Abstract
353 hospitalized children diagnosed with Mycoplasma pneumoniae (MP) pneumonia were included in the study. They were divided into MP co-infection group and MP single infection group. 143 (40.5%) of the enrolled children had MP co-infections. The most common co-infecting pathogen was Rhinovirus (30.8%). Among the MP co-infections, 82 cases (57.3%) involved one pathogen, 44 cases (30.7%) involved two pathogens, 12 cases (8.4%) involved three pathogens, 4 cases (2.8%) involved four pathogens, and 1 case (0.7%) involved five pathogens. Significant differences were observed between the two groups in terms of severe MP pneumonia, macrolide resistance, bronchial mucus plug, and hormone use, with P-values of 0.039, 0.000, 0.000, and 0.035. The MP mixed virus or bacteria infection group was more likely to develop drug resistance compared to the mixed virus and bacteria group (P = 0.007 and P = 0.046). The MP mixed virus and bacteria group was more likely to develop severe pneumonia compared to the mixed virus or bacteria infection group (P = 0.032 and P = 0.017). In conclusion, MP was most commonly co-infected with Rhinovirus. Children with MP co-infections tend to exhibit higher rates of macrolide resistance, require more frequent use of hormones, and are more likely to develop severe pneumonia and bronchial mucus plug.
Similar content being viewed by others
Introduction
Mycoplasma pneumoniae pneumonia (MPP) is a significant public health issue affecting children worldwide, prompting extensive research in recent years1. Epidemics of Mycoplasma pneumoniae (MP) occur every 3–7 years, accounting for more than 40% of pediatric community-acquired pneumonia (CAP) cases during epidemic years2. Most studies have focused on refractory MP pneumonia (RMPP), macrolide resistant MP pneumonia (MRMPP), and severe MP pneumonia (SMPP). Few studies have investigated the influence of respiratory pathogens co-infection on the clinical course of MPP and their results have been inconclusive3,4,5. This study aims to explore the effects of respiratory pathogen co-infections on MPP in children.
Methods
Study patients and data collection
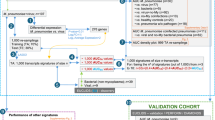
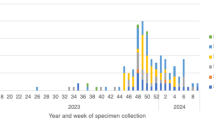
We retrospectively collected data from patients diagnosed with MPP who were admitted to the Women and Children’s Hospital of Ganzhou and underwent bronchoalveolar lavage (BAL) therapy between May 2023 and July 2024. MPP was defined as patients presenting with clinical signs and symptoms of pneumonia underwent chest radiography, and MP infection was confirmed through laboratory tests6. SMPP was defined as MPP with any one of the follows7: (1) a poor general condition; (2) fastidium or dehydration; (3) disturbance of consciousness; (4) an increased respiratory rate (infants > 70 breaths/min and older children > 50 breaths/min); (5) dyspnea; (6) cyanosis; (7) extent of infiltration on chest X-ray ≥ 2/3 of one lung or multilobe involvement; (8) extra-pulmonary complications; (9) pleural effusion; (10) oxygen saturation in room air ≤ 92%. RMPP was defined as clinical and radiographic deterioration after at least seven days of macrolide antibiotic treatment7. MRMPP was defined as persistent fever ≥ 3 days after macrolide treatment, accompanied by the A2063G/2064G mutation8. Bronchial mucus plug was defined as endogenous foreign bodies in the respiratory tract. Due to the retrospective nature of the study, the Ethics and Research Council of the Women and Children’s Hospital of Ganzhou waived the need for informed consent. All methods were performed in accordance with relevant guidelines and regulations, and all experimental protocols were approved by the Women and Children’s Hospital of Ganzhou.
Microbiological analyses
On the day of admission, 3 mL of venous blood was collected from patients, and the passive agglutination assay was used to detect MP antibody (MP Antibody Test Kit, Fujitsu Joint Stock Company). An MP antibody titer ≥ 1:160 was considered positive. BALF samples were collected using flexible fiberoptic bronchoscopy. Briefly, after the bronchoscope was inserted into the affected lung lobe, warm sterile saline was instilled and gently suctioned. A 1.5–2 mL aliquot of BALF from each patient was used for traditional bacterial culture within 30 min of collection9. Another 1.5–2 mL of BALF samples were sent to a third-party testing agency (Guangzhou KingMed Center for clinical laboratory) for metagenomic next generation sequencing (mNGS). Patients were included in the MP group only if both the agglutination assay and molecular detection of MP in BALF yielded positive results. The sensitivity of mNGS depends on sequencing depth and the abundance of pathogens10. False positives may arise from sample contamination or database alignment errors, while false negatives may result from low pathogen abundance or insufficient sequencing depth11. To minimize false positives, we implemented negative controls and optimized the bioinformatics analysis process (e.g., removing host sequences and filtering low-quality data). Results were interpreted in conjunction with clinical information. A bacterial infection was defined as the presence of bacteria detected in BALF culture and confirmed by mNGS. Colonization was ruled out based on clinical presentation, pathogen abundance, inflammatory markers, imaging findings, and repeated testing12. MP antibiotic resistance gene testing was also conducted by the third-party testing agency (Guangzhou KingMed Center for Clinical Laboratory). Full length sequencing of the 23S rRNA gene of MP was performed by polymerase chain reaction (PCR).
Statistical analyses
Data were analyzed using SPSS 20 software. Continuous variables were reported as median (range) and compared using Student’s t-test or the Mann–Whitney U-test. Categorical variables were presented as numbers (percentages) and compared using the Chi-square test. A P-value < 0.05 was considered statistically significant.
Results
Demographic characteristics and pathogens of the study population
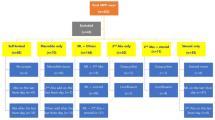
A total of 353 MPP patients who underwent BAL therapy were included. The male-to-female ratio was 1.34:1. Age distribution was as follows: < 1 year (31 cases, 8.8%), 1–3 years (66 cases, 18.7%), 3–6 years (113 cases, 32%), and 6–14 years (143 cases, 40.5%). There were 72 cases (20.4%) of RMPP, 86 cases (24.4%) of SMPP, and 183 cases (51.8%) of MRMPP. Among the patients, 210 (59.5%) had MP single infections, and 143 (40.5%) had co-infections. Co-infections included 111 cases (31.4%) of MP mixed with viruses and 73 cases (20.7%) of MP mixed with bacteria. The most common co-infecting pathogen was Rhinovirus (RV, 30.8%), followed by Streptococcus pneumoniae (SP, 27.3%) and Haemophilus influenzae (HI, 16.1%). Co-infections with Coronavirus (CoV), Adenovirus (ADV), Parainfluenza virus (PIV), Epstein-Barr virus (EBV), Human Bocavirus (HBoV), Influenza virus (IV), Respiratory syncytial virus (RSV), Staphylococcus aureus (SA), and Bordetella pertussis were also observed (Fig. 1). When patients were co-infected with more than two respiratory viruses, each pathogen was counted independently. Among MP co-infections, 82 cases (57.3%) involved one pathogen, 44 cases (30.7%) involved two pathogens, 12 cases (8.4%) involved three pathogens, 4 cases (2.8%) involved four pathogens, and 1 case (0.7%) involved five pathogens (Table 1).
Comparison between the MP single infection and MP co-infection group
No significant differences were observed between the MP co-infection and MP single infection groups in terms of gender, RMPP, fever, cough, wheezing, length of hospital stay, or complications (P > 0.05). However, patients in the MP single infection group were older than those in the MP co-infection group (P = 0.007). Significant differences were observed between the two groups in SMPP, macrolide resistance, bronchial mucus plug, and hormone use, with P-values of 0.039, 0.000, 0.000, and 0.035, respectively. No significant differences were found in laboratory findings between the two groups (Table 2).
Comparison between MP mixed virus and bacteria group
There were 70 cases (19.8%) of MP mixed with only viruses, 32 cases (9.1%) of MP mixed with only bacteria, and 41 cases (11.6%) of MP mixed with both viruses and bacteria. The MP mixed virus or bacteria infection group was more likely to develop drug resistance compared to the mixed virus and bacteria group (P = 0.007 and P = 0.046). The MP mixed virus and bacteria group was more likely to develop severe pneumonia compared to the mixed virus or bacteria infection group (P = 0.032 and P = 0.017). The MP mixed virus group had a longer hospital stay compared to the MP mixed bacteria group (P = 0.009). No significant differences were observed in gender, age, RMPP, bronchial mucus plug, fever, cough, wheezing, fever peak, fever duration, complications, or hormone use among the three groups. Additionally, no significant differences were found in laboratory findings among the three groups (Table 3).
Discussion
Currently, there are limited studies on mixed infections involving MP. Previous studies have reported respiratory virus co-infection rates in children with MPP ranging from 27.3 to 56.1%3,4,13. This study found a respiratory virus co-infection rate of 31.4%, consistent with previous findings. Martin et al. reported that the most common co-infection combination was MP and PIV (15%), followed by MP and IV14. In this study, the most common co-infecting pathogen was RV, followed by SP, HI, CoV, EBV, PIV, and ADV. Yun et al. reported that the most commonly co-detected bacteria were SP, SA, and Escherichia coli15. To date, there have been no reports of MP being frequently co-infected with RV. This discrepancy may be due to differences in study populations and specimen types. Most literature focuses on specific subgroups, such as patients with RMPP or SMPP. Zhang et al. reported that 27.0% of RMPP patients had co-infections with other pathogens, including SP, HI, SA, HBoV, RV, and RSV16. There was no consensus on the impact of co-infection on disease severity, which might depend on the specific pathogens involved17. However, this study found that MP co-infection was more likely to cause severe pneumonia. Co-infections with ADV have been associated with more severe clinical manifestations18. Some case studies have reported that patients co-infected with MP and EBV suffered more severe symptoms19. In this study, children with MP co-infection tended to exhibit higher rates of macrolide resistance. Co-infections may indirectly increase the risk of antibiotic resistance through mechanisms such as increased antibiotic selection pressure, promotion of gene transfer, or interference with immune clearance. Research suggested that co-infected patients have higher rates of macrolide resistance gene mutations (e.g., A2063G) in Japan and China20,21. Some studies suggested that drug resistance is primarily related to host variability and prior antibiotic misuse22. However, large-scale prospective studies directly linking co-infections to antibiotic resistance are lacking and require further investigation. Yan et al. found that co-infections with other respiratory pathogens were an independent risk factor for the development of resistance genes23. This study also found that MP co-infections were associated with a higher likelihood of bronchial mucus plug, which has rarely been reported.
Several studies have shown that the rate of MP mixed bacteria infection is relatively low. In Chen et al. reported that 10.9% (22/201) of RMPP patients had bacterial co-infections24. Jin et al. confirmed that bacterial co-infections in pediatric RMPP were rare9. This study also found a low rate of MP mixed with bacterial infections. In terms of co-infection with bacteria, MP was found to compete for nutrients to eliminate other bacteria25 and activate the host inflammatory response26, resulting in limited bacterial diversity in MPP27. In contrast, viral infections can damage the lung epithelium and suppress the immune response, promoting bacterial growth and leading to secondary bacterial infections28, which may increase bacterial diversity.
This study has several limitations. It was a retrospective study with a small sample size, which might introduce selection bias. Future research should involve prospective studies with larger sample sizes. In conclusion, MP was most commonly co-infected with RV. Children with MP co-infections tend to exhibit higher rates of macrolide resistance, more severe pneumonia, and a higher likelihood of bronchial mucus plug.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Liu, C. et al. Research status and challenges of Mycoplasma pneumoniae pneumonia in children: A bibliometric and visualization analysis from 2011 to 2023. Medicine https://doi.org/10.1097/MD.0000000000037521 (2024).
Zhe, S. et al. Global research trends of pneumonia in children: A bibliometric analysis. Front. Pediatr. https://doi.org/10.3389/fped.2023.1306234 (2023).
Zhao, M. C. et al. Impact and clinical profles of Mycoplasma pneumoniae co-detection in childhood community-acquired pneumonia. BMC Infect. Dis. https://doi.org/10.1186/s12879-019-4426-0 (2019).
Chiu, C. Y. et al. Impact of bacterial and viral coinfection on Mycoplasma pneumoniae in childhood community-acquired pneumonia. J. Microbiol. Immunol. Infect. https://doi.org/10.1016/j.jmii.2013.06.006 (2015).
Kim, J. H. et al. Clinical features of Mycoplasma pneumoniae coinfection and need for its testing in infuenza pneumonia patients. J. Thorac. Dis. https://doi.org/10.21037/jtd.2018.10.33 (2018).
Chao, Y. et al. Current status of Mycoplasma pneumoniae infection in China. World J. Pediatr. https://doi.org/10.1007/s12519-023-00783-x (2024).
Zheng, Y. et al. The level of D-dimer is positively correlated with the severity of pneumonia in children. Front. Cell Infect. Microbiol. https://doi.org/10.3389/fcimb.2021.687391 (2021).
He, M. et al. Clinical efficacy of macrolide antibiotics in Mycoplasma pneumoniae pneumonia carrying a macrolide-resistant mutation in the 23 S rRNA gene in pediatric patients. BMC Infect. Dis. 24(1), 758. https://doi.org/10.1186/s12879-024-09612-6 (2024).
Liu, J.-R. et al. Low bacterial co-infection invalidates the early use of non-anti-Mycoplasma pneumoniae antibiotics in pediatric refractory Mycoplasma pneumoniae pneumonia patients. Front Pediatr. https://doi.org/10.3389/fped.2018.00296 (2018).
Lv, T. et al. Utilizing metagenomic next-generation sequencing for pathogen detection and diagnosis in lower respiratory tract infections in real-world clinical practice. Infection 52(2), 625–636. https://doi.org/10.1007/s15010-024-02185-1 (2024).
Lai, L. M. et al. The value of metagenomic next-generation sequencing in the diagnosis of fever of unknown origin. Sci. Rep. 15(1), 1963. https://doi.org/10.1038/s41598-025-86295-2 (2025).
Qin, W. et al. Metagenomic next generation sequencing of bronchoalveolar lavage fluids for the identification of pathogens in patients with pulmonary infection: A retrospective study. Diagn. Microbiol. Infect Dis. 110(1), 116402. https://doi.org/10.1016/j.diagmicrobio.2024.116402 (2024).
Zhou, Y. et al. Impact of viral coinfection and macrolide-resistant mycoplasma infection in children with refractory Mycoplasma pneumoniae pneumonia. BMC Infect. Dis. https://doi.org/10.1186/s12879-020-05356-1 (2020).
Martin, E. T. et al. Multiple versus single virus respiratory infections: Viral load and clinical disease severity in hospitalized children. Influenza Other Respir Viruses. https://doi.org/10.1111/j.1750-2659.2011.00265.x (2012).
Yun, L. et al. Risk factors and prescription patterns analysis for macrolide-resistant Mycoplasma pneumoniae pneumonia in children. iScience. https://doi.org/10.1016/j.isci.2024.111503 (2025).
Xinxing, Z. et al. Viral and bacterial co-infection in hospitalised children with refractory Mycoplasma pneumoniae pneumonia. Epidemiol. Infect. https://doi.org/10.1017/S0950268818000778 (2018).
Liu, J. et al. Viral and bacterial coinfection among hospitalized children with respiratory tract infections. Am. J. Infect. Control. https://doi.org/10.1016/j.ajic.2020.01.013 (2020).
Jiaojiao, G. et al. Human adenovirus Coinfection aggravates the severity of Mycoplasma pneumoniae pneumonia in children. BMC Infect. Dis. https://doi.org/10.1186/s12879-020-05152-x (2020).
Xu, Y. et al. Impact of epstein-barr virus coinfection in Mycoplasma pneumoniae pneumonia. Medicine https://doi.org/10.1097/MD.0000000000019792 (2020).
Tsutomu, Y. & Tsuyoshi, K. Epidemiology of Mycoplasma pneumoniae infections in Japan and therapeutic strategies for macrolide-resistant M. Pneumoniae. Front Microbiol. https://doi.org/10.3389/fmicb.2016.00693 (2016).
Xinyu, J. et al. Increased in vitro antimicrobial resistance of Mycoplasma pneumoniae isolates obtained from children in Beijing, China, in 2023. Front. Cell Infect. Microbiol. https://doi.org/10.3389/fcimb.2024.1478087 (2025).
Francesco, F. et al. The challenge of antimicrobial resistance (AMR): Current status and future prospects. Naunyn Schmiedebergs Arch Pharmacol. https://doi.org/10.1007/s00210-024-03318-x (2024).
Mengzhen, Y. et al. Clinical characteristics and logistic regression analysis of macrolide-resistant Mycoplasma pneumoniae pneumonia in children. Eur. J. Clin. Microbiol. Infect. Dis. 43(9), 1825–1835. https://doi.org/10.1007/s10096-024-04902-y (2024).
Chen, L. L. et al. Mixed infections in children with Mycoplasma pneumoniae pneumonia. Zhonghua Er Ke Za Zhi 50(3), 211–215. https://doi.org/10.1007/s11783-011-0280-z (2012).
Yang, J. et al. Cytokines in Mycoplasma pneumoniae infections. Cytokine Growth Factor Rev 15, 157–168. https://doi.org/10.1016/j.cytogfr.2004.01.001 (2004).
Peteranderl, C. et al. Inflammatory responses regulating alveolar ion transport during pulmonary infections. Front. Immunol. 8, 446. https://doi.org/10.3389/fimmu.2017.00446 (2017).
Miller-Samuel, I., Ernst-Robert, K. & Bader-Martin, W. LPS, TLR4 and infectious disease diversity. Nat. Rev. Microbiol 3(1), 36–46. https://doi.org/10.1038/nrmicro1068 (2005).
Brealey, J. C. et al. Viral bacterial co-infection of the respiratory tract during early childhood. FEMS Microbiol. Lett. https://doi.org/10.1093/femsle/fnv062 (2015).
Acknowledgements
We thank all colleagues in the Department of Pediatric for collecting the clinical data. We also thank all the families for their enrollment in this study.
Funding
The project was supported by grants from Bethune Foundation (SCE041BS).
Author information
Authors and Affiliations
Contributions
Li Yuan wrote the main manuscript text and Lili Zhou prepared Figs. 1 and table 1–3. Diao Mingyue helped to analyzed and interpreted the data. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Due to the retrospective nature of the study, the Ethics and Research Council of Women and Children’s Hospital of Ganzhou waived the need of obtaining informed consent. All methods were performed in accordance with the relevant guidelines and regulations. All experimental protocols were approved by Women and Children’s Hospital of Ganzhou.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yuan, L., Mingyue, D. & Zhou, L. Analysis of the characteristics of mixed infections with Mycoplasma pneumoniae in children. Sci Rep 15, 9414 (2025). https://doi.org/10.1038/s41598-025-94292-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-94292-8