Abstract
This study investigates the impact of choroidal blood flow on pigment epithelial detachment (PED) and subretinal fluid (SRF) in central serous chorioretinopathy (CSC). We categorized CSC patients into groups with and without PED, alongside healthy controls. Using SS-OCT, we measured the three-dimensional choroidal vascular index (CVI), choroidal vascular volume (CVV), SRF and PED area and volume, subfoveal choroidal thickness (SFCT), and choroid thickness beneath the highest point of PED (PED-ct). Image-J was utilized to analyze flow void area (FVa) and flow void area percentage (FV%) in the choriocapillary. Results indicated that SFCT, CVI, CVV, FVa, and FV% were significantly higher in CSC patients compared to healthy individuals. Furthermore, CSC patients with PED exhibited a greater three-dimensional CVI than those without PED (P < 0.05). Within CSC patients, FV% positively correlated with SRF area, CVV, and SFCT. In the PED group, CVV positively correlated with PED-ct, FVa, FV%, and both PED and SRF area. Thus, the presence of PED and SRF in CSC is associated not only with low choriocapillary blood flow but also with increased vascular density and the expansion of Sattler and Haller layers.
Similar content being viewed by others
Introduction
The detachment of the retinal pigment epithelium and neural epithelium serves as the primary contributor to visual impairment and distortion in central serous chorioretinopathy (CSC)1,2,3. As the disease progresses, patients may experience photoreceptor degeneration, retinal pigment epithelium atrophy, choroidal neovascularization, and other tissue alterations4,5,6. Extensive research indicated that CSC patients show various ocular manifestations, including choroidal thickening, vortical vein congestion, vascular dilation7,8,9. In diagnosing and assessing CSC, choroidal thickness and serous detachment of the neuroepithelium are critical follow-up indicators10,11,12. Although the subfoveal choroidal thickness (SFCT) can reflect choroidal changes in CSC patients to some extent, it has limitations in assessing choroidal vasculature changes and is affected by circadian rhythm13,14,15,16. Recent studies suggest using parameters like flow void area (FVa), flow void area percentage (FV%),17,18,19,20 three-dimensional choroidal vascular index (CVI)7, choroidal vascular volume (CVV)7 for a more comprehensive evaluation of choriocapillary, sattler and haller layers. Notably, previous studies primarily utilized two-dimensional CVI to assess choroidal vessel changes in a unit area, which did not accurately reflect the entire choroidal vascular system21,22,23. In contrast, recent studies employing three-dimensional CVI have provided more detailed information about choroidal vessels changes in a unit volume7.
The pathogenesis of CSC is complex, and intricately linked to the dysfunction of the choroidal vasculature and retinal pigment epithelium pump9,24. During follow-up, both acute or chronic CSC may involve the occurrence of PED phenomenon23,25. Even when subretinal fluid (SRF) gradually diminishes, pigment epithelial detachment (PED) perhaps persist in some patients26. Some cases of CSC with flat irregular PED can progress to neovascular PED27, while a few finger-like PEDs may evolve polypoidal choroidal vasculopathy21,26,28. Nevertheless, the precise mechanisms behind the development and progression of PED and SRF are not fully understood, and there is a lack of research on the correlation between choroidal blood flow changes and PED/SRF formation. Consequently, this study aim to assess parameters such as FVa, FV%, three-dimensional CVI, and CVV in CSC patients with or without PED and to explore potential associations between these indicators and the development of PED and SRF, providing a scientific foundation for further understanding the pathogenesis of CSC.
Results
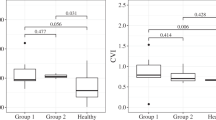
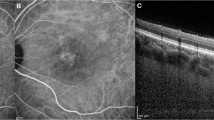
The SFCT in CSC with PED (496.593 ± 69.409 μm) and without PED (528.885 ± 100.251 μm) were significantly elevated compared to the normal group (381.556 ± 87.928 μm), with statistical significance (P < 0.05). However, there was no significant difference in SFCT between CSC without PED and with PED (P = 0.144). Choroidal thickness was noticeably increased in CSC, as shown in Fig. 1A. In the CSC cohort with PED, no significant variation in SFCT or choroidal thickness beneath the apical region of PED (488.593 ± 83.875 μm, t = 0.704). The orange line in Fig. 1A indicates the area of SRF, while the red triangle denotes the PED area. CSC with PED had greater SRF area and volume (9.685 ± 6.636mm2, 1.174 ± 1.133mm3) than those without PED (9.302 ± 5.861mm2, 1.076 ± 1.110mm3), but this difference was not statistically significant (t = 0.825, t = 0.753).
The macular foveal area was analyzed using the ETDRS, dividing it into 1, 3, and 6 mm circular regions. Figure 1A shows the orange region, illustrating the Sattler and Haller layer, where vessels dilated is evident. Red asterisks in Fig. 1A highlight notable enlargement of the vascular lumen beneath the PED, with increased pixel density in the choroidal vasculature shows in Fig. 1B red rectangle. Comparison to the normal group, three-dimensional CVI values in the 1, 3, and 6 mm regions were significantly elevated in CSC patients with PED (P < 0.05). In contrast, CSC patients without PED showed elevated the three-dimensional CVI only in the 6 mm region, also reaching statistical significance (P < 0.05). Three-dimensional CVI was greater in all circular regions for CSC with PED compared to those without PED, with significant differences (P < 0.05). The CVV measurements in all circular regions were notably increased in both CSC groups compared to the normal group (P < 0.05, Table 1).
OCTA images of CSC. CTA images centered on the macular foveal, Angio 6 × 6 mm 512 × 512 R4 mode. The patient is a 65-year-old male with a one-month disease history. (A) Orange line zone represents subretinal fluid; red zone represents retinal pigment epithelial detachment; red asterisks indicate the expanded choroidal vascular lumen below retinal pigment epithelial detachment; orange zone represents the distribution of the dilated Sattler and Haller vascular lumen. (B) Red rectangle indicating the retinal pigment epithelial detachment zone locating at the dilated choroidal vascular.
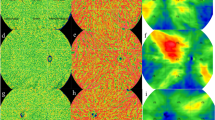
Figure 2 shows dark particles represents the FVa, indicating pixel inhomogeneity in the choriocapillary layer of CSC patients (Fig. 2B, C red rectangle zone). There were more dark particles in CSC compared to the normal group, and FVa and FV% significantly increased in both CSC groups (P < 0.05). However, no significant differences were found between CSC patients with and without PED regarding FVa and FV% (Table 1).
Regardless of PED presence, there was a strong positive correlation between CVV and SFCT in all regions. CVV showed an extremely strong positive correlation with choroidal thickness beneath the highest point of PED in CSC with PED, with a strong positive correlation at 6 mm, Conversely, CVV at 6 mm had a weak negative relationship with FV% across all CSC patients (r=-0.292, P = 0.034), indicating statistical significance (Table 2). A significant positive correlation was also observed between SRF area and FV% in CSC, with no correlation to FVa. In CSC with PED, a significant positive correlation existed between the area and volume of PED and both FVa and FV% (Table 3).
OCTA images of choriocapillary layer. Choriocapillary layer centered at macular foveal, Angio 6mmx6mm 512 × 512 R4 OCTA images: the light particles represent choriocapillary blood flow signal, the dark particles represent choriocapillary blood flow void area. (A) normal group: 52-year-old male; (B) CSC without PED group: 40-year-old male with a one-month disease history, Red rectangle zone locate at subretinal fluid zone performing the dark particles with low-pixel values; (C) CSC with PED group: 59-year-old male with a one-month disease history, red rectangle zone locate at subretinal fluid zone performing the dark particles with low-pixel values.
Discussion
Previous studies suggest that choroidal thickening in CSC may stem from to the expansion of outer choroidal blood vessels, corroborating our findings7,29. Our study reveal that CSC patients experience significant thickening of SFCT, notable increases in three-dimensional CVI and CVV within the 1, 3, and 6 mm ETDRS rings in the macular region compared to healthy individuals. This rise in three-dimensional CVI and CVV could disrupt the RPE function, as the increased density and lumen expansion of Sattler and Haller vessels heighten vascular permeability and hydrostatic pressure, allowing substantial fluid exudation through the RPE, contributing to SRF formation30. Notably, CVV did not differ between CSC patients with or without PED, however, those with PED displayed thinner choroidal thickness and significantly greater choroidal vessel density. The three-dimensional CVI is defined as the ratio of CVV to total choroidal volume. We hypothesize that the atrophy of the choroidal matrix in CSC with PED reduces total choroidal volume, which may lead to the increased relative choroidal vascular density also allowing impact RPE adhesion to Bruch’s membrane31, facilitating PED formation. We observed a strong positive correlation between SFCT, choroidal thickness at the peak of PED, and CVV(1, 3, 6 mm) in CSC with PED, suggesting that choroidal vascular expansion influences choroidal thickening, particularly at the peak of PED. PED primarily occurs in areas with expanded Sattler and Haller choroidal vasculature, consistent with prior findings that link PED to fluorescein leakage sites and regions of high vascular permeability7,9. Consequently, expanding blood vessels may create localized areas of weak RPE adhesion to Bruch’s membrane31, allowing fluid to infiltrate and form PED during RPE dysfunction.
We also noted diminished blood flow signals beneath PED in the choriocapillary layer, with a weak negative relationship between CVV and FV%, indicating dilation in Sattler and Haller layers exacerbates choriocapillary blood flow abnormalities. Some researchers believe this may result from mechanical compression of the CC layer by the dilated vessels24,29. Additionally, we found considerable increases in FVa and FV% in this layer among CSC patients, suggesting reduced choriocapillary perfusion32. As the choriocapillary layer is vital for supplying nutrients and metabolic support to the outer retina, reduced blood flow could impair RPE barrier and pump functions, weakening RPE adhesion to Bruch’s membrane31. Moreover, the elevated FV% in the choriocapillary layer correlates positively with the size of the SRF area, highlighting how hypoperfusion in the choriocapillary layer affects RPE barrier function. The increased density and lumen expansion of Sattler and Haller vessels contribute to heighten vascular permeability and hydrostatic pressure, leading to a significant influx of choroidal fluid from RPE layer to retinal neural epithelium24. It is important to note that the RPE fluid flow direction is normally from the retina to the choroid, so RPE pump dysfunction leads to fluid accumulation beneath the retinal neural epithelium. Previous studies aim to analyses abnormal blood flow in CC layer and CVI effect CSC recurrence and SRF formation33. However, our study mainly addresses the less-explored relationship between three-dimensional CVI, CVV, choriocapillary blood flow, and PED formation. In CSC patients with PED, we found a notable positive correlation between FV% and FVa of the choriocapillary layer with the area and volume of PED. Research indicates that some PEDs can evolve into choroidal neovascularization and polypoidal choroidopathy21,26,27,28, suggesting ischemia and hypoxia in choroidal capillaries, which aligns with our findings. We observed decreased choriocapillary perfusion, with localized increases in the density and lumen expansion of Sattler and Haller vessels contributing to RPE detachment by weakening adhesion with Bruch’s membrane and hindering the barrier and pump functions of the RPE, leading to fluid infiltration in the RPE-Bruch’s membrane region.
However, our reasearch has limitations. Expanding the sample size could enhance the study of choroidal vascular parameters and mitigate the age-related effects on choroidal blood vessels while highlighting differences in choroidal thickness between cases with and without PED. Additionally, the lack of follow-up on PED and SRF outcomes prevented us from monitoring changes in choroidal vascular parameters in relation to improvements or deteriorations in CSC. These shortcomings will be addressed in future research.
In summary, in CSC, lumen expansion in Sattler and Haller vessels may contribute to increased SFCT and choroidal thickness at the peak of PED, alongside a reduction in choriocapillary blood flow. Furthermore, the combined effects of localized increases in the density and lumen expansion of Sattler and Haller vessels, and hypoperfusion of choriocapillaris could lead to RPE layer barrier and pump dysfunction, weaken RPE layer adhesion to Bruch’s membrane, facilitating SRF and PED formation. Specifically, increased three-dimensional CVI, FVa and FV% in the choriocapillaris layer may significantly elevate the risk of PED occurrence. Consequently, these indicators could play a role in assessing the progression of PED and SRF in CSC, enhancing our understanding of CSC’s pathogenesis and serving as tracking metrics for disease outcomes.
Materials and methods
General information
This study adhered to the Declaration of Helsinki and received approval from the Ethical Review Committee of Renmin Hospital of Wuhan University (WDRY2021-k162). We conducted a cross-sectional study involving 53 patients with CSC admitted to the ophthalmology department of the Eastern Renmin Hospital of Wuhan University from March 2020 to June 2024. The CSC cohort was categorized into two distinct subgroups: a PED-associated subgroup of 27 individuals, ages 31 to 59 years, and a non-PED subgroup of 26 patients, including 21 males and 5 females, ages 29 to 69 years (Table 4). Patients with CSC presenting only with SRF were categorized as non-PED, while those with both SRF and PED showing a double-walled appearance were classified as PED-positive. Diagnosis involved multimode imaging, including Swept-source Optical Coherence Tomography Angiography (SS-OCTA) which revealed serous detachment of the retinal nerve epithelial layer, wavy RPE appearance and accompanied by finger-like serous detachments, and fluid separation between the RPE and Bruch membrane, with image signal intensity exceeding a threshold of 8. Fluorescein angiography revealed active leakage and pigment epithelial defects, and Indocyanine green angiography displayed characteristic hyperfluorescence of the choroid. All patients had a disease duration of less than 6 months with first onset symptoms. Exclusion criteria included previous photodynamic therapy or laser treatment, choroidal neovascularization, ametropia from − 3D to + 1D, poor image quality due to media opacity or lack of cooperation, alternative causes of SRF, prior systemic glucocorticoid treatment, age-related macular degeneration, polypoidal choroidal vasculopathy, and other retinal vascular diseases. We also included 27 healthy control eyes matched for age (30–59 years, Table 4). Inclusion criteria for controls were clear optical media, absence of any systemic diseases or other ocular pathologies, and ametropia from − 3D to 1D. No significant differences in gender or age existed between the control group and the PED-associated or non-PED groups.
Routine examination
All subjects underwent comprehensive eye examinations including visual acuity, optometry, and intraocular pressure measurement, and slit lamp microscopy of the anterior segment. Retinal assessments utilized scanning laser ophthalmoscopy, and detailed medical histories were obtained.
OCTA examination
All subjects underwent SS-OCTA using a VG100 device (Henan, China: MicroVision Gmbh), with experienced doctors performing 6mmx6mm 512 × 512 R4 scanning at a wavelength of 1050 nm and speed of 200,000 A/s, 56°wide-angle scans, a scanning line length of 16 mm, a tissue scanning depth of 2.7 mm, an image signal intensity was maintained at a level greater than 8. Images were analyzed with built-in MicroVision software to measure structural layers and regions, and manual verification was performed. The measurement of SFCT refers to the distance extending from the Bruch membrane to the scleral junction of the choroid at subfoveal; the measurement of choroidal thickness beneath the highest point of PED signifies the distance between the Bruch membrane to the scleral junction of the choroid; the measurement of PED liquid dark area involves quantifying the area and volume between the elevated RPE layer to Bruch membrane; the measurement of SRF entails determining the area and volume occupied by the space the retinal nerve epithelial layer to REP layer; three-dimensional CVI is defined as the ratio of the volume occupied by Sattler and Haller vascular volume within the total choroidal volume, mainly reflecting the vessel density of choroidal Sattler and Haller layer; CVV is defined as the Sattler and Haller vascular distribution volume of choroid; choriocapillary layer measurement was 25 μm below Bruch membrane. Quantification of SFCT, choroidal thickness beneath the highest point of PED, PED, as well as SRF areas and volumes, three-dimensional CVI and CVV were automatically measured and calculated by MicroVision OCT system. We used Early Treatment Diabetic Retinopathy Study (ETDRS) circular regions (1 mm, 3 mm, and 6 mm in diameter) centered on the macular fovea for further analysis.
Choriocapillary layer image processing
The choriocapillary layer image was scanned and exported utilizing the software SS-OCTA. Within this image, the presence of bright particles exhibiting a high pixel density serves as an indication of blood flow signals. Conversely, dark particles occupying low-pixel areas represent vascular FVa. The proportion of these dark particles with low-pixel areas, relative to the overall image area, is designated as FV%. To analyze and process the dark regions of the image, as well as calculate FVa and FV%, the ImageJ software is employed. The specific steps28 involved are outlined as follows: (1) The initial 6*6 mm image is converted to an 8-bit format. Subsequently, automatic local thresholding is applied to eliminate minor variations in image brightness. (2) The Phansalkar method is then utilized to mark the low-pixel dark areas within the image. (3) The Analyze Particles function is subsequently utilized to conduct a comprehensive analysis and measurement of the image. Within this analysis, FVa is defined as the cumulative area occupied by the dark particles with low-pixel values. Conversely, FV% represents the ratio of the total area occupied by these dark particles with low-pixel values, relative to the entire image area.
Statistical analysis
SPSS 27.0 software was used for statistical analysis. The distribution of variables was rigorous tested by P-P plot, and data with normal distribution were expressed as mean ± SD. In the case of comparing three or more groups, a one-way ANOVA was conducted. Additionally, Levene’s test was employed to assess the homogeneity of variance. If the variance was deemed homogeneous, LSD was chosen as the appropriate post hoc analysis method. Conversely, in the absence of homogeneity of variance, Dunnett’s T3 test was utilized for post hoc analysis. For comparisons involving two groups, independent sample t-tests were appropriately applied. Furthermore, Pearson correlation analysis was performed to investigate the correlation between variables, provided that the distribution of the statistical variables adhered to normal distribution (statistical significance was determined at P < 0.05).
Data availability
The data from this study are available form the corresponding author upon request.
References
Sarah, M. et al. Long-term visual outcomes and causes of vision loss in chronic central serous chorioretinopathy. Ophthalmology 126, 4 (2019).
Filippos, V. Quantitative contrast sensitivity test to assess visual function in central serous chorioretinopathy. Br. J. Ophthalmol. 107, 8 (2022).
Enrico, B. et al. Prevalence and morphologic biomarkers of metamorphopsia in eyes with resolved chronic central serous chorioretinopathy. Retina 43, 9 (2023).
von der Burchard, C. et al. AI-based photoreceptor length segmentation and influence on disease progression and visual acuity changes in central serous chorioretinopathy (CSCR). Invest. Ophthalmol. Vis. Sci. 65 (7), 5659 (2024).
Jia, Y., Chunhui, J. & Gezhi, X. Correlations between changes in photoreceptor layer and other clinical characteristics in central serous chorioretinopathy. Retina 39, 6 (2018).
Maria Cristina, S. et al. Central serous chorioretinopathy by autofluorescence, enface and SLO-Retromode imaging. Life (Basel). 13, 6 (2023).
Jingyuan, Y., Erqian, W., Mingzhen, Y. & Youxin, C. Three-dimensional choroidal vascularity index in acute central serous chorioretinopathy using swept-source optical coherence tomography. Graefes Arch. Clin. Exp. Ophthalmol. 258, 2 (2019).
Bei, X., Ming, Y., Yan-Ping, S., Ya, Y. & Zhen, H. Quantitative assessment of choroidal parameters and retinal thickness in central serous chorioretinopathy using ultra-widefield swept-source optical coherence tomography: a cross-sectional study. BMC Ophthalmol. 24, 1 (2024).
Takahiro, K. et al. Pigment epithelial detachment and leak point locations in central serous chorioretinopathy. Am. J. Ophthalmol. 261, 0 (2024).
Dong-Geun, P., Seongyong, J., Donghyoun, N. & Min, S. Optimal fluence rate of photodynamic therapy for chronic central serous chorioretinopathy. Br. J. Ophthalmol. 105, 6 (2020).
Baek-Lok, O. & Hyeong Gon, Y. Choroidal thickness after full-fluence and half-fluence photodynamic therapy in chronic central serous chorioretinopathy. Retina 35, 8 (2015).
Alejandra, D. et al. Acute central serous chorioretinopathy: factors influencing episode duration. Retina 37, 10 (2017).
Takamasa, K. et al. Diurnal variations in luminal and stromal areas of choroid in normal eyes. Br. J. Ophthalmol. 101, 3 (2016).
Shinichi, U. et al. Circadian changes in subfoveal choroidal thickness and the relationship with circulatory factors in healthy subjects. Invest. Ophthalmol. Vis. Sci. 53, 4 (2012).
Seung Won, L., Seung-Young, Y., Kyung Hoon, S., Eung Suk, K. & Hyung Woo, K. Diurnal variation in choroidal thickness in relation to sex, axial length, and baseline choroidal thickness in healthy Korean subjects. Retina 34, 2 (2013).
Hannah, J. B., Nimesh, B. P. & Lisa, A. O. Ocular biometric diurnal rhythms in emmetropic and myopic adults. Invest. Ophthalmol. Vis. Sci. 59, 12 (2018).
Cédric, R. et al. Optical coherence tomography angiography quantitative assessment of choriocapillaris blood flow in central serous chorioretinopathy. Am. J. Ophthalmol. 194, 0 (2018).
Fabio, S., Francesca Romana, P., Eliana, C. & Mariacristina, P. Relationship of choroidal vasculature and choriocapillaris flow with alterations of salivary α-Amylase patterns in central serous chorioretinopathy. Invest. Ophthalmol. Vis. Sci. 62, 15 (2021).
Bo-Een, H., Joo-Young, K. & Young-Hoon, P. The effect of heart rate variability on the choroidal vascularity of the optical coherence tomography and angiography in central serous chorioretinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 262(12), 3825–3835 (2024).
Cheolmin, Y. et al. Choriocapillaris flow features and choroidal vasculature in the fellow eyes of patients with acute central serous chorioretinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 257, 1 (2018).
Iovino, C. et al. Choroidal anatomic alterations after photodynamic therapy for chronic central serous chorioretinopathy: A multicenter study. Am. J. Ophthalmol. 217, 104–113 (2020).
Tatti, F. et al. En face choroidal vascularity in both eyes of patients with unilateral central serous chorioretinopathy. J. Clin. Med. 12, 1 (2022).
Rae-Young, K., Doh Hoon, C., Mirinae, K. & Young-Hoon, P. Use of choroidal vascularity index for choroidal structural evaluation in central serous chorioretinopathy with choroidal neovascularization. Retina 40, 7 (2019).
Federica, F. et al. Pathogenesis of central serous chorioretinopathy and the link between choroidal hyperpermeability and retinal pigment epithelium pump reversal. Am. J. Ophthalmol. 266, 0 (2024).
Rabih, H. et al. Flat irregular retinal pigment epithelium detachments in chronic central serous chorioretinopathy and choroidal neovascularization. Am. J. Ophthalmol. 159, 5 (2015).
Hisataka, F. et al. Morphologic changes in acute central serous chorioretinopathy evaluated by fourier-___domain optical coherence tomography. Ophthalmology 115, 9 (2008).
Richard, F. S. Choriocapillaris flow features follow a power law distribution: implications for characterization and mechanisms of disease progression. Am. J. Ophthalmol. 170, 0 (2016).
Yuki, M. et al. Genome-wide survival analysis for macular neovascularization development in central serous chorioretinopathy revealed shared genetic susceptibility with polypoidal choroidal vasculopathy. Ophthalmology 129, 9 (2022).
Hideki, S. et al. Quantitative analyses of diameter and running pattern of choroidal vessels in central serous chorioretinopathy by En face images. Sci. Rep. 10, 1 (2020).
Michiyuki, S., Kousuke, N., Wataru, S. & Susumu, I. Relationship between choroidal blood flow velocity and choroidal thickness in patients with regression of acute central serous chorioretinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 256, 1 (2017).
Dou, R., Chu, Y., Han, Q., Zhang, W. & Bi, X. Giant retinal pigment epithelium tears with membranous nephropathy: a case report and literature review. BMC Ophthalmol. 24 (1), 177 (2024).
Rebecca, K. et al. Central serous chorioretinopathy: an update on risk factors, pathophysiology and imaging modalities. Prog Retin Eye Res. 79, 0 (2020).
Eoi, J. et al. Yoon.Abnormal choroidal flow on optical coherence tomography angiography in central serous chorioretinopathy. Clin. Exp. Ophthalmol. 47 (4), 0 (2018).
Acknowledgements
Supported by Eye Center, Renmin hospital of Wuhan University.
Author information
Authors and Affiliations
Contributions
Conceptualization, A.M.X. and Q.L.L.; methodology, A.M.X. and G.P.S.; software, Q.L.L. and G.P.S.; validation, A.M.X. and Q.L.L.; formal analysis, A.M.X. and Q.L.L.; investigation, A.M.X., Q.L.L.and C.Z.C.; resources, Z.C.; data curation, A.M.X. and Q.L.L.; writing—original draft preparation, A.M.X. and Q.L.L.; writing—review and editing, Z.C. and C.Z.C.; visualization, Q.L.L.; supervision, C.Z.C.; project administration, Z.C.; funding acquisition, Z.C. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Informed consent
Informed consent was obtained form all subjects involved in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xu, A., Lai, Q., Sun, G. et al. Choroidal blood flow changes in central serous chorioretinopathy. Sci Rep 15, 9907 (2025). https://doi.org/10.1038/s41598-025-94096-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-94096-w