hospital mortality51. Numerous studies have consistently demonstrated a higher mortality rate among males with COVID-19 in comparison to females, even when adjusted for cardiovascular comorbidities within a 30–90 days period9,10,11. Our study further substantiates that male sex constitutes an independent risk factor for mortality during long-term follow-up. Nonetheless, no significant differences were observed in survival rates between male and female COVID-19 patients in subgroups with concurrent HF, AH, AF, DM, or CAD. Furthermore, no differences in 42 months mortality were found across the entire study cohort, nonetheless males below the age of 65 exhibited poorer survival outcomes.
Conclusions
In patients with COVID-19, male sex, the presence of cardiovascular comorbidities, and advanced age are determinants that adversely affect survival at the 42 months follow-up. Elevated levels of laboratory indicators of myocardial injury and a thrombotic state are associated with worse outcomes in long-term follow-up. Patients with COVID-19 exhibiting increased levels of D-dimer, troponin I, and NT-proBNP are at a heightened risk of mortality and warrant more rigorous supervision and medical monitoring.
During SARS-CoV-2 infection, female sex over the age of 65 years is no longer a factor improving prognosis; hence, older women with comorbidities should be considered a special medical group.
Data availability
The data underlying this article may potentially be shared on reasonable request to the corresponding author and with appropriate permissions and oversight.
References
Del Sole, F. et al. Features of severe COVID-19: A systematic review and meta-analysis. Eur. J. Clin. Invest. 50(10), e13378. https://doi.org/10.1111/ECI.13378 (2020).
Figliozzi, S. et al. Predictors of adverse prognosis in COVID-19: A systematic review and meta-analysis. Eur. J. Clin. Invest. 50(10), e13362. https://doi.org/10.1111/ECI.13362 (2020).
Wang, W., Wang, C. Y., Wang, S. I. & Wei, J. C. C. Long-term cardiovascular outcomes in COVID-19 survivors among non-vaccinated population: A retrospective cohort study from the TriNetX US collaborative networks. eClinicalMedicine 53, 101619. https://doi.org/10.1016/j.eclinm.2022.101619 (2022).
Clerkin, K. J. et al. COVID-19 and cardiovascular disease. Circulation 141(20), 1648–1655. https://doi.org/10.1161/CIRCULATIONAHA.120.046941/ASSET/869E6314-869F-4C2D-9F6D-87FF1087C3E0/ASSETS/IMAGES/LARGE/CIRCULATIONAHA.120.046941.FIG04.JPG (2020).
Chlabicz, M. et al. Cardiovascular risk and the COVID-19 pandemic: A population-based and case-control studies. Popul. Health Metr. 22(1), 1–10. https://doi.org/10.1186/S12963-024-00338-W/TABLES/5 (2024).
Warren-Gash, C. et al. Severe COVID-19 outcomes by cardiovascular risk profile in England in 2020: A population-based cohort study. Lancet Reg. Heal Eur. 1, 27. https://doi.org/10.1016/J.LANEPE.2023.100604 (2023).
Xie, Y., Evan, X., Bowe, B. & Al-Aly, Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 28(3), 583–590. https://doi.org/10.1038/s41591-022-01689-3 (2022).
Pijls, B. G. et al. Temporal trends of sex differences for COVID-19 infection, hospitalisation, severe disease, intensive care unit (ICU) admission and death: A meta-analysis of 229 studies covering over 10M patients. F1000Research 11, 5. https://doi.org/10.12688/f1000research.74645.1 (2022).
Alkhouli, M. et al. Sex differences in case fatality rate of COVID-19: Insights from a multinational registry. Mayo Clin. Proc. 95(8), 1613–1620. https://doi.org/10.1016/J.MAYOCP.2020.05.014 (2020).
Pastor-Barriuso, R. et al. Infection fatality risk for SARS-CoV-2 in community dwelling population of Spain: Nationwide seroepidemiological study. BMJ 27, 371. https://doi.org/10.1136/BMJ.M4509 (2020).
Williamson, E. J. et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 584(7821), 430–436. https://doi.org/10.1038/S41586-020-2521-4 (2020).
Tejpal, A. et al. Sex-based differences in COVID-19 outcomes. J. Women’s Health 30(4), 492–501. https://doi.org/10.1089/jwh.2020.8974 (2021).
Zettersten, E. et al. Long-term outcome after intensive care for COVID-19: Differences between men and women—a nationwide cohort study. Crit. Care https://doi.org/10.1186/s13054-021-03511-x (2021).
Günster, C. et al. 6-month mortality and readmissions of hospitalized COVID-19 patients: A nationwide cohort study of 8679 patients in Germany. PLoS ONE 16(8), e0255427. https://doi.org/10.1371/journal.pone.0255427 (2021).
Sieurin, J. et al. A population-based cohort study of sex and risk of severe outcomes in covid-19. Eur. J. Epidemiol. https://doi.org/10.1007/S10654-022-00919-9 (2022).
Flisiak, R. et al. Recommendations of management in SARS-CoV-2 infection of the polish association of epidemiologists and infectiologists. Pol. Arch. Intern. Med. https://doi.org/10.20452/pamw.15270 (2020).
Flisiak, R. et al. Management of SARS-CoV-2 infection: Recommendations of the polish association of epidemiologists and infectiologists. Annex no. 1 as of June 8, 2020. Pol. Arch. Intern. Med. 130(6), 557–558. https://doi.org/10.20452/PAMW.15424 (2020).
Hong, K. H. et al. Guidelines for laboratory diagnosis of coronavirus disease 2019 (COVID-19) in Korea. Ann. Lab. Med. 40(5), 351–360. https://doi.org/10.3343/ALM.2020.40.5.351 (2020).
Hosmer, D., Lemesow, S. & May, S. Applied Survival Analysis: Regression Modeling of Time-to-Event Data (Wiley, 1999).
Terlecki, M. et al. Association between cardiovascular disease, cardiovascular drug therapy, and in-hospital outcomes in patients with COVID-19: Data from a large single-center registry in Poland. Kardiol. Pol. 79(7–8), 773–780. https://doi.org/10.33963/KP.15990 (2021).
Rademacher, J. et al. Association of respiratory infections and the impact of vaccinations on cardiovascular diseases. Eur. J. Prev. Cardiol. 31(7), 877–888. https://doi.org/10.1093/EURJPC/ZWAE016 (2024).
Bekkering, S. & Burgner, D. Viruses and cardiovascular disease: From bad to worse. Nat. Cardiovasc. Res. 1(7), 601–602. https://doi.org/10.1038/s44161-022-00101-0 (2022).
Hulme, K. D., Noye, E. C., Short, K. R. & Labzin, L. I. Dysregulated inflammation during obesity: Driving disease severity in influenza virus and SARS-CoV-2 infections. Front. Immunol. 28, 12. https://doi.org/10.3389/FIMMU.2021.770066 (2021).
Bekkering, S. et al. Trained immunity: Linking obesity and cardiovascular disease across the life-course?. Trends Endocrinol. Metab. 31(5), 378–389. https://doi.org/10.1016/J.TEM.2020.01.008/ASSET/1827E738-D3D9-4DC6-BE3E-34A157E04F13/MAIN.ASSETS/GR3.SML (2020).
Othman, H. Y. et al. A systematic review of thromboembolic complications and outcomes in hospitalised COVID-19 patients. BMC Infect. Dis. 24(1), 1–16. https://doi.org/10.1186/S12879-024-09374-1/TABLES/5 (2024).
Puri, A. et al. Comparison of comorbidities among severe and non-severe COVID-19 patients in Asian versus non-Asian populations: A systematic review and meta-analysis. Nurs. Open 9(1), 733–751. https://doi.org/10.1002/NOP2.1126 (2022).
Gebhard, C. et al. Impact of sex and gender on COVID-19 outcomes in Europe. Biol. Sex. Differ. https://doi.org/10.1186/S13293-020-00304-9 (2020).
Bechmann, N. et al. Sexual dimorphism in COVID-19: Potential clinical and public health implications. Lancet Diabetes Endocrinol. 10(3), 221–230. https://doi.org/10.1016/S2213-8587(21)00346-6 (2022).
Ho, J. Q. et al. The immune response to COVID-19: Does sex matter?. Immunology 166(4), 429–443. https://doi.org/10.1111/IMM.13487 (2022).
Dumanski, J. P. et al. Immune cells lacking Y chromosome show dysregulation of autosomal gene expression. Cell. Mol. Life Sci. 78(8), 4019–4033. https://doi.org/10.1007/S00018-021-03822-W (2021).
Abassi, Z. et al. ACE2, COVID-19 infection, inflammation, and coagulopathy: Missing pieces in the puzzle. Front. Physiol. 6, 11. https://doi.org/10.3389/FPHYS.2020.574753 (2020).
Potluri, T. et al. Age-associated changes in the impact of sex steroids on influenza vaccine responses in males and females. npj Vaccines https://doi.org/10.1038/s41541-019-0124-6 (2019).
Ballini, A. et al. Multiparametric correlation of laboratory biomarkers to multiorgan failure outcome in hospitalized COVID-19 patients: A retrospective observational study. Eur. Rev. Med. Pharmacol. Sci. 27(18), 8962–74 (2023).
Minhas, A. S. et al. The role of sex and inflammation in cardiovascular outcomes and mortality in COVID-19. Int. J. Cardiol. 8(337), 127. https://doi.org/10.1016/J.IJCARD.2021.05.011 (2021).
Megiorni, F. et al. Sex-related factors in cardiovascular complications associated to COVID-19. Biomolecules 12(1), 21. https://doi.org/10.3390/biom12010021 (2021).
Lau, E. S. et al. Sex differences in inflammatory markers in patients hospitalized with COVID-19 infection: Insights from the MGH COVID-19 patient registry. PLoS ONE 16(4), e0250774. https://doi.org/10.1371/journal.pone.0250774 (2021).
Zwaenepoel, B. et al. The prognostic value of cardiac biomarkers and echocardiography in critical COVID-19. Front. Cardiovasc. Med. 5, 8. https://doi.org/10.3389/FCVM.2021.752237 (2021).
Jin, X. et al. Elevated high sensitivity cardiac troponin T is nonlinearly associated with poor prognosis in aging COVID-19 patients: A retrospective study. Infect. Drug Resist. 16, 5155–5163. https://doi.org/10.2147/IDR.S422492 (2023).
Liu, A. et al. Normal high-sensitivity cardiac troponin for ruling-out inpatient mortality in acute COVID-19. PLoS ONE 18(4), e0284523. https://doi.org/10.1371/journal.pone.0284523 (2023).
Shi, S. et al. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur. Heart J. 41(22), 2070–2079. https://doi.org/10.1093/EURHEARTJ/EHAA408 (2020).
Caro-Codón, J. et al. Characterization of NT-proBNP in a large cohort of COVID-19 patients. Eur. J. Heart Fail. 23(3), 456–464. https://doi.org/10.1002/EJHF.2095 (2021).
Yoo, J. et al. Admission NT-proBNP and outcomes in patients without history of heart failure hospitalized with COVID-19. ESC Hear. Fail. 8(5), 4278–4287. https://doi.org/10.1002/EHF2.13548 (2021).
Klocek, M. et al. Cardiac biomarkers on admission and in-hospital mortality in COVID-19 patients with or without concomitant heart failure. Pol. Arch. Intern. Med. https://doi.org/10.20452/pamw.16256 (2022).
Cozzolino, D. et al. Long-Term prognosis among COVID-19 patients: The predictive role played by hyperinflammation and arrhythmic disorders in fatal outcome. J. Clin. Med. 12(17), 5691. https://doi.org/10.3390/jcm12175691 (2023).
Mu, S. et al. NT-proBNP reference intervals in healthy U.S. children, adolescents, and adults. J. Appl. Lab. Med. 8(4), 700–712. https://doi.org/10.1093/jalm/jfad024 (2023).
Sabatino, J., De Rosa, S., Di Salvo, G. & Indolfi, C. Impact of cardiovascular risk profile on COVID-19 outcome. A meta-analysis. PLoS ONE 15(8), e0237131. https://doi.org/10.1371/journal.pone.0237131 (2020).
Hernandez-Hernandez, M. E. et al. The effects of biological sex and cardiovascular disease on COVID-19 mortality. Am. J. Physiol. Heart Circ. Physiol. 323(3), H397–H402. https://doi.org/10.1152/AJPHEART.00295.2022 (2022).
Phelps, M. et al. Cardiovascular comorbidities as predictors for severe COVID-19 infection or death. Eur. Hear. J. Q. Care Clin. Outcomes 7(2), 172. https://doi.org/10.1093/EHJQCCO/QCAA081 (2021).
Lashgari, N. A. et al. Statins: Beneficial effects in treatment of COVID-19. Adv. Exp. Med. Biol. 1412, 457–476. https://doi.org/10.1007/978-3-031-28012-2_25 (2023).
Matsumoto, S. et al. Sex differences in clinical outcomes among patients with COVID-19 and cardiovascular disease - insights from the CLAVIS-COVID registry. Circ. Rep. 4(7), 315–321. https://doi.org/10.1253/CIRCREP.CR-22-0047 (2022).
Funding
This publication was supported by the National Center for Research and Development CRACoV-HHS project (Model of multispecialist hospital and nonhospital care for patients with SARS-CoV-2 infection) through the initiative “Support for specialist hospitals in fighting the spread of SARS-CoV-2 infection and in treating COVID-19” (contract number SZPITALE-JEDNOIMIENNE/18/2020). The described research was implemented by a consortium of the University Hospital in Cracow and the Jagiellonian University Medical College.
Author information
Authors and Affiliations
Consortia
Contributions
All the authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by M.K.T., M.T., A.O., C.P. and M.R. The first draft of the manuscript was written by M.K.T., and all the authors commented on previous versions of the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The study was a retrospective analysis of the medical records of discharged patients and did not affect patient safety or the occurrence of possible complications, therefore obtaining consent from the study participants was not necessary. The study was conducted in accordance with the guidelines of the Declaration of Helsinki and with the aforementioned methodology was approved by the Bioethics Committee of Jagiellonian University in Krakow (Poland) (decision number 1072.6120.278.2020). The authors confirm that the obtaining of informed consent from the study participants was waived by the Bioethics Committee of the Jagiellonian University in Krakow (Poland).
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kocowska-Trytko, M., Terlecki, M., Olszanecka, A. et al. Sex and other predictors of mortality in long-term follow-up of patients with cardiovascular disease and COVID-19: a single-center retrospective study. Sci Rep 15, 13245 (2025). https://doi.org/10.1038/s41598-025-93402-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-93402-w