Abstract
To explore whether Methamphetamine-related disorders (MRDs) will cause the risk of cardiomyopathy in the future. This study used Taiwan’s Longitudinal Generation Tracking Database (LGTD) to conduct a 1:4 paired analysis of sex, age, and inclusion year. 17,071 patients with MRDs and 153 patients with cardiomyopathy were selected; 68,264 patients without MRDs and 274 patients with cardiomyopathy were also selected. This study used SPSS 22 statistical software to conduct Cox regression analysis. Patients with MRDs had a 3.421-folds higher risk of cardiomyopathy than patients without MRDs. Men have a 0.735-fold lower risk of developing cardiomyopathy than women. In terms of age group, aged 50–64 and ≧ 65 have a 1.145- and 1.332-folds higher risk of cardiomyopathy, respectively, compared to those aged 20–49. For each one-point increase in Charlson Comorbidity Index (CCI), the risk of cardiomyopathy rises by 58.3%. Specifically, for three types of Methamphetamines (Methamphetamine and other psychostimulant dependence, Methamphetamine or related acting sympathomimetic abuse, Methamphetamine psychosis), the HR for cardiomyopathy in patients with MRDs was 3.864 (p < 0.001), 2.916 (p < 0.001), and 2.295 (p = 0.016) times higher, respectively, compared to patients without MRDs. The Kaplan-Meier log-rank test was used to calculate the cumulative risk of MRDs, showing a significant difference in the cumulative cardiomyopathy incidence between the MRDs and non-MRDs groups (long-rank test, p < 0.001). MRDs will increase the risk of cardiomyopathy. Women are more susceptible to cardiomyopathy than men, and the risk escalates for individuals aged 50–64 and those 65 years or older, compared to the 20–49-year age group. Additionally, an increase in the CCI correlates with a heightened risk of cardiomyopathy. There are important differences between these groups in terms of duration, frequency, and severity of use, with longer exposure and more frequent use increasing the risk of dependence and psychosis, but individual susceptibility, dose, and use patterns also play key roles.
Similar content being viewed by others
Introduction
Amphetamines were first synthesized in the late 1920s as an analogue of the popular drug ephedrine1. These drugs are mainly used for nasal congestion relief, asthma, narcolepsy, depression, and weight loss, and less frequently for heart block, myasthenia gravis, dysmenorrhea, and persistent hiccups2. Amphetamine patents in the 1920s forced competitors to synthesize methamphetamine, which was used for many of the same indications as amphetamine3. However, recognition of their serious addictive potential led to production restrictions, but the drugs entered the black market, thus starting an epidemic. The estimated prevalence of illicit drug use among people aged 12 years and older in the United States was 13.0% in 2019, and 0.6% (approximately 1.5 million people) of people aged 12 years and older reported having methamphetamine use disorder in 20204,5. The average duration of methamphetamine use before a diagnosis of congestive heart failure was five years, with almost half (18%) of reported diagnoses made within the previous 12 months. In some cases, congestive heart failure was diagnosed even after a single use6. Methamphetamine use induces potent vasoconstriction, resulting in severe vasospasm of the coronary arteries and microvasculature, resulting in myocardial ischemia. In the heart, methamphetamine promotes myocardial structural and electrical remodeling, which may promote arrhythmias. Ultimately, methamphetamine induces severe mitochondrial dysfunction and cardiomyocyte death, leading to dilated cardiomyopathy and heart failure7,8. Amphetamine use causes cardiomyopathy through catecholamine-mediated effects such as tachycardia, hypertension, vasoconstriction, and direct cardiotoxic effects9,10.
Cardiomyopathy results from changes in the structure and function of the myocardium caused by a variety of potential causes, which can be localized to the heart or cause systemic disease11. Cardiomyopathy can be classified as primary (e.g., hereditary, mixed, or acquired) or secondary (e.g., infiltrative, toxic, inflammatory). The main types include dilated cardiomyopathy, hypertrophic cardiomyopathy, restrictive cardiomyopathy and arrhythmogenic right ventricular cardiomyopathy11. Hypertrophic cardiomyopathy is the most common primary cardiomyopathy and can cause active dyspnea, syncope, atypical chest pain, HF, and sudden cardiac death. Dilated cardiomyopathy can be hereditary or acquired and usually presents with classic symptoms of HF with reduced ejection fraction of the heart. Restrictive cardiomyopathy is less common and is usually associated with systemic disease12.
Cardiomyopathy is a heterogeneous disease and a leading cause of heart failure (HF) and is associated with significant morbidity and mortality13. HF often coexists with many comorbidities, among which decreased renal function is particularly important. The interaction between the heart and kidneys is complex and bidirectional14, and 75% of patients have left ventricular hypertrophy at the start of dialysis15.
There are currently limited studies on the relationship between Methamphetamine-related disorders (MRDs) and cardiomyopathy. Therefore, we hypothesized that MRDs and cardiomyopathy have related risk effects, and we used Taiwan’s Longitudinal Generation Tracking Database (LGTD) from 2000 to 2015 to analyze the impact of MRDs and cardiomyopathy through long-term follow-up.
Materials and methods
Data source
Since 1995, Taiwan’s Ministry of Health and Welfare has maintained the National Health Insurance Research Database (NHIRD), This study utilized data from the Longitudinal Health Insurance Database (LHID), a subset of the NHIRD which encompasses medical services provided to all insured individuals under the National Health Insurance (NHI) scheme. This includes 23 million Taiwanese citizens and covers disease diagnoses, examinations, drug prescriptions, outpatient and hospital visit codes, medical procedures, and charges. The NHI’s coverage rate has surpassed 99%, making NHIRD a significant source of empirical data for medical and health research. NHI, a compulsory and comprehensive health insurance program, has been in effect since 1995, covering over 23 million beneficiaries, which is more than 99% of the population, and is contracted with 97% of medical service providers. The program’s specifics have been detailed in numerous prior studies. NHIRD holds extensive data on outpatient and inpatient numbers. The inpatient dataset from 2000 to 2015, coded according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), was selected for analysis.
NHIRD served as the primary data source for this study, standing as Taiwan’s most exhaustive healthcare research database. Diagnoses and procedures within NHIRD are classified following ICD-9-CM. The database is also connected to the National Death Registry and employs encryption of national identification numbers to safeguard individual privacy. Personal data within NHIRD is encrypted to maintain patient confidentiality. This study adhered to the Declaration of Helsinki and received approval from the Research Ethics Committee of the Tri-Service General Hospital, National Defense Medical College. (TSGHIRB: E202416046).
Research design and participants
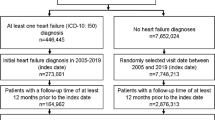
Starting January 1, 2000, NHI enrollees were included in the study. Exclusions were made for those with an MRDs prior to 2000, cardiomyopathy before tracking, drug disorders other than Amphetamine, no tracking, under 20 years of age, and unknown sex (n = 6,178). A diagnosis was established with at least one medical visit or hospitalization. Initially, individuals with an MRDs as of January 2000 were identified; subsequently, the rest were categorized as the unexposed group (non-MRDs) and matched in a 1:4 ratio by age, sex, and year of inclusion. The exposure date for the MRDs was set as the index date for the exposed group, with the same date applied to the matched subjects in the unexposed group. “Non-MRDs” refers to individuals not exposed to Methamphetamine (true control group). In February 2000, those selected for the study pairs and any individuals who had passed away in January were removed, and the same matching and indexing procedures were applied. This method was repeated monthly until the end of the observation period in December 2015.
To classify the three types of amphetamine use—dependence, abuse, and psychosis—it is essential to understand the diagnostic criteria and distinctions drawn from psychiatric and medical literature. These classifications align with diagnostic frameworks such as the DSM-5 (Diagnostic and Statistical Manual of Mental Disorders, 5th edition) and the ICD-10/ICD-11 (International Classification of Diseases).
-
1.
Amphetamine and other psychostimulant dependence.
-
This category falls under substance use disorder (SUD) in DSM-5 and corresponds to F15.2 (Mental and Behavioral Disorders due to Use of Other Stimulants: Dependence Syndrome) in ICD-10.
-
Dependence is characterized by compulsive drug-seeking behavior, tolerance, withdrawal symptoms, and the inability to control use despite negative consequences.
-
Research indicates that chronic and high-frequency use of amphetamines leads to neuroadaptive changes, reinforcing compulsive drug-seeking behaviors16.
-
2.
Amphetamine or related acting sympathomimetic abuse.
-
This category refers to harmful or problematic use that does not meet the criteria for dependence. In DSM-4, this was called Substance Abuse, but DSM-5 now includes it under the broader spectrum of SUD. In ICD-10, this aligns with F15.1 (Harmful Use of Stimulants).
-
It typically includes episodic, recreational, or binge use that leads to short-term harm, such as cardiovascular effects, aggression, or risky behaviors.
-
Studies show that intermittent, high-dose amphetamine use can still cause cognitive and psychiatric impairments without full-blown dependence17.
-
3.
Amphetamine psychosis.
-
Amphetamine-induced psychosis is recognized in both DSM-5 (Substance/Medication-Induced Psychotic Disorder) and ICD-10 (F15.5: Psychotic Disorder due to Stimulants).
-
It involves symptoms like hallucinations, delusions, paranoia, and agitation, often resembling schizophrenia but directly triggered by amphetamine use.
-
Research suggests that prolonged high dose use, or binge consumption significantly increases the risk of psychosis, but even short-term use in vulnerable individuals can trigger symptoms18.
Rationale for classification
The differentiation between these groups implies that:
-
Dependence results from prolonged and frequent amphetamine use, leading to addiction.
-
Abuse involves intermittent, non-dependent use that still causes harm.
-
Psychosis can emerge independently of dependence, often linked to high doses or individual susceptibility.
The three types of MRDs—dependence, abuse, and psychosis—classify different levels and consequences of Methamphetamine use. Here’s how they relate to types of use and why distinguishing them is important:
Clarification of types of methamphetamine use
Each MRD category relates to different aspects of Methamphetamine use.
Mode of use
These categories do not directly classify how Methamphetamine is used (e.g., oral, snorted, injected, smoked), but different modes of use may increase the likelihood of certain disorders.
For example, injections and smoking have a higher risk of dependence and psychosis compared to oral use.
Length of use
Dependence usually results from chronic, long-term use.
Abuse can occur after short- or long-term use, depending on the pattern and severity.
Psychosis can emerge after both short- and long-term use, though chronic heavy use increases the risk.
Significance of comparing these codes
Clinical Implications—Helps determine the severity of the patient’s condition and the appropriate treatment approach.
Treatment & Intervention—Differentiating between abuse, dependence, and psychosis guides medical decisions, such as detox, rehabilitation, or antipsychotic treatment.
Legal & Insurance Considerations—Different diagnoses impact medical coding, insurance coverage, and legal outcomes (e.g., disability claims, criminal responsibility).
Research & Public Health—Understanding patterns of MRDs helps in tracking trends, designing prevention programs, and guiding policy decisions.
A flowchart of the study design is depicted in Fig. 1.
Covariates
Covariates included sociodemographic characteristics and comorbidities. Sociodemographic characteristics include sex, age (20–49, 50–64, ≧ 65 years old), monthly insurance premium, urbanization level, and area of residence. The monthly insurance premium has been divided into three categories in New Taiwan Dollars [NT$]: < 18,000, 18,000–34,999, and ≥ 35,000.
Season” refers to a specific time frame corresponding to the four calendar seasons: spring, summer, fall, and winter. For example, spring spans from March to May, summer from June to August, fall from September to November, and winter from December to February. Season was included as a demographic variable to explore potential temporal influences on the incidence and severity of methamphetamine-related cardiomyopathy (MRCM). This decision was based on evidence suggesting that seasonal factors—ranging from behavioral patterns of drug use to environmental stressors—may impact cardiovascular health. The rationale for including season as a variable in this study is threefold:
-
1.
Seasonal variations in methamphetamine use: Previous research suggests that stimulant use, including methamphetamine, may fluctuate seasonally. Increased use has been reported in colder months, potentially due to higher rates of depression and social isolation, while summer months may see higher use in recreational settings such as festivals and nightlife19. If methamphetamine use follows seasonal patterns, fluctuations in exposure intensity could influence the incidence and severity of MRCM.
-
2.
Winter-associated viral infections and myocardial injury: Viral infections, particularly influenza and other respiratory viruses, are more prevalent in winter and have been linked to increased cardiovascular morbidity, including myocarditis and myocardial dysfunction20. Given that methamphetamine exacerbates oxidative stress and cardiac inflammation, co-occurring viral infections could act as an additional trigger for myocardial injury, potentially worsening MRCM outcomes.
-
3.
Environmental and physiological stressors: Cold weather increases sympathetic nervous system activity, vasoconstriction, and cardiac workload, leading to higher blood pressure and heart rate21. These physiological changes may compound methamphetamine’s known cardiotoxic effects, potentially leading to seasonal variations in MRCM severity.
The level of urbanization is the level of development of a city defined by population and certain indicators. The first-level urbanization rate is defined as a population exceeding 1,250,000 people. Secondary urbanization is defined as a population between 500,000 and 1,250,000. Urbanization levels 3 and 4 are defined as populations between 150,000 and 500,000 and less than 150,000, respectively. Charlson Comorbidity Index (CCI) is one of the most important widely used comorbidity index. The ICD codes for cardiomyopathy and comorbidities are listed in Table S1.
Statistical analysis
The study utilized SPSS 22.0 software (SPSS Inc., Chicago, IL, USA) for statistical analysis, considering a p-value < 0.05 as statistically significant. Demographic characteristics and common comorbidities between patients with and without MRDs were compared using Pearson chi-square tests. Continuous variables were presented as means (± SD), and the mean age of patients was determined for two column pairs via a two-sample t-test. Age, sex, and concurrent comorbidities were adjusted for in the multivariable model analyses. Hazard ratios (HR) and 95% confidence intervals (CI) were derived from multivariate Cox proportional hazards models. The Kaplan-Meier method assessed the cardiomyopathy risk in patients with and without MRDs.
Results
Basic characteristics of patients in the study
Table 1 presents the demographic characteristics of patients with MRDs and their common comorbidities. From 2000 to 2015, the study included 17,071 patients with ARDs and 68,264 control subjects. The average age in the MRDs group was 43.82 ± 18.40 years; males constituted 70.25%, and females 29.75%. Significant differences between the MRDs group and controls were observed in monthly insurance premiums, CCI, residential area, urbanization level, and level of care, all with p-values less than 0.001.
Characteristics of patient endpoints in the study
Table 2 shows the data of demographic characteristics for MRDs and associated comorbidities. From 2000 to 2015, the study included 17,071 MRDs patients and 68,264 controls. The MRDs group had an average age of 51.02 ± 18.97 years; males constituted 70.25%, while females accounted for 29.75%. Significant differences between the study and control groups included cardiomyopathy, age, age groups, monthly insurance premiums, CCI, season, residential area, urbanization level, and level of care, all with p-values less than 0.001.
Years from methamphetamine-related disorders to cardiomyopathy
The mean time after diagnosis from the index date to the diagnosis of cardiomyopathy was 10.24 (SD = 9.86) years. Patients with MRDs developed cardiomyopathy at a mean age of 5.62 (SD = 3.89) years earlier than patients without MRDs (6.21 [SD = 4.20] years) (Table S2-1, Table S2-2).
Use Cox regression to analyze the influencing factors of cardiomyopathy in methamphetamine-related disorders
Table 3 presents the results of the cox regression analysis on risk factors within the MRDs study cohort and the comparison group. The study reveals that after adjusting for sex, age, insurance premiums, CCI, season, ___location, urbanization level, and level of care, individuals with MRDs have a 3.421 times higher risk of developing cardiomyopathy compared to non-MRDs. Gender-wise, men have a 0.735 times lower risk of developing cardiomyopathy than women. Age-wise, individuals aged 50–64 and those 65 or older have a 1.145- and 1.332-times higher risk, respectively, compared to those aged 20–49. For each one-point increase in CCI, the risk of cardiomyopathy rises by 58.3%. Seasonally, the risk in winter is 1.672 times higher than in spring. Regarding urbanization, the first and second levels have a 2.210- and 1.563-times higher risk, respectively, compared to the fourth level. Finally, concerning the level of care, hospital centers and regional hospitals have a 1.985- and 1.301-times higher risk, respectively, compared to local hospitals.
Methamphetamine—related disorders using Cox regression to stratify cardiomyopathy factors by the variables listed in the table
Table 4 presents the incidence and hazard ratio (HR) of cardiomyopathy among individuals with and without MRDs relative to controls. Accounting for variables such as sex, age, insurance premiums, CCI, season, ___location, urbanization level, and level of care, the study found that patients with MRDs had a 3.421-fold higher incidence of cardiomyopathy compared to those without MRDs (p < 0.001). Gender-wise, male MRDs patients had a 3.178-fold higher HR for cardiomyopathy than non-MRDs patients (p < 0.001), while female ARDs patients had a 4.011-fold increase (p < 0.001). Age-wise, MRDs patients aged 20–49 had a 2.978-fold higher risk, those 50–64 had a 3.402-fold increase, and those aged 65 and above had a 4.551-fold higher risk of cardiomyopathy compared to non-MRDs patients (p < 0.001).
Factors of cardiomyopathy across various subgroups of methamphetamine e-related disorders using Cox regression and applying bonferroni correction for multiple comparisons
Table 5 presents the incidence and hazard ratio (HR) of cardiomyopathy among individuals with and without MRDs subgroups relative to controls. Accounting for variables such as sex, age, insurance premiums, CCI, season, ___location, urbanization level, and level of care, the study found that the incidence of cardiomyopathy was 0.77 cases per 100,000 person-years (PYs) in patients with MRDs, compared to 0.34 cases per 100,000 PYs in those without MRDs. The HR for cardiomyopathy was 3.421 times higher in patients with MRDs than in those without (p < 0.001). Specifically, for three types of Methamphetamines (Methamphetamine and other psychostimulant dependence, Methamphetamine or related acting sympathomimetic abuse, Methamphetamine psychosis), the HR for cardiomyopathy in patients with MRDs was 3.864 (p < 0.001), 2.916 (p < 0.001), and 2.295 (p = 0.016) times higher, respectively, compared to patients without MRDs.
Kaplan-Meier cumulative incidence curve of cardiomyopathy in methamphetamine-related disorders
Figure 2 reveals the cumulative incidence of cardiomyopathy (long-rank test, p < 0.001) in the MRDs group (n = 17,071) and the non-MRDs group (n = 68,264) during follow-up. There was a significant difference in the cumulative incidence of cardiomyopathy between the MRDs group and the non-MRDs group (long-rank test, p < 0.001).
Discussion
This population-based cohort study showed that patients with MRDs had a 3.421-folds higher risk of cardiomyopathy than patients without MRDs. Men have a 0.735-fold lower risk of developing cardiomyopathy than women. In terms of age group, aged 50–64 and ≧ 65 have a 1.145- and 1.332-folds higher risk of cardiomyopathy, respectively, compared to those aged 20–49. For each one-point increase in CCI, the risk of cardiomyopathy rises by 58.3%. Specifically, for three types of Methamphetamines (Methamphetamine and other psychostimulant dependence, Methamphetamine or related acting sympathomimetic abuse, Methamphetamine psychosis), the HR for cardiomyopathy in patients with MRDs was 3.864 (p < 0.001), 2.916 (p < 0.001), and 2.295 (p = 0.016) times higher, respectively, compared to patients without MRDs. The Kaplan-Meier log-rank test was used to calculate the cumulative risk of MRDs, showing a significant difference in the cumulative cardiomyopathy incidence between the MRDs and non-MRDs groups (long-rank test, p < 0.001).
Methamphetamine-induced cardiomyopathy, a rare yet serious heart condition, arises from prolonged Methamphetamine use. Journal of Cardiology Cases reported on a 37-year-old individual who suffered from dilated cardiomyopathy as a result of chronic Methamphetamine consumption22. Another published case report describes a patient with severe Methamphetamine-induced cardiomyopathy who was successfully treated with heart transplantation. This study re-emphasizes the critical role of early intervention and appropriate management in improving patient outcomes23. Although the exact mechanism of Methamphetamine-induced cardiomyopathy is not fully understood, several possible pathways have been proposed, including oxidative stress, mitochondrial dysfunction, and neurohormonal priming24. Methamphetamine use causes cardiomyopathy through catecholamine-mediated effects such as tachycardia, hypertension, vasoconstriction and direct cardiotoxic effects25. Stimulants such as Methamphetamine exert negative effects through catecholamines, causing tachycardia, hypertension, vasoconstriction, and vasospasm26,27. Sustained exposures to catecholamines can lead to cardiotoxic effects, including changes in myocardial contractility and myocardial fibrosis28,29. Structural changes may include dilated cardiomyopathy, with or without reduced ejection fraction, or hypertrophic cardiomyopathy30. Emerging evidence suggests that acute Epstein-Barr virus (EBV) infection, identified through virological screening, may contribute to the development of cardiomyopathy, particularly in individuals with a history of Methamphetamine use. This highlights a potential interplay between viral myocarditis and Methamphetamine-induced cardiotoxicity, warranting further investigation into their combined effects on myocardial function25. Recent studies indicate that the incidence of Methamphetamine-induced cardiomyopathy appears to be increasing, possibly due to increased availability and use of Methamphetamines, particularly in younger adults. Additionally, there is growing evidence that the combined use of Methamphetamines with other drugs, such as heroin or opioids, may further increase the risk of cardiomyopathy8. Some studies suggest that early identification and cessation of Methamphetamine use may improve outcomes, while others find that long-term prognosis for these patients remains poor even with aggressive drug treatment31,32.
Comparison of clinical features and outcomes of patients with reversible and persistent methamphetamine-associated cardiomyopathy showed that MAC reversal is not uncommon and is associated with significant clinical improvement including reduced mortality. It can be facilitated by MA cessation when the cardiac chambers, especially the right ventricle, are not severely dilated33,34,35,36,37,38,39,40,41. Clinical correlates and outcomes of methamphetamine-associated cardiovascular disease among hospitalized patients in California; methamphetamine use was associated with a similar magnitude of CVD risk compared with alcohol and heroin42. The CCI is associated with the risk of 30-day mortality in patients with myocardial injury after non-cardiac surgery43. Studies have identified three types of Methamphetamine use—dependence, abuse, and psychosis—as being associated with an increased hazard ratio (HR) for cardiomyopathy44,45. The distinction between these three types of Methamphetamine use—dependence, abuse, and psychosis—suggests important differences in the duration, frequency, and severity of use.
-
1.
Methamphetamine and other psychostimulant dependence: This category refers to chronic and compulsive use, where individuals develop tolerance, experience withdrawal symptoms, and prioritize drug use over other aspects of life. Dependence typically implies long-term, high-frequency exposure to Methamphetamines.
-
2.
Methamphetamine or related acting sympathomimetic abuse: This classification includes sporadic or less frequent misuse that does not necessarily lead to full dependence but still results in harmful consequences. It suggests that individuals in this group engage in episodic or recreational use, potentially with lower cumulative exposure compared to dependent users.
-
3.
Methamphetamine psychosis: This refers to a state where amphetamine use leads to psychotic symptoms, such as hallucinations or paranoia. While psychosis can occur in both short- and long-term users, it is more commonly associated with high doses, chronic use, or binge patterns. However, some individuals may develop psychosis even with relatively short-term but intense use.
The distinction between these groups implies that longer exposure and more frequent use increases the risk of dependence and psychosis, but individual susceptibility, dosage, and patterns of use also play key roles. The above research results are similar to our research results.
In Taiwan, the majority of amphetamines abused are methamphetamine, which accounts for 40% of illicit drug use, second only to heroin46. Additionally, Methamphetamine drugs (such as dextroamphetamine) are not licensed or reimbursed by Taiwan’s NHI program. Additionally, people who had poor impulse control in childhood may be prone to abusing methamphetamine because its effects occur more quickly and last longer than amphetamine drugs [47.48]. The long-term prognosis of patients with Methamphetamine-induced cardiomyopathy remains poor, highlighting the need for increased awareness and prevention efforts around this growing public health problem8. “Our findings align with global trends, as demonstrated by Raja et al. (2024), who reported an increase in substance-induced cardiomyopathy-related mortality among older adults in the United States from 1999 to 202049. This reinforces the need for early cardiovascular screening in individuals with MRDs.”
Strengths
Currently, there is limited research on the relationship between MRDs and its impact on the development of cardiomyopathy in the general population. To our knowledge, our study is the largest and longest nationwide cohort study investigating the association between MRDs and cardiomyopathy risk in Taiwan from 2000 to 2015. Our study shows that people with MRDs have a higher risk of cardiomyopathy than people without MRDs, women have a greater risk of cardiomyopathy than men, those aged 50–64 and ≧ 65 years have a greater risk of injury than those aged 20–49 years. For each one-point increase in CCI, the risk of cardiomyopathy rises by 58.3%. Specifically, for three types of Methamphetamines (Methamphetamine and other psychostimulant dependence, Methamphetamine or related acting sympathomimetic abuse, Methamphetamine psychosis), the HR for cardiomyopathy in patients with MRDs was higher compared to patients without MRDs. Kaplan-Meier showed a significant difference in the incidence of cumulative cardiomyopathy between the MRDs and non-MRDs groups. Distinctions between the three types of Methamphetamine use (dependence, abuse, and psychosis) suggest that there are important differences between these groups in terms of duration, frequency, and severity of use, with longer exposure and more frequent use increasing the risk of dependence and psychosis, but individual susceptibility, dose, and use patterns also play key roles.
Limitations
This study has some limitations. First, this is a generational follow-up study based on the Taiwanese population, so our findings may not be generalizable to other regions and ethnic groups. Second, we were unable to assess the lifestyle, behavioural patterns, alcohol consumption, genetic factors, psychosocial factors, environmental factors, severity, or psychological assessment of patients with MRDs because these data were not recorded in the NHI research repository. Third, we used data from the NHIRD rather than self-reports from patients with MRDs. Therefore, the prevalence of MRDs or the incidence of cardiomyopathy may still be underestimated because the database only contains data on those who seek medical care. There were no reported cases of patients with MRDs who did not present to clinics or emergency rooms, which may have increased bias in the study. Finally, we were unable to account for the onset and duration of comorbidities, which may influence changes in MRDs.
Conclusion
The study indicates that individuals with MRDs have a significantly higher risk of developing cardiomyopathy compared to those without MRDs. It also suggests that women are more susceptible to cardiomyopathy than men, and the risk escalates for individuals aged 50–64 and those 65 years or older, compared to the 20–49-year age group. Additionally, an increase in the CCI correlates with a heightened risk of cardiomyopathy. The study found that HR for cardiomyopathy is elevated in cases of three types of MRDs: dependence on Methamphetamines and other psychostimulants, abuse of Methamphetamines or related sympathomimetics, and Methamphetamine psychosis. These findings could inform the creation of cardiomyopathy prevention strategies. There are important differences between these groups in terms of duration, frequency, and severity of use, with longer exposure and more frequent use increasing the risk of dependence and psychosis, but individual susceptibility, dose, and use patterns also play key roles.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Change history
10 May 2025
The original online version of this Article was revised: In the original version of this Article, Wu-Chien Chien and Sung-Sen Yang were incorrectly affiliated with “Department of Medical Research, Tri-Service General Hospital, National Defense Medical Center, 7115R, No.325, Section 2, Cheng-Kung Road, Nei-hu District, Taipei City 11490, Taiwan”.
The correct affiliations for Wu-Chien Chien are ‘Graduate Institute of Medicine, National Defense Medical Center, Taipei 11490, Taiwan’, ‘Department of Medical Research, Tri-Service General Hospital, Taipei 11490, Taiwan’, ‘School of Public Health, National Defense Medical Center, Taipei 11490, Taiwan’, ‘Taiwanese Injury Prevention and Safety Promotion Association (TIPSPA), Taipei 11490, Taiwan’ and ‘Graduate Institute of Life Sciences, National Defense Medical Center, Taipei 11490, Taiwan’. The correct affiliations for Sung-Sen Yang are ‘Graduate Institute of Medicine, National Defense Medical Center, Taipei 11490, Taiwan’ and ‘Department of Nephrology, Tri-Service General Hospital, Taipei 11490, Taiwan’.
The article has been corrected.
References
Rasmussen, N. Making the first anti-depressant: amphetamine in American medicine, 1929–1950. J. Hist. Med. Allied Sci. 61, 288–323 (2006).
Vearrier, D., Greenberg, M. I., Miller, S. N., Okaneku, J. T. & Haggerty, D. A. Methamphetamine: history, pathophysiology, adverse health effects, current trends, and hazards associated with the clandestine manufacture of methamphetamine. Dis. Mon. 58, 38–89 (2012).
Rasmussen, N. Amphetamine-type stimulants: the early history of their medical and non‐medical uses. Int. Rev. Neurobiol. 120, 9–25 (2015).
Centers for Disease Control and Prevention. Centers for Disease Control and Prevention: FastStats: Illegal drug use (2022). https://www.cdc.gov/nchs/fastats/drug-use-illicit.htm.
National Institute on Drug Abuse. What is the scope of methamphetamine use in the United. https://nida.nih.gov/publications/research-reports/methamphetamine/what-scope-methamphetamine-misuse-in-united-states (2024).
Science, X. Medical xpress: prevalence of Meth heart failure now seen in a wide range of socioeconomic and Racial groups (2022). https://medicalxpress.com/news/2022-12-prevalence-meth-heart-failure-wide.html.
Kevil, C. G., Goeders, N. E., Woolard, M. D. et al. 7. Methamphetamine use and cardiovascular disease. Arterioscler Thromb. Vasc. Biol. 39, 1739–1746. https://doi.org/10.1161/ATVBAHA.119.312461 (2019).
Breier, Y., Azrak, E., Banks, W., Kushmakov, R. & Aleshinskaya, I. Management of new onset Amphetamine-Induced acute decompensated heart failure in a young adult: case report. Cureus 15(8), e43854. https://doi.org/10.7759/cureus.43854 (2023).
Lucy Chapman, I. et al. Amphetamine-induced cardiomyopathy complicated by embolic stroke: a case report. Eur. Heart J. Case Rep. 6(2), ytac044. https://doi.org/10.1093/ehjcr/ytac044 (2020).
Won, S., Hong, R. A., Shohet, R. V., Seto, T. B. & Parikh, N. I. Methamphetamine-associated cardiomyopathy. Clin. Cardiol. 36(12), 737–742 (2013).
Maron, B. J. et al. Contemporary definitions and classification of the cardiomyopathies: an American heart association scientific statement from the Council on clinical cardiology, heart failure and transplantation committee; quality of care and outcomes research and functional genomics and translational biology interdisciplinary working groups; and Council on epidemiology and prevention. Circulation 113(14), 1807–1816 (2006).
Brieler, J., Breeden, M. A. & Tucker, J. Cardiomyopathy: an overview. Am. Family Phys. 96(10), 640–646 (2017).
Seferović, P. M. et al. Heart failure in cardiomyopathies: a position paper from the heart failure association of the European society of cardiology. Eur. J. Heart Fail. 21(5), 553–576 (2019).
Tsuruya, K. & Eriguchi, M. Cardiorenal syndrome in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 24(2), 154–162 (2015).
Foley, R. N. et al. Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int. 47(1), 186–192 (1995).
Everitt, B. J. & Robbins, T. W. Drug addiction: updating actions to habits to compulsions ten years on. Ann. Rev. Psychol. 67(1), 23–50 (2016).
Sulzer, D., Sonders, M. S., Poulsen, N. W. & Galli, A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog. Neurobiol. 75(6), 406–433 (2005).
Herbst, C., O’Connell, M., Melton, B. L. & Moeller, K. E. Initiation of antipsychotic treatment for amphetamine induced psychosis and its impact on length of stay. J. Pharm. Pract. 36(6), 1324–1329 (2023).
Cunningham, J. K. et al. Mexico’s precursor chemical controls: emergence of less potent types of methamphetamines in the united States. Drug Alcohol Depend. 129(1–2), 125–136 (2013).
Guzik, T. J. et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc. Res. 116(10), 1666–1687 (2020).
Goel, H., Shah, K., Kumar, A., Hippen, J. T. & Nadar, S. K. Temperature, cardiovascular mortality, and the role of hypertension and renin–angiotensin–aldosterone axis in seasonal adversity: a narrative review. J. Hum. Hypertens. 36(12), 1035–1047 (2022).
Segawa, T., Arita, Y., Ogasawara, N. & Hasegawa, S. Hypertensive heart disease associated with methamphetamine abuse. J. Cardiol. Cases 19, 47–50. https://doi.org/10.1016/j.jccase.2018.10.001. (2019).
Qutrio et al. Methamphetamine-induced cardiomyopathy (MACM) in a middle-aged man; a case report. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5827055/Emerg(Tehran);6:0 (2018).
Dridi, H. et al. Mitochondrial calcium overload plays a causal role in oxidative stress in the failing heart. Biomolecules 13(9), 1409. https://doi.org/10.3390/biom13091409 (2023).
Chapman, L., Khalifa, I., Sheriff, N. & Colwell, N. Amphetamine-induced cardiomyopathy complicated by embolic stroke: a case report. Eur. Heart J. Case Rep. 6(2), ytac044. https://doi.org/10.1093/ehjcr/ytac044 (2022).
Kevil, C. et al. Methamphetamine use and cardiovascular disease. Arterioscler. Thromb. Vasc Biol. 39, 1739–1746 (2019).
Mladěnka, P. et al. TOX-OER and CARDIOTOX Hradec Králové researchers and collaborators. Comprehensive review of cardiovascular toxicity of drugs and related agents. Med. Res. Rev. 38, 1332–1403 (2018).
Kaye, S., McKetin, R., Duflou, J. & Darke, S. Methamphetamine and cardiovascular pathology: a review of the evidence. Addiction 102, 1204–1211 (2007).
Jellis, C., Martin, J., Narula, J. & Marwick, T. H. Assessment of nonischemic myocardial fibrosis. J. Am. Coll. Cardiol. 56, 89–97 (2010).
Richards, J. R., Harms, B. N., Kelly, A. & Turnipseed, S. D. Methamphetamine use and heart failure: prevalence, risk factors, and predictors. Am. J. Emerg. Med. 36, 1423–1428 (2018).
Shoptaw, S. J., Kao, U., Heinzerling, K. & Ling, W. Treatment for amphetamine withdrawal. Cochrane Database Syst. Rev. 2009(2), CD003021. https://doi.org/10.1002/14651858.CD003021.pub2 (2009).
Siefried, K. J., Acheson, L. S., Lintzeris, N. & Ezard, N. Pharmacological treatment of methamphetamine/amphetamine dependence: a systematic review. CNS Drugs 34(4), 337–365. https://doi.org/10.1007/s40263-020-00711-x (2020).
Zhao, S. X., Seng, S., Deluna, A., Yu, E. C. & Crawford, M. H. Comparison of clinical characteristics and outcomes of patients with reversible versus persistent Methamphetamine-Associated cardiomyopathy. Am. J. Cardiol. 125(1), 127–134 (2020).
Kaye, S., McKetin, R. & Duflou, J. Methamphetamine and cardiovascular pathology: a review of the evidence. Addiction 102, 1204–1211 (2007).
Yeo, K.-K., Wijetunga, M. & Ito, H. The association of methamphetamine use and cardiomyopathy in young patients. Am. J. Med. 120, 165–171 (2007).
Wijetunga, M., Seto, T. & Lindsay, J. Crystal methamphetamine-associated cardiomyopathy:tip of the iceberg? J. Toxicol. Clin. Toxicol. 41, 981–986 (2003).
Schürer, S., Klingel, K. & Sandri, M. Clinical characteristics, histopathological features, and clinical outcome of methamphetamine-associated cardiomyopathy. JACC Heart Fail. 5, 435–445 (2017).
Ito, H., Yeo, K.-K. & Wijetunga, M. A comparison of echocardiographic findings in young adults with cardiomyopathy: with and without a history of methamphetamine abuse. Clin. Cardiol. 32, E18–E22 (2009).
Won, S., Hong, R. A. & Shohet, R. V. Methamphetamine-associated cardiomyopathy. Clin. Cardiol. 36, 737–742 (2013).
Jafari Giv, M. Exposure to amphetamines leads to development of Amphetamine Type Stimulants Associated Cardiomyopathy (ATSAC). Cardiovasc. Toxicol. 17, 13–24 (2017).
Zhao, S. X., Kwong, C. & Swaminathan, A. et al. Clinical characteristics and outcome of methamphetamine-associated pulmonary arterial hypertension and dilated cardiomyopathy. JACC Heart Fail. 6, 209–218 (2018).
Curran, L. et al. Clinical correlates and outcomes of Methamphetamine-Associated cardiovascular diseases in hospitalized patients in California. J. Am. Heart Assoc. 11(16), e023663. https://doi.org/10.1161/JAHA.121.023663 (2022).
Kim, S. et al. The Charlson comorbidity index is associated with risk of 30-day mortality in patients with myocardial injury after non-cardiac surgery. Sci. Rep. 11(1), 18933. https://doi.org/10.1038/s41598-021-98026-4 (2021).
Martin, D. & Le, J. K. Amphetamine. In StatPearls [Internet] (StatPearls Publishing, 2024). https://www.ncbi.nlm.nih.gov/books/NBK556103/. Accessed 31 Jul 2023.
Tzeng, N. S. et al. Association between amphetamine-related disorders and dementia-a nationwide cohort study in Taiwan. Ann. Clin. Transl Neurol. 7(8), 1284–1295. https://doi.org/10.1002/acn3.51113 (2020).
Ministry of Health and Welfare. The Annual of Statistical Data of Cases and Laboratory Examinations for the Drug Abuse 2018: Taiwan Food and Drug Administration, Ministry of Health and Welfare (2018).
Fowler, J. S. et al. Fast uptake and long-lasting binding of methamphetamine in the human brain: comparison with cocaine. NeuroImage 43, 756–763 (2008).
Barr, A. M. et al. The need for speed: an update on methamphetamine addiction. J. Psychiatry Neurosci. 31, 301–313 (2006).
Raja, A. et al. Trends in substance-induced cardiomyopathy-related mortality among older adults in the united States from 1999 to 2020. Curr. Probl. Cardiol. 49(2), 102355 (2024).
Acknowledgements
We appreciate the support from the Tri-Service General Hospital Research Foundation and the Medical Affairs Bureau, Ministry of Defense, Taiwan, ROC. We also appreciate the database provided by the Health and Welfare Data Science Center, Ministry of Health and Welfare (HWDC, MOHW).
Funding
This study was funded by the Tri-Service General Hospital, grant number: TSGH-A-114010, TSGH-B-114022, TSGH-D-114196.
Author information
Authors and Affiliations
Contributions
P.-C.Y., T.-H.W., W.-T. L., Y.-C.H., S.-H.H., B.-L.W., L.-Y. F., H.-T.H., C.-H.C., S.-S.Y. and W.-C.C. wrote the main manuscript text and P.-C.Y., T.-H.W., W.-T. L., Y.-C.H., S.-H.H., B.-L.W., L.-Y. F., H.-T.H., C.-H.C., S.-S.Y. prepared Figs. 1 and 2. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was conducted according to the Code of Ethics of the World Medical Association (Declaration of Helsinki). The Institutional Review Board of the Tri-Service General Hospital approved this study (TSGHIRB No. E202416046)) and waived the need for individual consent since all the identification data were encrypted in the NHIRD.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yu, PC., Hsin, HT., Lin, WT. et al. Increased risk of cardiomyopathy in individuals with methamphetamine related disorders in Taiwan. Sci Rep 15, 11449 (2025). https://doi.org/10.1038/s41598-025-94591-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-94591-0